2022 Volume 47 Issue 2 Pages 93-99
2022 Volume 47 Issue 2 Pages 93-99
Orius insidiosus, known as the pirate bug, is widely distributed throughout the Americas. It is employed for the biological control of Frankliniella occidentalis in organic berry crops in Mexico. In conventional crops, spinosad is the main control method for this pest. The LD50 of spinosad on O. insidiosus was determined. In addition, we monitored the population density of F. occidentalis in blackberry crops under two types of management (biochemical+mass trapping, and biological control). The LD50 was 225.65 ppm 3.8 times greater than the 60 ppm dose commonly used in blackberry crops. Both types of control are efficient; however, spinosad is less effective and should be combined with other environmentally friendly strategies. The possibility of combining chromatic traps+spinosad application and chromatic traps+strategic release of O. insidiosus to effectively control thrips without compromising fruit quality is discussed.

Orius insidiosus Say (Hemiptera: Anthocoridae), the “flower bug”, is an insect widely distributed throughout the United States, Mexico, and Central and South America.1) This generalist predator feeds on aphids, lepidopteran eggs, coleoptera, mites, and pollen or flowers during periods of prey scarcity.2) It establishes itself readily on corn, sorghum, cotton, strawberries, and alfalfa.3,4) O. insidiosus is used as one of the main controllers of thrips populations, as it feeds on thrips in all stages of the life cycle5) and is effective on both open-field and protected crops.6) In Michoacán, O. insidiosus is used to control Frankliniella occidentalis (Thysanoptera: Thripidae) and other thrips species that are problematic in blackberry production.7)
Control of F. occidentalis is based on high-cost pesticides as well as the ability of F. occidentalis to develop resistance to these compounds.8–12) Currently, efforts are being made to reduce the negative impact of pesticides by promoting the use of biorational insecticides. For example, large areas are treated with spinosad to control fruit flies and Aedes spp.,13) as well as to manage thrips in mango,14) blackberry, and avocado crops (Cruz-Esteban, personal observation). Spinosad is considered to be a biological insecticide; its mode of action is by contact or ingestion, which causes paralysis in the insect.15) It is degraded rapidly in plant leaves by photolysis, hydrolysis, and oxidation.16) Spinosad is classified as an environmentally low hazardous and toxicologically reduced compound.17) However, due to the constant use of this biopesticide, thrips attacking strawberry and avocado crops in Michoacán (F. occidentalis, mainly) are becoming increasingly resistant as reported in other species18–20) and with other pesticides.11) Resistance may also be due to the biology of the insect, which does not allow spinosad to contact thrips all stages of the life cycle during application, making spinosad alone less efficient over time for the management of this pest (Cruz-Esteban, personal communication).
Although spinosad was initially considered relatively safe for non-target organisms and effective against insect pests, studies have reported toxic side effects in mammals, oxidative stress in fish, reduced mobility, and a decrease in the number of hatchlings per female of the crustacean Daphnia magna.21) There have also been reported of reduced reproductive rates, longevity, fecundity and emergence rates related to other biological parameters in Trichogramma brassicae Bezdenko (Hymenoptera: Trichogrammatidae).22)
It is, therefore, necessary to know the lethal or sublethal effects of pesticides and biopesticides on natural enemies that are used regularly to control insect pests, as this allows us to program both biopesticide applications and the release of insect natural enemies (such as predators and parasitoids).23) It is advisable to evaluate and re-evaluate the resistance of natural enemies to the effect of these pesticides and biopesticides, specifically the susceptibility of predators such as O. insidiosus, which plays a key role in the management of various agricultural pests.24) It should be noted that, even though biological control strategies are focused on the release of specialized natural enemies, generalist insects have been considered to be an integral part of biological control programs.25) Predator can be included in an integrated pest-management program for populations of various thrip species limiting various agricultural crops in Mexico such as strawberries, blackberries, avocados, and mangoes, among others.
Therefore, this study revaluates the mean lethal dose (LD50) of spinosad on the predator O. insidiosus and discusses a possible strategy that would allow the combined use of chromatic traps+spinosad application, as well as the application of spinosad+strategic release of O. insidiosus to make thrip control effective without compromising fruit quality in blackberry crops.
The experiments were carried out at the Instituto de Ecología A.C. in Pátzcuaro, Michoacán, Mexico, from November to December 2020 with groups of 20 O. insidiosus (Thripor-I, Koppert Biological System), at 22±3°C and 60% RH. The following spinosad was used: Spinosyn A and Spinosyn D, Spintor™ 12SC 120 g active ingredient/L, Dow AgroSciences de México, S.A. de C.V. This commercial product (SpinTor™ 12SC) was prepared in a solution of 100 mL/200 L of water. This information was obtained from three blackberry farms, and all three agreed to use the same amount of this commercial product in the same volume of water to control thrip populations, mainly F. occidentalis. This solution is equivalent to 60 mg/L (60 ppm of spinosad), and the indicated dose was confirmed during applications on the studied farms. The application was made once a week with motorized pumps and, when the population was very dense, every 5 days. The equipment and the form of application were similar between the studied farms.
2. Laboratory experimentsTwo experiments were conducted: (1) the effects of two doses, 60 and 80 ppm of spinosad, on O. insidiosus populations were evaluated, and (2) the 50% lethal dose (LD50) of the O. insidiosus population was determined. Sterile distilled water was used to prepare the experimental spinosad solutions.
In the first experiment, three treatments were evaluated: (A) 20 µL of a 60 ppm solution of spinosad applied to the thorax of each insect with the aid of a micropipette15,26); to avoid mobility, insects were numbed by refrigeration at 4°C for 5 min. The same method of application was used for the following experiments: (B) 20 µL of a solution of 80 ppm, and (C) 20 µL of distilled water on the thorax (control). Each treatment was performed in five replicates. One experimental unit consisted of a group of 20 O. insidiosus in a transparent plastic container of 1 L (14 cm height×10.5 cm diameter), with a pantyhose netting on the lid that prevented thrips from exiting (DuPont, USA), together with 70–100 thrips inside the container (100 thrips/20 O. insidiosus) so that they would not starve to death. To ensure that they were not contaminated by pesticides, the thrips were collected from blackberry crops after more than one month without pest management (abandoned). Mortality was recorded after 24 hr with the aid of a stereomicroscope (Leica EZ4, Leica Microsystems, Morrisville, NC, USA), and O. insidiosus that had lost complete mobility in their limbs were considered dead.
In the second experiment (LD50), a similar procedure was followed. Different doses of spinosad (0, 20, 40, 60, 80, 100, 120, 140, 160, 180, 200, 220, 250, 300, 350, 400, 450, 500, 550 and 600 ppm) were evaluated with 5 replicates; 20 µL of each evaluated dose was applied on the thorax of each insect of the corresponding experimental unit. At dose 0, only sterile water was applied as a control. The data were recorded after 24 hr with the aid of a stereomicroscope, and insects that had lost complete mobility in their limbs were considered dead.
3. Biological and biochemical control of Frankliniella occidentalis in blackberry cropsThe control of F. occidentalis was monitored on two different blackberry crops. In the first area, blackberries were grown in tunnels belonging to Koppert Development Institute Berries, located in Tiripetío, Municipality of Morelia, Michoacán (19°31′55″N, 101°22′10″W). The experimental area was 1 ha surrounded by strawberry and corn crops. For thrip management, O. insidiosus (Thripex, Koppert Biological Systems, Mexico) were used; 50 individuals/m2 were released every 15 days, and 10 blue chromatic traps (30×20 cm) were placed as monitors, 8 perimeter traps 10 m inside the crop and separated by 40 m and 2 traps within the crop separated by 20 m between them (Fig. 1A). In the second zone, blackberries were grown in open fields of the Domillo orchard, located in Barrio Alto Taretan, Michoacán (19°20′54.1″N, 101°55′46.9″W). The experimental area was 2 ha surrounded by other blackberry crops. Spinosad (SpinTor, Corteva Agriscience, Guadalajara, Jalisco, Mexico) (60 mg/L), Proxy and Protecprid (Koor Intercomercial, Morelos, Mexico), neem potassium soap, and Bio A6 (Echeri Tzippity, Michoacán, Mexico) were constantly used to control F. occidentalis in this area. In addition, 200 blue chromatic traps/ha (30×20 cm) separated by 5 m between traps were installed within the crop to capture adult thrips (Fig. 1B).7) In both experimental areas, blackberry plants had flowers as well as green and ripe fruits when the experiments were conducted. Experiments were conducted in both experimental areas between October 2020 and January 2021, which is when F. occidentalis populations increase in these regions (Cruz-Esteban, unpublished data). At both study sites, plants were 7 months from transplanting, plant height was approximately 2 m, and row spacing was 1.7 m. It was not possible to have a control plot as no farmer agreed to this.
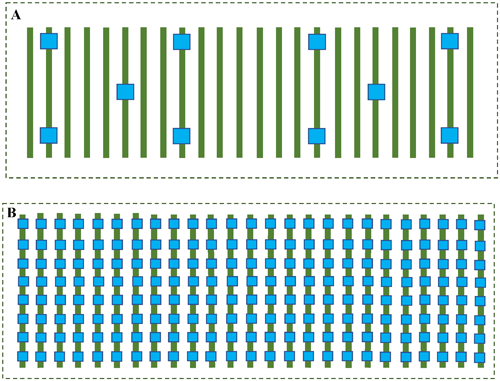
Twenty flowers/ha were collected randomly every 7 days and deposited in 10 mL vials with alcohol to be transported to the Chemical Ecology Laboratory of the Institute of Ecology, Patzcuaro, Michoacan, to count thrips with the support of a stereomicroscope (Leica EZ4, Leica Microsystems, Morrisville, NC, USA) in order to determine the number of thrips/flower. The first monitoring session was carried out one day before the beginning of the control. It was not possible to obtain other measurement variables because the cultures were patent protected.
4. Data analysisData were analyzed with R statistical software, version 4.0.5.27) Mortality results of the first experiment were analyzed by one-way analysis of variance (ANOVA), and means were compared by Tukey's test (α=0.05). In addition, a generalized linear regression analysis of the binomial family (probit) (α=0.05) was performed to determine the correlation between the doses evaluated and mortality (LD50). We analyzed thrip captures as captures/trap/day using a repeated measures ANOVA. Prior to the analyses, we tested the assumptions of normality and homoscedasticity of the data, and it was necessary to perform a square root transformation. We compared means with Tukey’s test (α=0.05).
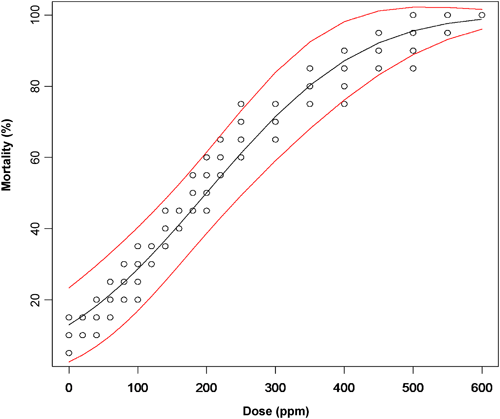
The thoracic application of spinosad at doses of 60 and 80 ppm caused mortality ranging from 21–25% (21±1.9 and 25±1.6, mean±S.E., respectively), which differed from the observed mortality in the control (9±1.9, mean±S.E.) (F=21.89; df=2, 12; P<0.0001). Although mortality did not differ between doses, a numerical increase in mortality was observed as the dose was increased. This effect was corroborated by the data from the second experiment. The generalized linear regression model indicated an increase in mortality as the evaluated dose increased (mortality=0.005664dose–1.132051), and the fitted model was significant (χ2=40.39; df=1; P<0.0001). Based on the fitted model, 225.65 mg/L (225.65 ppm; 95% CI=149.4, 250.4) was required to kill 50% of the O. insidiosus population, and 477.59 mg/L (477.59 ppm; 95% CI=325.3, 527) kill 90% of the population (Fig. 2). Thus, the LD50 is quite high (3.8 times higher) as compared to the dose commonly applied to blackberry crops for thrip control (Fig. 2).
The number of F. occidentalis nymphs at the start of biological control using O. insidiosus was high and differed significantly from week to week within the treatment period (F=310.56; df=7, 152; P<0.0001); the same behavior was observed with respect to females (F=184.34; df=7, 152; P<0.0001) and males (F=297.61; df=7, 152; P<0.0001) (Fig. 3). In general, there was a significant decrease in the number of thrips/flower (Fig. 3). The total presence (nymphs and adults) of thrips/flower at the beginning of the biological control had a mean of 38.3±7.2; one week later, a decrease of 71.5% was recorded, and this population was maintained until it decreased to a total of 2±0.5 thrips/flower.
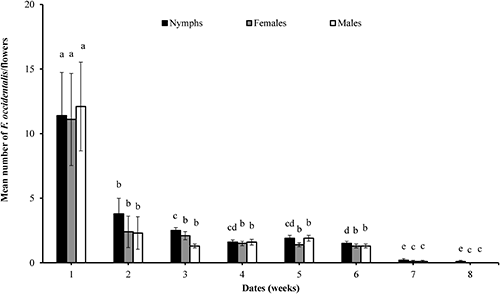
The number of F. occidentalis nymphs at the start beginning of the chemical control was high and differed significantly from week to week within the treatment period (F=671.48; df=7, 152; P<0.0001), the same behavior was observed with respect to females (F=173.8; df=7, 152; P<0.0001) and males (F=407.15; df=7, 152; P<0.0001) (Fig. 4). The mean number of thrips/flower decreased significantly; however, the population of F. occidentalis persisted until the last sampling (Fig. 4). The total presence of thrips/flower (nymphs and adults) at the beginning of the chemical control averaged 62.4±9.2; one week later a decrease of 49.5% was recorded, until a decrease of 80.2% was reached in the fourth week, maintaining this population during the experiment at a rate of 5±2 thrips/flower. The chromatic traps captured an average of 602±87 thrips per day (females and males) until the third week, and then the captures decreased, fluctuating between 80 and 120 thrips per day (females and males) and remained constant until the last monitoring.

In this study, we observed that the susceptibility of O. insidiosus to spinosad increases as the dose increases. We verified that the standard application between 60 and 80 ppm is below the median lethal dose, which is three times higher (225.65 ppm). Our results are similar to the effect of spinosad on O. insidiosus with a LD50 of 200 ppm in 24 hr as reported in laboratory studies.28) However, our results differ from those reported for other insects, where topical application and ingestion of spinosad by nymphs of Podisus maculiventris Say (Hemiptera: Pentatomidae), a generalist predator, caused mortality with concentrations of 15–50 ppm.29) Also, spinosad is reported to be highly toxic to the predator Doru taeniatum Dohrn (Dermaptera: Forficulidae), both in the laboratory and in the field.30) In contrast, other studies have reported the safe use of spinosad in the control of Spodoptera exigua (Lepidoptera: Noctuidae) for the predators Harmonia axyridis (Coleoptera: Coccinellidae) and Chrysoperla sinica Tjeder (Neuroptera: Chrysopidae), while it is toxic to the parasitoids Snelleniua manilae Ashmead and Telenomus remus Nixon (Hymenoptera: Scelionidae), confirming that among natural enemies, Hymenoptera is the most susceptible.31,32) In this study, only topical applications of spinosad were made on O. insidiosus, which showed tolerance to the standard application dose. However, progressively increasing this amount or consecutive applications on crops without addressing label recommendations would possibly pose a risk to O. insidiosus populations.
Regarding thrip management using two different strategies, it has been observed that biological control through the release of O. insidiosus maintains populations below the economic threshold reported for bell peppers and eggplants,33) achieving a significant drop in populations from the first week of release. These results are promising, if safe populations are maintained in crops at a rate of at least one O. insidiosus per 40 thrips.34,35) In this study, we performed preventive releases of 50 O. insidiosus/m2, which was sufficient to keep the crop free of thrips during the season. The chromatic traps used in this type of management as monitoring devices showed very low captures (3–5 thrips/day), and most showed no captures. These results are similar to those of other studies conducted in cucumber crops, where one individual of O. insidiosus for every 180 thrips was sufficient to control the population.36) However, studies of other predators, such as Neoseiulus cucumeris Oudemans (Acari: Pythoseiidae), have shown that the consumption rate of those predators can be affected by prey density and the presence of conspecifics.37) The strategy of Koppert Biological Systems is to sell biological control packages for managing populations of certain insect pests. In the case of thrips on blackberry crops, one options is to control them with the predator O. insidiosus for a full season (7–8 months), performing preventive releases from the first signs of the presence of the pest. The cost of the thrip control package varies, averaging around US$3,500; however, the presence of other pests increases the cost if their integration into the control package is desired. The package includes releases as required based on pest behavior, so that releases of up to 100 O. insidiosus/m2 could be made every 15 days without increasing the cost.
On the other hand, biochemical control using spinosad in combination with blue chromatic traps (200 traps/ha) has shown promising results, since it has reduced the thrip population below the economic threshold.33) However, captures in the chromatic traps were maintained throughout the experiment; although they decreased significantly, the thrip population was never completely eliminated. The effectiveness of blue chromatic traps at capturing thrips is high as compared to that of other colors7,38); moreover, the blue color does not attract predators or non-target parasitoids that are attracted to yellow and other colors.39) Although the presence of F. occidentalis does not decline to extinction levels as compared to crops where biological control is employed, this result should be considered in relation to the levels of resistance shown by F. occidentalis in blackberry crops treated with spinosad in the state of Michoacán,40) since no predators were found in any of the samples collected in these crops (Cruz-Esteban, personal observation). The total cost of spinosad application during a season can vary from US$1,800–2,000 according to the intensity of application by the farmer. Applications are generally performed once a week, and the number of applications is increased when thrip populations do not subside. To this must be added the cost of labor (US$12–15/day/worker), equipment wear, the development of thrip resistance to spinosad due to constant use, unknown adverse effects to non-target insects, and possible damage to the environment, among others. In addition, the cost of the polymer used for the traps must be added (US$150/roll of polymer to make 200 traps).
Biological control alone is highly effective; the cost of its implementation may seem expensive, but this can be reduced by combining it with other strategies such as the use of traps, semiochemicals, resistant plants, cultural control, and other alternatives41) to increase economic viability and reduce the impact of pesticides that, although economical in the short and long term generate resistance or adverse effects in non-target organisms.8–12) On the other hand, the costs of both methods of control are similar, but the investment in biological control is high at the beginning, since it is necessary to pay for the whole package, taking into account that culturally this technique is still unreliable, and farmers doubt its efficiency. In addition, the initial cost can result in a final benefit by bringing an increase and improvement in production quality (Ayala-Ortiz, personal comment; Senior Consultant, Koppert Biological Systems). In integrated thrip management programs, it is recommended to avoid repeated applications of spinosad in areas where resistance has developed so that populations can return to the field susceptibility rate while environmentally friendly technologies are implemented.33,36,42) Therefore, it is of great importance to propose management strategies that can be used and tested by farmers.
In conclusion, findings that the LD50 of spinosad is below the dose commonly used in berry crops and that it rapidly degrades upon exposure to sunlight16) could lead us to reconsider using it without reservation; however, these results should be taken with extreme caution and responsibility. Field applications differ from laboratory ones; additionally, it is necessary to carry out studies that allow us to determine the effect on O. insidiosus when feeding on thrips with spinosad residues and the effects on their biological cycle or on their predation capacity. It is also necessary to determine the timeframe in which spinosad remains active when applied on protected crops (under tunnels). The fact that neither O. insidiosus nor any other predator was found in crops treated with spinosad could be an adverse result of the constant use of spinosad, since this bioinsecticide has always been part of agronomic management during the last five years (farmer's comment). Preliminary studies have shown that spinosad (60 ppm) is not compatible with commercial natural enemies in the field, as more than 70% of the released population dies. However, individuals from the third and subsequent generations have been shown to be more compatible, as less than 30% of the exposed population dies (Cruz-Esteban and Castañeda, unpublished data). Therefore, the use of chromatic traps+biological control could be the best alternative. The chromatic traps along the crop perimeter capture adult thrips that visit it for the first time, and thrips that are not trapped but that penetrate the crop are eaten by the O. insidiosus. Moreover, if they reproduce before being eaten, the eggs and larvae will be predated more easily since they are less mobile and, in the case of the eggs, mobility is null. Traps within a crop also capture adults of both sexes. In general, these results are of importance in the integrated pest management program for thrips in regions where thrips are a limiting pest in agricultural production, mainly in countries where they have become one of the main pests of blackberry and avocado crops.
We thank Julio C. Rojas (ECOSUR) for his comments on the first draft of this manuscript.