2022 Volume 47 Issue 3 Pages 118-124
2022 Volume 47 Issue 3 Pages 118-124
Fifteen novel pyridine carboxamide derivatives bearing a diarylamine-modified scaffold were designed, synthesized, and their antifungal activity was evaluated. Preliminary bioassay results showed that some of the synthesized compounds exhibited moderate to good in vitro antifungal activity. Further, compound 6-chloro-N-(2-(phenylamino)phenyl)nicotinamide (3f) displayed good in vivo antifungal activity against Botrytis cinerea. The enzymatic test on B. cinerea succinate dehydrogenase (SDH) showed that the inhibitory activity possessed by compound 3f equally matches that of thifluzamide. Molecular docking results demonstrated that compound 3f could commendably dock with the active site of SDH via stable hydrogen bonds and hydrophobic interactions, suggesting the possible binding modes of the title compounds with SDH. The results above revealed that the target compounds would be the leading fungicide compound for further investigation.
Fungi have been known to be an extensive threat to plant species for a long time, accounting for more than 70% of plant diseases, and pathogenic fungi cause about 10% of the world’s major crops to be reduced each year.1–3) Moreover, fungi are easily spread through air and water flow, which increases the difficulty of their prevention.4) Currently, people are mainly using chemical agents for the prevention and control of fungal diseases in agricultural production crops. As a matter of fact, the use of fungicides has been one of the most economical and effective ways to reduce huge losses in crop productivity. However, the long-term and increasing application of chemical fungicides has caused ever-increasing fungicide resistance in plant pathogenic fungi. It is necessary to develop novel scaffolds or action mode fungicides to control these crop diseases and solve the problem of fungicide resistance.
Succinate dehydrogenase inhibitors (SDHIs) have been developed for more than 50 years, since the first commercial product, carboxin, was launched in 1966.5) The mode of action of these fungicides is based on disruption of the mitochondrial tricarboxylic acid cycle and respiratory chain.6) The SDHIs have common heterocyclic acid scaffolds, such as pyrazole, pyridine and thiazole rings. Recent studies have mainly focused on modification of the amine moiety and retaining the core moieties of commercial SDHIs, which may lead to structural similarity and better cross-resistance.7) Pyridine carboxamide has attracted great attention since boscalid, the first pyridine carboxamide fungicide, was commercialized by the chemical company BASF.8)
The diarylamine group represents an important structure in many agrochemicals,9) and it may be a promising group to be integrated with some pharmacophores.10) In our previous work, six novel nicotinamide derivatives with a diarylamine-modified scaffold were synthesized and evaluated for their antifungal activity.11) To continue our ongoing work on the discovery of novel nicotinamide fungicides, a series of novel nicotinamide derivatives were synthesized and widely investigated (Fig. 1).

All reagents and solvents were commercially available and used directly without further purification unless specified otherwise. 1H nuclear magnetic resonance (1H NMR) was obtained with chloroform-d as a solvent and tetramethylsilane as an internal standard by using a 400 MHz Bruker NMR spectrometer (Bruker Co., Switzerland). Electrospray ionization mass spectrometry (ESI-MS) were recorded on a Thermo Scientific ISQ™ EC spectrometer (Thermo Fisher Technology Co., Ltd., China). Thin-layer chromatography was performed on silica gel 60 F254 (Qingdao Marine Chemical Co., Ltd., Qingdao, China). Column chromatography purification was conducted on silica gel (200–300 mesh, Qingdao Marine Chemical Co., Ltd., Qingdao, China).
2. Synthesis 2.1. The synthetic procedure for intermediates 1Anhydrous potassium carbonate (20 mmol) was added to a mixture of 1-chloro-2-nitrobenzene (20 mmol) and substituted anilines (30 mmol) in macrogol 1000 (2 mmol), and the resulting mixture was heated at 180°C for 12 hr. The mixture was cooled and quenched with water at room temperature and then extracted with ethyl acetate (3×30 mL). The organic phase was washed with brine, dried with anhydrous sodium sulfate, and concentrated under reduced pressure. The crude product was subjected to flash column chromatography to produce intermediates 1.
2.2. The synthetic procedure for intermediates 2Intermediates 1 (15.0 mmol), reductive iron powder (15.0 mmol), ammonia chloride (45.0 mmol), and aqueous ethanol solution (75%, 30 mL) were added to a flask. The reaction was refluxed at 90°C for 3 hr. When the reaction was finished, the mixture was cooled at room temperature, filtered, and extracted with ethyl acetate (3×20 mL). The organic phase was evaporated in vacuo to obtain intermediates 2.
2.3. The synthetic procedure for title compounds 3Intermediates 2 (1.1 mmol) were dissolved in 5 mL of dichloromethane solvent, and the mixture was cooled to 0°C. The mixture was initially supplemented with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (1.2 mmol) and 4-dimethylaminopyridine (0.2 mmol), subsequently supplemented with intermediate 2 (1.0 mmol), and reacted for 5 hr. When the reaction was completed, the mixture was quenched with water, and the water phase was extracted with dichloromethane (2×5 mL). Then the dichloromethane phase was combined, washed with brine (2×5 mL), dried with anhydrous sodium sulfate, and filtered. The solvent was removed in vacuo, and the crude product was purified through column chromatography to obtain pyridine carboxamides 3a–3o.
Data for N-(2-(phenylamino)phenyl)nicotinamide (3a): white solid; mp 122.2–125.0°C; 1H NMR (400 MHz, Chloroform-d) δ 9.15 (s, 1H), 9.04 (s, 1H), 8.63 (s, 1H), 8.27 (d, J=7.6 Hz, 1H), 8.08 (d, J=5.4 Hz, 1H), 7.51 (dd, J1=8.9 Hz, J2=3.6 Hz, 1H), 7.32–7.23 (m, 2H), 7.17 (m, 4H), 6.83 (m, 3H); Anal. Calcd. for C18H15N3O: C, 74.72; H, 5.23; N, 14.52; Found: C, 74.52; H, 5.27; N, 14.48; ESI-MS m/z 288.12 [M]−.
Data for N-(2-(p-tolylamino)phenyl)nicotinamide (3b): white solid; mp 136.1–138.2°C; 1H NMR (400 MHz, Chloroform-d) δ 8.91 (s, 1H), 8.70 (d, J=4.6 Hz, 1H), 8.61 (s, 1H), 8.19 (d, J=7.7 Hz, 1H), 8.05 (d, J=7.8 Hz, 1H), 7.44–7.32 (m, 1H), 7.25–7.11 (m, 4H), 7.03 (d, J=7.9 Hz, 2H), 6.73 (d, J=8.0 Hz, 2H), 2.26 (s, 3H); Anal. Calcd. for C19H17N3O: C, 75.23; H, 5.65; N, 13.85; Found: C, 75.31; H, 5.55; N, 13.83; ESI-MS m/z 302.13 [M]−.
Data for N-(2-((4-fluorophenyl)amino)phenyl)nicotinamide (3c): off-white solid; mp 149.5–149.9°C; 1H NMR (400 MHz, Chloroform-d) δ 8.96 (s, 1H), 8.73 (s, 1H), 8.53 (s, 1H), 8.10 (t, J=8.3 Hz, 2H), 7.41 (d, J=5.8 Hz, 1H), 7.24–7.08 (m, 4H), 6.93 (t, J=8.5 Hz, 2H), 6.78 (dd, J1=8.6 Hz, J2=4.4 Hz, 2H); Anal. Calcd. for C18H14FN3O: C, 70.35; H, 4.59; N, 13.67; Found: C, 70.30; H, 4.62; N, 13.63; ESI-MS m/z 307.18 [M]−.
Data for N-(2-((4-chlorophenyl)amino)phenyl)nicotinamide (3d): brown solid; mp 155.4–156.8°C; 1H NMR (400 MHz, Chloroform-d) δ 9.04 (s, 1H), 8.73 (s, 1H), 8.62 (s, 1H), 8.12 (m, 2H), 7.44 (d, J=2.6 Hz, 1H), 7.28 (d, J=8.0 Hz, 2H), 7.24–7.18 (m, 2H), 7.16 (d, J=8.0 Hz, 2H), 6.74 (d, J=8.2 Hz, 2H); Anal. Calcd. for C18H14ClN3O: C, 66.77; H, 4.36; N, 12.98; Found: C, 66.74; H, 4.32; N, 12.92; ESI-MS m/z 323.12 [M]−.
Data for N-(2-((2,4-dimethylphenyl)amino)phenyl)nicotinamide (3e): gray solid; mp 142.4–143.1°C; 1H NMR (400 MHz, Chloroform-d) δ 8.97 (s, 1H), 8.69 (s, 2H), 8.18 (d, J=7.1 Hz, 1H), 8.09 (d, J=7.6 Hz, 1H), 7.48–7.09 (m, 4H), 6.98 (d, J=8.0 Hz, 1H), 6.63 (s, 1H), 6.57 (d, J=7.9 Hz, 1H), 2.17 (s, 6H); Anal. Calcd. for C20H19N3O: C, 75.69; H, 6.03; N, 13.24; Found: C, 75.65; H, 6.07; N, 13.19; ESI-MS m/z 317.17 [M+H]+.
Data for 6-chloro-N-(2-(phenylamino)phenyl)nicotinamide (3f): light red solid; mp 137.8–139.0°C; 1H NMR (400 MHz, Chloroform-d) δ 8.85 (s, 1H), 8.52 (d, J=2.2 Hz, 1H), 8.37 (s, 1H), 8.26 (d, J=7.6 Hz, 1H), 8.17 (d, J=6.0 Hz, 1H), 7.87 (dd, J1=8.3 Hz, J2=2.4 Hz, 1H), 7.62 (d, J=7.9 Hz, 1H), 7.46 (d, J=8.3 Hz, 1H), 7.40 (d, J=7.7 Hz, 1H), 7.37–7.31 (m, 1H), 7.21 (dd, J1=12.8 Hz, J2=6.8 Hz, 2H), 6.91 (t, J=7.3 Hz, 1H), 6.76 (d, J=7.7 Hz, 1H); Anal. Calcd. for C18H14ClN3O: C, 66.77; H, 4.36; N, 12.98; Found: C, 66.71; H, 4.34; N, 12.88; ESI-MS m/z 322.99 [M]−.
Data for 6-chloro-N-(2-(p-tolylamino)phenyl)nicotinamide (3g): light yellow solid; mp 138.1–139.2°C; 1H NMR (400 MHz, Chloroform-d) δ 8.89 (s, 1H), 8.61 (s, 1H), 8.46 (s, 1H), 8.28 (d, J=7.3 Hz, 1H), 7.94 (d, J=8.3 Hz, 1H), 7.52 (m, 1H), 7.40 (d, J=8.3 Hz, 1H), 7.29 (d, J=7.0 Hz, 1H), 7.28–7.22. (m, 1H), 7.08 (d, J=8.1 Hz, 2H), 6.73 (d, J=8.1 Hz, 2H), 2.31 (s, 3H); Anal. Calcd. for C19H16ClN3O: C, 67.56; H, 4.77; N, 12.44; Found: C, 67.52; H, 4.75; N, 12.24; ESI-MS m/z 337.07 [M]−.
Data for 6-chloro-N-(2-((4-fluorophenyl)amino)phenyl)nicotinamide (3h): light red solid; mp 182.3–183.1°C; 1H NMR (400 MHz, Chloroform-d) δ 8.62 (s, 1H), 8.37 (s, 1H), 8.15 (s, 1H), 7.99 (d, J=8.1 Hz, 1H), 7.40 (d, J=8.1 Hz, 1H), 7.25–7.11 (m, 4H), 6.95 (d, J=8.2 Hz, 1H), 6.75 (d, J=8.2 Hz, 1H); Anal. Calcd. for C18H13ClFN3O: C, 63.26; H, 3.83; N, 12.30; Found: C, 63.22; H, 3.73; N, 12.26; ESI-MS m/z 341.12 [M]−.
Data for 6-chloro-N-(2-((4-chlorophenyl)amino)phenyl)nicotinamide (3i): light yellow solid; mp 128.4–130.1°C; 1H NMR (400 MHz, Chloroform-d) δ 8.56 (s, 1H), 8.40 (s, 1H), 8.25 (s, 1H), 7.90 (d, J=8.3 Hz, 1H), 7.36 (d, J=8.3 Hz, 1H), 7.25–7.11 (m, 4H), 7.04 (d, J=8.0 Hz, 1H), 6.68 (d, J=8.2 Hz, 1H); Anal. Calcd. for C18H13Cl2N3O: C, 60.35; H, 3.66; N, 11.73; Found: C, 60.30; H, 3.67; N, 11.68; ESI-MS m/z 357.09 [M]−.
Data for 6-chloro-N-(2-((2,4-dimethylphenyl)amino)phenyl)nicotinamide (3j): light brown solid; mp 150.2–150.6°C; 1H NMR (400 MHz, Chloroform-d) δ 8.64 (s, 1H), 8.48 (s, 1H), 8.28 (s, 1H), 7.95 (d, J=8.2 Hz, 1H), 7.40 (d, J=8.2 Hz, 1H), 7.30–7.13 (m, 1H), 7.03 (d, J=7.9 Hz, 1H), 6.64 (s, 1H), 6.56 (d, J=7.7 Hz, 1H), 2.22 (s, 6H); Anal. Calcd. for C20H18ClN3O: C, 68.28; H, 5.16; N, 11.94; Found: C, 68.25; H, 5.14; N, 11.88; ESI-MS m/z 351.09 [M]−.
Data for N-(2-(phenylamino)phenyl)-6-(trifluoromethyl)nicotinamide (3k): yellow solid; mp 139.4–139.8°C; 1H NMR (400 MHz, Chloroform-d) δ 8.91 (s, 1H), 8.61 (s, 1H), 8.30 (d, J=8.1 Hz, 1H), 8.08 (d, J=8.2 Hz, 1H), 7.71 (d, J=8.1 Hz, 1H), 7.34–7.15 (m, 6H), 6.95 (d, J=7.4 Hz, 1H), 6.80 (d, J=7.9 Hz, 2H); Anal. Calcd. for C19H14F3N3O: C, 63.86; H, 3.95; N, 11.76; Found: C, 68.88; H, 3.94; N, 11.74; ESI-MS m/z 357.13 [M]−.
Data for N-(2-(p-tolylamino)phenyl)-6-(trifluoromethyl)nicotinamide (3l): yellowish green solid; mp 113.6–114.7°C; 1H NMR (400 MHz, Chloroform-d) δ 8.94 (s, 1H), 8.60 (s, 1H), 8.29 (d, J=8.0 Hz, 1H), 8.12 (d, J=9.2 Hz, 1H), 7.73 (s, 1H), 7.45–7.14 (m, 4H), 7.04 (d, J=12.4 Hz, 2H), 6.70 (d, J=12.4 Hz, 2H), 2.28 (s, 3H); Anal. Calcd. for C20H16F3N3O: C, 64.69; H, 4.34; N, 11.32; Found: C, 64.74; H, 4.33; N, 11.35; ESI-MS m/z 371.11 [M]−.
Data for N-(2-((4-fluorophenyl)amino)phenyl)-6-(trifluoromethyl)nicotinamide (3m): gray white solid; mp 183.8–185.2°C; 1H NMR (400 MHz, Chloroform-d) δ 8.98 (s, 1H), 8.54 (s, 1H), 8.20 (s, J=5.2 Hz, 2H), 7.76 (d, J=8.4 Hz, 1H), 7.30–7.12 (m, 2H), 6.94 (d, J=10.4 Hz, 2H), 6.75 (d, J=10.0 Hz, 2H), 3.61 (m, 2H); Anal. Calcd. for C19H13F4N3O: C, 60.80; H, 3.49; N, 11.20; Found: C, 60.82; H, 3.46; N, 11.22; ESI-MS m/z 375.11 [M]−.
Data for N-(2-((4-chlorophenyl)amino)phenyl)-6-(trifluoromethyl)nicotinamide (3n): gray white solid; mp 190.7–191.2°C; 1H NMR (400 MHz, Chloroform-d) δ 9.01 (s, 1H), 8.50 (s, 1H), 8.26 (d, J=7.2 Hz, 1H), 8.22 (d, J=7.6 Hz, 1H), 7.79 (d, J=8.0 Hz, 1H), 7.36–7.24 (m, 4H), 7.21 (d, J=8.4 Hz, 2H), 6.73 (d, J=8.0 Hz, 2H); Anal. Calcd. for C19H13ClF3N3O: C, 58.25; H, 3.34; N, 10.73; Found: C, 58.21; H, 3.34; N, 10.75; ESI-MS m/z 392.02 [M]−.
Data for N-(2-((2,4-dimethylphenyl)amino)phenyl)-6-(trifluoromethyl)nicotinamide (3o): yellow solid; mp 119.7–121.5°C; 1H NMR (400 MHz, Chloroform-d) δ 8.80 (s, 1H), 8.34 (s, 1H), 7.93 (d, J=7.8 Hz, 1H), 7.74–7.65 (m, 1H), 7.37 (m, 1H), 7.25–7.16 (m, 4H), 7.14 (m, 2H), 7.04 (d, J=7.5 Hz, 1H), 2.38 (s, 3H), 2.32 (s, 3H); Anal. Calcd. for C21H18F3N3O: C, 65.45; H, 4.71; N, 10.90; Found: C, 65.41; H, 4.72; N, 10.88; ESI-MS m/z 386.12 [M+H]+.
2.4. Antifungal activity assay in vitroThe antifungal activities of target compounds 3a–3o against eight plant pathogens, Fusarium solani, Sclerotinia sclerotiorum, Botrytis cinerea, Phytophthora capsici, Fusarium oxysporum, Cytospora ambiens, Gibberella zeae, and Alternaria alternata, were tested in vitro according to the mycelium growth rate method. The test pathogens grown on PDA medium slants were subcultured for 48 hr in petri dishes prior to testing and used for inoculation of fungal strains on PDA plates. The commercially available fungicides boscalid and thifluzamide were used as the positive control, whereas acetone was set as the negative control. The compounds were dissolved in acetone to prepare a 1000 mg/L stock solution for the following antifungal test. The diameter of each strain was measured after the mycelia were incubated at 25°C for a certain duration. The percentage of inhibition was calculated as follows:
 |
where I is the inhibition percentage, A is the average mycelial diameter (mm) with the compounds in petri dishes, and B is the average mycelial diameter with the compounds in blank petri dishes. The inhibition percentage of the compounds was determined at 50 mg/L, and these compounds with an inhibition rate of more than 70% against B. cinerea were further determined for their median effective concentration (EC50) values according to the method described above. The stock solution was mixed with the autoclaved PDA medium to prepare a set of media containing 1.5625, 3.125, 6.25, 12.5, 25, and 50 µg/mL of the test compounds.
2.5. Antifungal activity assay in vivoTomato fruits were collected from the Sanhe sub-district of Huainan City. Healthy tomatoes were washed and treated with water and 75% aqueous ethyl alcohol in advance and rinsed with water, which was then evaporated at room temperature. The fruits were wounded (d=5 mm) using an inoculating needle and then inoculated with each pathogen. The test compound 3f was dissolved in 200 µL of dimethyl sulfoxide; then 0.2% Tween-80 aqueous water was added at concentrations of 50, 100, and 200 mg/L. The fruits treated with aqueous dimethyl sulfoxide (2%) containing Tween-80 (0.5%) were used as the control. The commercial fungicide boscalid was used as the positive control. All of the treated fruits were then placed into an illumination incubator (25±2°C and 95% relative humidity) for 4 days. These experiments were repeated three times. The protective activity rate (%) was calculated using the following formula: (diameter of lesion in the negative control−diameter of lesion in the treatment)/diameter of lesion in the negative control×100.
2.6. SDH inhibition assay in vitroThe in vitro inhibitory effects of title compound 3f and thifluzamide against B. cinerea SDH were determined using a succinate dehydrogenase assay kit that was purchased from the Nanjing Jiancheng Bioengineering Institute. Cultures were inoculated at 0.05 OD600 nm and grown on a reciprocal shaker (180 rpm, 28°C) for 7 days in Sabouraud Maltose Broth (SMB), and the mycelia of B. cinerea was collected for preparing the tested mitochondrial suspension. The tested compounds were added to the working solution according to the operating instructions for the purchased SDH assay kit. After adding mitochondrial suspensions containing B. cinerea SDH to the above-mentioned solution, the absorbance values of the obtained mixtures were monitored at 600 nm to calculate the median inhibitory concentration (IC50) against fungal SDH.12)
2.7. Molecular dockingMolecular docking studies were performed to investigate the binding modes of compound 3f and thifluzamide to SDH (PDB Code: 2FBW13,14)) using the molecular docking software Ledock (http://www.lephar.com), which was chosen for its high speed and accuracy.15,16) The 3D structures of compound 3f and thifluzamide were drawn using ChemBio3D Ultra 19.0 software, and the docking results were analyzed and visualized using the PyMOL Molecular Graphics System, Version 1.8.0.0, from Schrödinger, LLC (http://www.pymol.org/pymol). The dimension of the binding box was set as [Xmin=36.5, Xmax=53.3, Ymin=−23.0, Ymax=−8.5, Zmin=−2.7, Zmax=13.5]. The conformations of the test compounds were generated by energy minimization using the MM2 force field.
Scheme 1 provides the details of the synthesis route of compounds 3a–3o. The diarylamine intermediate 2 was obtained through the reduction of intermediate 1, which can be easily prepared with high yields. The title compounds were prepared by reacting pyridine carboxylic acid with intermediate 2 via a classic synthetic approach called condensation reaction. The structure of 3a–3o was confirmed through 1H NMR, ESI-MS, and elemental analysis. The 1H NMR and ESI-MS spectra of representative compounds are given in the supplemental material.

The preliminary in vitro screening results for the antifungal activities of compounds 3a–3o against eight plant pathogens are listed in Table 1. The bioassay results indicated that these synthesized compounds have different degrees of activity against the eight tested plant pathogens at 50 mg/L. Among them, compound 3f showed a 76.9% inhibition rate against B. cinerea, and compound 3g had an inhibition rate of 84.1% against C. ambiens. Although most compounds showed low to moderate antifungal activity, with the inhibition ranging from 0 to 50%, compound 3f impressed me most for its similar in vitro antifungal activity against B. cinerea to thifluzamide (Table 2), and compound 3g also had better antifungal activity against C. ambiens than the positive control.
| Compd. | Inhibition ratea) (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| F.s.b ) | S.s. | B.c. | P.c. | F.o. | C.a. | G.z. | A.a. | |
| 3a | 2.1±0.1 | 8.5±1.3 | 22.4±0.5 | 23.7±0.2 | 14.6±1.7 | 35.9±1.8 | 31.1±0.7 | 9.6±0.7 |
| 3b | 1.1±0.2 | 5.9±0.8 | 13.1±1.2 | 11.2±0.3 | 1.8±0.1 | 48.1±1.9 | 26.4±1.1 | 3.1±0.4 |
| 3c | 0 | 0 | 19.4±0.8 | 18.1±1.1 | 2.8±0.1 | 38.7±1.6 | 11.3±1.2 | 0 |
| 3d | 2.7±0.2 | 1.6±0.2 | 13.8±0.7 | 5.6±0.3 | 0 | 26.6±0.8 | 0 | 0 |
| 3e | 1.8±0.2 | 0 | 22.7±1.9 | 7.2±1.0 | 9.9±0.3 | 49.1±0.2 | 27.1±0.4 | 7.8±0.9 |
| 3f | 16.4±0.4 | 57.5±2.2 | 76.9±0.9 | 10.8±0.5 | 20.9±0.4 | 29.8±0.4 | 7.4±0.1 | 31.7±1.2 |
| 3g | 2.7±0.3 | 14.9±0.5 | 24.9±1.7 | 10.6±1.0 | 20.1±1.2 | 84.1±1.5 | 22.3±1.2 | 17.6±0.7 |
| 3h | 3.3±0.2 | 0 | 28.5±1.0 | 8.8±0.6 | 10.0±1.1 | 9.2±0.3 | 23.7±0.9 | 11.7±0.6 |
| 3i | 0 | 0 | 29.4±0.3 | 5.0±0.6 | 8.9±0.5 | 42.1±1.2 | 24.4±0.9 | 13.2±1.1 |
| 3j | 1.8±0.2 | 0 | 33.6±3.0 | 11.8±2.6 | 12.9±1.0 | 9.0±0.4 | 15.2±0.5 | 8.3±0.7 |
| 3k | 12.1±1.6 | 27.4±1.7 | 53.9±2.3 | 11.5±0.7 | 4.3±2.4 | 16.7±1.9 | 46.4±0.9 | 18.9±1.3 |
| 3l | 10.5±1.1 | 28.7±1.7 | 48.9±4.4 | 23.4±0.6 | 5.3±1.5 | 27.7±2.2 | 44.7±2.0 | 19.4±1.2 |
| 3m | 3.0±0.4 | 13.5±0.7 | 19.8±1.5 | 0 | 0 | 13.8±2.2 | 33.7±5.5 | 0 |
| 3n | 12.4±0.4 | 22.7±0.7 | 28.9±2.3 | 6.3±0.5 | 0 | 8.2±0.3 | 27.5±1.1 | 0 |
| 3o | 13.0±1.2 | 25.8±1.0 | 40.0±0.1 | 25.5±0.5 | 6.5±1.7 | 15.1±1.3 | 38.1±2.7 | 11.7±0.8 |
| Thifluzamide | 1.1±0.2 | 100 | 75.5±1.1 | 11.1±0.5 | 6.9±0.8 | 11.0±0.5 | 10.8±0.8 | 81.1±2.1 |
| Boscalid | 7.9±0.7 | 100 | 100 | 15.0±1.3 | 10.5±1.4 | 63.4±1.5 | 21.3±1.3 | 100 |
a) Values were the mean±standard deviation (SD) of three replicates. b) Abbreviations: F.s., Fusarium solani; S.s., Sclerotonia sclerotiorum; B.c., Botrytis cinerea; P.c., Phytophythora capsici; F.o., Fusarium oxysporum; C.a., Cytospora ambiens; G.z., Gibberella zeae; A.a., Alternaria alternata.
| Compd. | Regression equation | EC50 (mg/L) | 95% Confidence interval (mg/L) | R 2 |
|---|---|---|---|---|
| 3f | y=−3.12+2.34x | 21.72 | 18.01–27.03 | 0.952 |
| Thifluzamide | y=−1.80+1.56x | 14.71 | 11.36–19.86 | 0.948 |
| Boscalid | y=−1.33+2.42x | 3.52 | 2.78–4.29 | 0.989 |
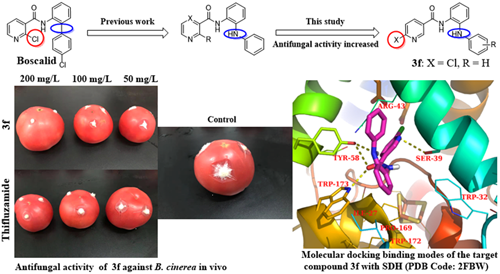
Compound 3f demonstrated the best antifungal activity against B. cinerea during the in vitro tests, and the efficacy was compared with that of the commercial fungicide thifluzamide. Table 3 presents the results of in vivo tests. The preventative efficacies of compound 3f were 53.9%, 49%, and 27.1% at concentrations of 200 mg/L, 100 mg/L, and 50 mg/L, respectively, whereas those of thifluzamide were 55.2%, 41.2%, and 33.8%. The above results confirmed that compound 3f had antifungal activity against B. cinerea similar to that of thifluzamide, both in vivo and in vitro. Further, compound 3f showed preventative efficacy against B. cinerea on tomato fruits, and its effects exhibited concentration-dependent properties (Fig. 2). In fact, we could conclude that compound 3f has potential applications as a fungicide.
| Compd. | Treatment (mg/L) | Protective activitya) (%) |
|---|---|---|
| 3f | 200 | 53.9±5.9 |
| 100 | 49.0±2.2 | |
| 50 | 27.1±1.5 | |
| Thifluzamide | 200 | 55.2±1.5 |
| 100 | 41.2±1.3 | |
| 50 | 33.8±3.1 | |
| Negative Control | — | — |
a) Values were the mean±standard deviation (SD) of three replicates; “—”, not tested.

To further prove the possible antifungal mechanism of the novel pyridine carboxamide derivatives, compound 3f, with promising fungicidal activity against B. cinerea, was selected and evaluated for SDH enzymatic inhibition determination for its target site validation. As shown in Table 4, compound 3f exhibited preferable SDH inhibition with an IC50 value of 5.6 mg/L (17.3 µM), which is the same level of inhibitory activity as thifluzamide (IC50=7.61 mg/L, 14.4 µM). This proved that the pyridine carboxamide derivatives designed in this paper displayed as good inhibitory effects against B. cinerea SDH as other successfully developed carboxamide fungicides.
| Compd. | IC50 | |
|---|---|---|
| mg/L | μM | |
| 3f | 5.60±0.49 | 17.3±1.5 |
| Thifluzamide | 7.61±0.58 | 14.4±1.1 |
Since compound 3f and thifluzamide had similar inhibitory effects on B. cinerea SDH, they were selected for molecular docking analysis. The three-dimensional schematic diagrams clearly explained the possible optimal combination between the ligands and the receptor protein. As shown in Fig. 3A and B, compound 3f and thifluzamide could be embedded well into the protein cavity. The docking total scores for compound 3f and thifluzamide were −6.87 and −7.65 kcal/mol, respectively. Compound 3f was well bound to the receptor protein by forming four hydrogen bonds with TYR-58, TRP-173, and SER-39 (Fig. 3C), and the pyridine ring and diphenylamine structure played a key role in the bond formation. In addition, the hydrophobic cavities formed by amino acid residues such as ILE-27, PRO-169, TRP-172, and TRP-32 could create a good hydrophobic interaction with the phenyl fragment of compound 3f. In fact, thifluzamide’s better docking results were related not only to its ability to form two hydrogen bonds with TYR-58 and TRP-173 but, more importantly, to the good hydrophobic interaction between thifluzamide and its surrounding amino acid residues (Fig. 3D). Docking analysis revealed that to design target compounds with a higher docking score, hydrophobic substituents should be introduced into the structure of compound 3f to increase the hydrophobic interaction between compound 3f and its surrounding amino acid residues so that target compounds can be better embedded into the protein cavity.

Fifteen novel pyridine carboxamides bearing the substituted diarylamine-modified scaffold were designed, synthesized, and evaluated for their antifungal activity based on our previous study. The results of preliminary fungicidal bioassays revealed that some of the target compounds exhibited a certain degree of antifungal activity against the tested pathogens. Among them, compound 3g had an inhibition rate of 84.1% against C. ambiens, and 3f showed 76.9% inhibition rate against B. cinerea in vitro. Meanwhile, it showed a good in vivo control effect against B. cinerea that was comparable to that of thifluzamide. The test of inhibitory activity against B. cinerea SDH showed that compound 3f had enzyme inhibitory activity equal to that of thifluzamide. The molecular docking results demonstrated that compound 3f could dock well into the active site of SDH via stable hydrogen bonds and hydrophobic interactions, suggesting the possible binding modes of the title compounds with SDH. Compared with the structure–activity relationship reported in our previous work, it was found that the activity of these compounds was slightly better than that of 2-substituted compounds when 6-substituted on the pyridine ring. In addition, the absence of substitution on the terminal benzene ring may be beneficial to the activity. These results suggested that individual compounds should be the lead fungicide compounds for further investigation.
This work was supported by the Natural Science Research Key Project of the Universities of Anhui Province, China (Grant No. KJ2020A0334), university-level key projects of the Anhui University of Science and Technology (Grant No. QN2019107), and the Collaborative Innovation Project of Colleges and Universities of Anhui Province (Grant No. GXXT-2020-058).
The online version of this article contains supplementary materials, which are available at https://www.jstage.jst.go.jp/browse/jpestics/.