2023 Volume 48 Issue 4 Pages 211-217
2023 Volume 48 Issue 4 Pages 211-217
Flupentiofenox, which has a unique chemical structure, is a novel acaricide that has been developed by the Kumiai Chemical Industry Co., Ltd. Flupentiofenox exerted significant acaricidal activities against spider mites Tetranychus urticae and Panonychus citri at all developmental stages even at extremely low concentrations, as compared with its practical concentration (80 ppm) for use in mites and was effective against spider mite populations that are resistant to widely used commercial acaricides. These results suggested that flupentiofenox could be used effectively for the control and prevention of spider mite infestation. Additionally, flupentiofenox had a more rapid effect than acetyl CoA carboxylase inhibitors, but it had a relatively slower effect than mitochondrial electron transport inhibitors and glutamate-gated chloride channel modulators. Overall, flupentiofenox is assumed to have a new mode of action.
Spider mites, broad mites, and rust mites are agriculturally troublesome phytophagous pests. Particularly, spider mites cause severe damage to several crops, including vegetables, fruit trees, tea, and flowers.1) Additionally, spider mites have unique biological characteristics, including arrhenotokous parthenogenesis, high fertility, short generation time, and enhanced metabolic detoxification, which facilitates the development of pesticide resistance.2) Several populations of spider mites show resistance to most commercially available acaricides,3) making their control difficult. Recently, spider mites have been controlled using integrated pest management (IPM) technology that combines not only chemical pesticides but also spiracle-blocking insecticides,4) natural enemies,5,6) UV-B irradiation,7) CO2 fumigation,8) and other control measures. Presently, chemical pesticides still play an important role as components of IPM, as other IPM components possess some limitations, including high control costs and low stability. Thus, there is an urgent need to develop novel acaricides that are effective against resistant spider mites.
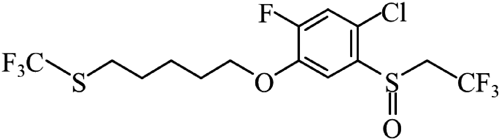
Flupentiofenox, a novel acaricide discovered and developed by Kumiai Chemical Industry Co., Ltd., has a phenyl sulfide structure that has never been observed in other commercial acaricides (Fig. 1).9) The recommended concentration for the practical use of spider mite control is 80 ppm.
In controlling phytophagous mites, it is of very important to show acaricidal activity against the field population of mites, but it is also important to determine which stage of mite show acaricidal activity and the effective time. Among commercially available acaricides for spider mites, inhibitors of the mitochondrial electron transport system, such as complex I, II, and III inhibitors, and glutamate-gated chloride channel (GluCl) allosteric modulators, show lethal activities in all developmental stages of mites, with rapid effects after treatment. In contrast, some acaricides, including growth inhibitors of mites that affect chitin synthase (CHS1) and inhibitors of acetyl CoA carboxylase, are only effective at some developmental stages. Specifically, the acaricidal effect of these inhibitors is slow, providing a time window for mites to damage crops unabated. Therefore, it is important to evaluate the biological activities of novel acaricides at different growth stages and elucidate their effective time.
Here, we examined the acaricidal activities of flupentiofenox not only against spider mites at each developmental stage but also against populations that are resistant to widely used acaricides. And also, we further evaluated the effective time of flupentiofenox against spider mites.
Flupentiofenox (4-chloro-2-fluoro-5-[(RS)-(2,2,2-trifluoroethyl)sulfinyl]phenyl 5-[(trifluoromethyl)thio]pentyl ether) was synthesized and formulated as a 100 g/kg wettable powder by Kumiai Chemical Industry Co., Ltd. Pyflubumid SC, cyenopyrafen SC, acequinocyl SC, tebufenpyrad EW, bifenazate SC, etoxazole SC, milbemectin EC, and spiromesifen SC were purchased from Japan Agricultural Cooperatives. The mode of action of each commercial acaricide is as follows: pyflubumid and cyenopyrafen are mitochondrial complex II electron transport inhibitors categorized as Group 25 of the Insect Resistance Action Committee (IRAC); acequinocyl and bifenazate are mitochondrial complex III electron transport inhibitors categorized in Group 20; tebufenpyrad is a mitochondrial complex I electron transport inhibitor categorized in Group 21 of the IRAC; etoxazole is a mite growth inhibitor affecting CHS1 categorized in the Group 10; milbemectin is a GluCl allosteric modulator categorized in Group 6; and spiromesifen is an acetyl-CoA carboxylase inhibitor categorized in Group 23 in IRAC.
2. Spider mitesTetranychus urticae and Tetranychus kanzawai were collected from Kakegawa, Shizuoka, Japan. Both species were reared on kidney beans (Phaseolus vulgaris) for more than 30 years under controlled conditions (temperature: 25±1°C; 16L : 8D photoperiod) at the Life Science Research Institute, Kumiai Chemical Industry Co., Ltd. Panonychus citri was collected in Kikugawa, Shizuoka, Japan, and reared on mandarin oranges (Citrus unshiu) for more than 10 years under controlled conditions (temperature: 25±1°C; 16L : 8D photoperiod) at the same place as above. They were used as the acaricide-susceptible populations in the experiments.
To compare the acaricidal (ovicidal) activities of flupentiofenox and other commercial acaricides against field-collected populations, T. urticae was collected from chrysanthemum, strawberry, and apple fields in Japan from 2017 to 2020, and reared on kidney beans to increase the population. The year and location of collection for each population are as follows: apple field in Goshogawara city, Aomori Prefecture in 2017; chrysanthemum field in Toyohashi city, Aichi Prefecture in 2018; strawberry field in Kakegawa city, Shizuoka Prefecture in 2018; and strawberry field in Chino city, Nagano Prefecture, in 2020.
3. Leaf disc assayA leaf disc assay10) was performed to investigate the acaricidal activity of candidate compounds against the mite species at different growth stages. Briefly, three leaf discs (approximately 20 mm in diameter) of kidney bean or citrus were placed on wet filter paper (55 mm in diameter) in a 60-mL plastic cup and inoculated with mites at a predetermined developmental stage. Thereafter, the leaf discs containing the mites were sprayed with solutions containing chemical compounds with a spreader (Kumiten®, 0.2 mL/L) for Tetranychus or without a spreader for Panonychus, using an automatic spraying device (applied amount: 15 mg/cm2). Water containing only the spreader for T. urticae and T. kanzawai and water without the spreader for P. citri were used as controls. After spraying, the treated leaf discs were placed in a room under controlled conditions of 25±1°C and 16L : 8D photoperiod.
3.1. Evaluation of the acaricidal activity of flupentiofenox at each developmental stage of T. urticae, T. kanzawai, and P. citriTo evaluate the acaricidal activity of flupentiofenox at each developmental stage of T. urticae, T. kanzawai, and P. citri, eggs within 24 hr after oviposition, larvae, protonymphs, and deutonymphs within 24 hr after molting, and female adults within 24 hr of emergence were inoculated on leaf discs, followed by treatment with flupentiofenox. Protochrysalises, deutochrysalises, and tritochrysalises were prepared within 24 hr of quiescence. Three cups (nine leaf discs in total) were used for each treatment. In detail, eggs were prepared by inoculating female adults from a stock culture onto leaf disc using a brush and removing the female adults after 24 hr. Larvae were prepared by allowing female adults to lay eggs on leaf discs and removing eggs that did not hatch within 24 hr. The protonymphs, deutonymphs, and female adults were reared from eggs within 24 hr after oviposition. Protochrysalises, deutochrysalises, and tritochrysalises were prepared by inoculating leaf discs with a brush with larvae, protonymphs, and deutonymphs developed from eggs within 24 hr after oviposition, and removing mites other than respective chrysalises 24 hr later. The number of unhealthy and dead mites was counted 3 day after the treatment, while the number of unhatched eggs was counted 10 day after treatment. Unhealthy mites were those that had difficulty walking normally, those that had convulsions, and those that were on the verge of death. Eggs at 3 and 5 day after oviposition and female adults at 5 day after emergence were additionally tested for T. urticae.
3.2. Evaluation of ovicidal activities of flupentiofenox and commercial acaricides against resistant T. urticae populationsThe ovicidal activities of flupentiofenox and other commercial acaricides against field-collected populations of T. urticae collected from chrysanthemum, strawberry, and apple fields were evaluated using leaf disc assay. Briefly, two leaf discs (one cup) were inoculated with eggs within 24 hr after oviposition at 25°C, followed by treatment with the acaricides.
3.3. Determination of the effective time of flupentiofenox and commercial acaricides against T. urticae and P. citriBriefly, female adult T. urticae and P. citri were inoculated in leaf discs at 25 and 15°C, respectively. One cup (three leaf discs in total) was used for each treatment. After spraying with a solution containing flupentiofenox or commercial acaricides, the number of unhealthy or dead mites was counted at 1, 3, 6, 10, 24, 48, and 72 hr. Unhealthy mites were those that had difficulty walking normally, those that had convulsions, and those that were on the verge of death.
4. Data analysisTo determine the concentration-mortality response of flupentiofenox, probit analysis11) was applied using PriProbit v.1.63,12) and 50% lethal concentrations (LC50) were obtained. Variances and covariances in the probit mortality response to flupentiofenox at each developmental stage were calculated using the SAS Equivalent. An all-or-nothing model with a natural response rate was used as the regression model.
To determine the 50% effective time (ET50), data for the lethal time fitted to a log-normal distribution were analyzed by parametric survival analysis using the JMP software ver. 5.0.1 (SAS Institute, Inc.). Adult females that survived for 72 hr after treatment were included in the analysis as censored cases.
Flupentiofenox showed high acaricidal activity at each developmental stage of T. urticae. Specifically, the LC50 of flupentiofenox against the egg, larvae, protochrysalises, protonymphs, deutochrysalises, deutonymphs, tritochrysalises, and female adults of T. urticae were 0.33, 0.02, 0.07, 0.06, 0.15, 0.13, 0.32, and 0.16 ppm, respectively (Table 1). Additionally, the LC50 of flupentiofenox against the eggs of T. urticae at 24 hr, 3 day, and 5 day after oviposition were 0.33, 0.36, and 0.78 ppm, respectively, indicating that the ovicidal activity of flupentiofenox decreased with egg development. Moreover, untreated eggs began to hatch 5–7 hr after treatment at 5 day after oviposition, suggesting that embryogenesis was almost complete. A previous study reported that the larvae of T. urticae tend to detach from the eggshell before complete embryogenesis and hatching.13) Therefore, it could be speculated that the lower activity of flupentiofenox 5 day after oviposition could be attributed to the little ability of flupentiofenox on the surface of the egg to reach inside the egg. Additionally, the LC50 of flupentiofenox against female adult T. urticae at 24 hr and 5 day after molting was the same (0.16 ppm), indicating that the acaricidal activity of flupentiofenox was not influenced by the number of days after molting.
| Developmental stage | N | Slope±S.E. | Natural response±S.E. | LC50 (mg a.i./L) | Goodness-of-fit test | |||
|---|---|---|---|---|---|---|---|---|
| Estimate | 95%CI | d.f. | χ2 | p | ||||
| Egg (within 24 hr after oviposition) | 1,119 | 12.59 | 0.03±0.01 | 0.33 | 0.31–0.32 | 51 | 67.10 | 0.06 |
| Egg (3 days after oviposition) | 1,469 | 15.71±0.47 | 0.04±0.01 | 0.36 | 0.34–0.37 | 51 | 80.12 | <0.01 |
| Egg (5 days after oviposition; just before hatching) | 1,169 | 5.38 | 0.08±0.003 | 0.78 | 0.74–0.88 | 42 | 178.80 | <0.01 |
| Larva | 1,002 | 4.53±0.71 | 0.05±0.03 | 0.02 | 0.01–0.02 | 33 | 94.13 | <0.01 |
| Protochrysalis | 1,196 | 10.63 | 0.05±0.01 | 0.07 | — | 33 | 71.99 | <0.01 |
| Protonymph | 594 | 7.08±1.52 | 0.06±0.02 | 0.06 | 0.04–0.07 | 33 | 44.85 | 0.08 |
| Deutochrysalis | 1,156 | 5.45±0.63 | 0.08±0.02 | 0.15 | 0.13–0.18 | 42 | 103.86 | <0.01 |
| Deutonymph | 663 | 3.51±0.40 | 0.03±0.01 | 0.13 | 0.11–0.15 | 42 | 72.67 | <0.01 |
| Tritochrysalis | 600 | 3.59 | 0.11±0.05 | 0.32 | — | 42 | 108.38 | <0.01 |
| Female Adult (within 24 hr after moulting) | 265 | 13.79 | 0.09 | 0.16 | — | 24 | 17.73 | 0.82 |
| Female Adult (5 days after moulting) | 360 | 17.46 | 0.11±0.02 | 0.16 | — | 33 | 30.55 | 0.59 |
In Slope, Natural response (untreated mortality), LC50, plots without standard error (SE) or 95% confidence interval (95%CI) indicated that SE and 95%CI were not calculated due to the poor fit of the data to the statistical model.
The LC50 of flupentiofenox against the egg, larvae, protochrysalises, protonymphs, deutochrysalises, deutonymphs, tritochrysalises, and female adults of T. kanzawai were 0.29, 0.05, 0.15, 0.09, 0.15, 0.11, 0.21, and 0.29 ppm, respectively (Table 2). Additionally, the LC50 of flupentiofenox against the egg, larvae, protochrysalises, protonymphs, deutochrysalises, deutonymphs, tritochrysalises, and female adults of P. citri were 0.68, 0.02, 0.09, 0.06, 0.08, 0.12, 0.15, and 0.10 ppm, respectively (Table 3).
| Developmental stage | N | Slope±S.E. | Natural response±S.E. | LC50 (mg a.i./L) | Goodness-of-fit test | |||
|---|---|---|---|---|---|---|---|---|
| Estimate | 95%CI | d.f. | χ2 | p | ||||
| Egg (within 24 hr after oviposition) | 964 | 3.68±0.62 | 0.03±0.01 | 0.29 | 0.23–0.35 | 51 | 150.47 | <0.01 |
| Larva | 1,179 | 7.55 | 0.12±0.003 | 0.05 | — | 60 | 234.02 | <0.01 |
| Protochrysalis | 1,827 | 5.83±0.45 | 0.05±0.01 | 0.15 | 0.14–0.17 | 42 | 78.20 | <0.01 |
| Protonymph | 540 | 3.95±0.59 | 0.02±0.01 | 0.09 | 0.07–0.11 | 51 | 66.61 | 0.07 |
| Deutochrysalis | 595 | 2.15±5.39 | 0±1.68 | 0.15 | — | 42 | 120.37 | <0.01 |
| Deutonymph | 360 | 19.54±0.73 | 0.01±0.01 | 0.11 | 0.11–0.12 | 33 | 26.10 | 0.8 |
| Tritochrysalis | 613 | 4.26±0.41 | 0.02±0.01 | 0.21 | 0.18–0.23 | 42 | 49.56 | 0.2 |
| Female Adult | 396 | 10.65 | 0.07±0.02 | 0.29 | — | 33 | 42.17 | 0.13 |
In Slope, Natural response (untreated mortality), LC50, plots without standard error (SE) or 95% confidence interval (95%CI) indicated that SE and 95%CI were not calculated due to the poor fit of the data to the statistical model.
| Developmental stage | N | Slope±S.E. | Natural response±S.E. | LC50 (mg a.i./L) | Goodness-of-fit test | |||
|---|---|---|---|---|---|---|---|---|
| Estimate | 95%CI | d.f. | χ2 | p | ||||
| Egg (within 24 hr after oviposition) | 711 | 11.38 | 0.08 | 0.68 | — | 51 | 43.02 | 0.78 |
| Larva | 1,080 | 4.29±0.37 | 0.03±0.01 | 0.02 | 0.02–0.02 | 60 | 55.26 | 0.65 |
| Protochrysalis | 994 | 5.08±0.64 | 0.01±0.01 | 0.09 | 0.08–0.10 | 42 | 69.14 | <0.01 |
| Protonymph | 540 | 4.00±0.94 | 0.13±0.04 | 0.06 | 0.04–0.08 | 51 | 135.63 | <0.01 |
| Deutochrysalis | 596 | 4.16±0.46 | 0.01±0.01 | 0.08 | 0.07–0.09 | 42 | 62.81 | 0.02 |
| Deutonymph | 765 | 3.87±0.34 | 0.02±0.01 | 0.12 | 0.11–0.14 | 42 | 41.82 | 0.48 |
| Tritochrysalis | 483 | 4.23±0.6 | 0.01±0.01 | 0.15 | 0.12–0.18 | 42 | 89.44 | <0.01 |
| Female Adult | 354 | 12.08 | 0.08±0.03 | 0.10 | 0.10–0.10 | 33 | 59.9 | <0.01 |
In Slope, Natural response (untreated mortality), LC50, plots without standard error (SE) or 95% confidence interval (95%CI) indicated that SE and 95%CI were not calculated due to the poor fit of the data to the statistical model.
Spider mite infestation is difficult to identify during the early stages until severe crop damage is evident owing to the small body size of the species. Additionally, the population of spider mites in infested fields is usually high owing to their high fecundity. Since flupentiofenox showed high acaricidal activity at even low concentrations to all development stages of T. urticae, T. kanzawai, and P. citri, it can be expected to quickly stop the damage even in such fields and show high control effects as an acaricide.
2. Ovicidal activities of flupentiofenox against T. urticae populations that are resistant to commercial acaricidesTo elucidate the activities of flupentiofenox against field-collected T. urticae populations, eggs of susceptible and field-collected T. urticae populations were treated with acaricides, and the mortality rate was recorded. The eggs of a susceptible population of T. urticae collected from a rose field in Kakegawa, Shizuoka Prefecture, Japan. in 1980 were highly susceptible to all tested acaricides, including flupentiofenox, with 100% egg mortality observed. In contrast, the eggs of T. urticae populations recently sampled showed reduced susceptibility to commercial acaricides (Table 4). Notably, although the eggs of T. urticae populations collected from a chrysanthemum field in Toyohashi, Aichi Prefecture, and strawberry fields in Kakegawa, Shizuoka Prefecture in 2018 showed reduced susceptibility to commercial acaricides, they were highly susceptible (100% mortality) to flupentiofenox even at 3.2 ppm (Table 4). Overall, these results indicate that flupentiofenox could be effective against field-collected mites, and could overcome the cross-resistance of mite against different types of acaricides.
 |
Over the years, mite populations that are resistant to commercial acaricides have been reported in various crops around the world, including strawberries and flowers. Previous studies have examined the resistance mechanisms of mites to the commercial acaricides tested in the present study. For instance, Sugimoto and Osakabe showed that cytochrome P450 and carboxyl/cholinesterase, which are involved in acquired metabolic resistance, were mainly responsible for the low effect of cyenopyrafen against a cyenopyrafen-resistant population of T. urticae.14) Additionally, Inak et al. reported that enhanced metabolism by detoxification enzymes (especially P450), target-site mutations, and high expression of the target enzyme acetyl-CoA carboxylase was responsible for the low effect of spiromesifen against T. urticae collected from strawberry fields in Turkey.15) Moreover, Van Nieuwenhuyse et al. suggested that amino acid mutations in cytochrome b of complex III in the mitochondrial electron transport system were responsible for the weak effect of bifenazate against T. urticae.16) The findings above indicate that acquired metabolic resistance and altered target-site resistance are the most common mechanisms in mites.17,18)
The T. urticae populations collected in 2017–2020 could be considered multiple-acaricide-resistant because the commercial acaricides tested in this study, which have different modes of action, did not show high acaricidal activity against these populations. However, since flupentiofenox was highly active against these populations, it was assumed that flupentiofenox metabolism was not developed in the acaricide-resistant populations.
3. Effective time of flupentiofenox against T. urticae and P. citriWe determined the effective time of the acaricides, including flupentiofenox, against T. urticae and P. citri. The ET50 of the mitochondrial complex I electron transport inhibitor tebufenpyrad, the mitochondrial complex III electron transport inhibitor acequinocyl, and the GluCl allosteric modulator milbemectin against T. urticae and P. citri under 25°C conditions was 1 hr (Figs. 2 and 3, Tables 5 and 6). Overall, these acaricides exhibited the most rapid effects against the mites. Additionally, the ET50 of the mitochondrial complex II electron transport inhibitors pyflubumide and cyenopyrafen against T. urticae were 4.1 and 4.2 hr, respectively, while their ET50 against P. citri was 5.3 and 3.1 hr, respectively. In contrast, the effect of the acetyl CoA carboxylase inhibitor spiromesifen was extremely slow, with ET50 of 37.7 and 40.2 hr against T. urticae and P. citri, respectively. However, the effect of flupentiofenox was relatively rapid, with ET50 of 6.5 and 10.2 hr against T. urticae and P. citri, respectively.


| Treatment | Concentration (mg a.i./L) | ET50 (50% Effective Time) | |||||
|---|---|---|---|---|---|---|---|
| 25°C | 15°C | ||||||
| N | Time (h) | 95%CI | N | Time (h) | 95%CI | ||
| Flupentiofenox WP | 80 | 30 | 6.5 | 7.37–5.72 | 31 | 18.9 | 20.86–17.04 |
| Pyflubumide SC | 100 | 31 | 4.1 | 4.65–3.62 | 32 | 5.8 | 6.38–5.23 |
| Cyenopyrafen SC | 150 | 33 | 4.2 | 4.74–3.72 | 33 | 6.1 | 6.74–5.54 |
| Tebufenpyrad EW | 100 | 32 | 1.0 | 1.13–0.88 | 31 | 1.0 | 1.11–0.90 |
| Acequinocyl SC | 150 | 30 | 1.0 | 1.13–0.88 | 31 | 1.0 | 1.11–0.90 |
| Milbemectin EC | 10 | 32 | 1.0 | 1.13–0.88 | 32 | 1.0 | 1.10–0.91 |
| Spiromesifen SC | 150 | 33 | 37.7 | 42.62–33.35 | 32 | 90.0 | 102.88–78.76 |
| Treatment | Concentration (mg a.i./L) | ET50 (50% Effective Time) | |||||
|---|---|---|---|---|---|---|---|
| 25°C | 15°C | ||||||
| N | Time (hr) | 95%CI | N | Time (hr) | 95%CI | ||
| Flupentiofenox WP | 80 | 30 | 10.2 | 11.52–9.11 | 32 | 20.8 | 24.13–17.99 |
| Pyflubumide SC | 100 | 31 | 5.3 | 5.95–4.72 | 31 | 15.0 | 17.40–12.91 |
| Cyenopyrafen SC | 150 | 32 | 3.1 | 3.44–2.74 | 32 | 7.2 | 8.31–6.20 |
| Tebufenpyrad EW | 100 | 33 | 1.0 | 1.12–0.89 | 33 | 1.0 | 1.16–0.87 |
| Acequinocyl SC | 150 | 33 | 1.0 | 1.12–0.89 | 33 | 1.1 | 1.23–0.93 |
| Milbemectin EC | 10 | 33 | 1.0 | 1.12–0.89 | 32 | 1.0 | 1.16–0.86 |
| Spiromesifen SC | 150 | 33 | 40.2 | 45.01–35.93 | 32 | 69.3 | 81.67–58.73 |
Additionally, the ET50 of tebufenpyrad, acequinocyl, and milbemectin at 15°C were similar to those at 25°C, whereas those of flupentiofenox, pyflubumide, cyenopyrafen, and spiromesifen were longer at 15°C than at 25°C (Tables 5 and 6). Moreover, the effect of flupentiofenox was similar to or slightly slower than that of the mitochondrial complex II electron transport inhibitor but was distinctly slower than those of the mitochondrial complex I and III electron transport inhibitors and the GluCl allosteric modulators and more rapid than that of the acetyl CoA carboxylase inhibitor. The effective time of acaricides is thought to be related to their modes of action, and flupentiofenox had a clearly different effective time from the mitochondrial complex I and III electron transport inhibitors, the GluCl allosteric modulators and the acetyl CoA carboxylase inhibitor. Furthermore, considering that flupentiofenox was effective against spider mite populations that are resistant to other well-known commercial acaricides as mentioned above, it could be speculated that flupentiofenox possesses a different mode of action from those of other acaricides evaluated in the present study.
Conclusively, the results of the study showed that flupentiofenox exerted acaricidal activities against spider mites at all developmental stages even at low concentrations. Additionally, flupentiofenox was effective against spider mite populations that are resistant to other well-known commercial acaricides. Moreover, flupentiofenox had a more rapid effect than acetyl CoA carboxylase inhibitors, but a relatively slower effect than the mitochondrial electron transport inhibitors and glutamate-gated chloride channel modulators. These results suggest that flupentiofenox is an effective acaricide with a novel mode of action for the control and prevention of spider mite infestation of agricultural fields and products.
The online version of this article contains supplementary material (Table S1) which is available at https://www.jstage.jst.go.jp/browse/jpestics/.