2024 Volume 49 Issue 4 Pages 203-209
2024 Volume 49 Issue 4 Pages 203-209
Flometoquin (FLO) is a novel quinoline-type insecticide that elicits a quick knock-down effect against target pests; however, its mode of action (MoA) remains unknown. In this study, we investigated its MoA systematically, using varying biochemical techniques. Since FLO-treated insects exhibited symptoms similar to those induced by respiratory inhibitors, we examined the effect of FLO on respiratory enzyme complexes using mitochondria isolated from different insects (housefly, diamondback moth, and western flower thrips). We found that FLO itself is not active; however, its deacylated metabolite, FloMet, specifically inhibits the activity of ubiquinol-cytochrome c oxidoreductase (complex III) in mitochondria at the nM level. Ligand binding assays and monitoring the reduction kinetics of cytochrome hemes b and c1 clearly revealed that FloMet inhibits complex III by binding to the Qi site.
Respiratory inhibition is one of the most common mode of action (MoA) for insecticides; for example, Groups 12, 13, 20, 21, 24, 25 and 34 in the IRAC (Insecticide Resistance Action Committee) classification.1) Insecticides of this MoA act ultimately as mitochondrial oxidative phosphorylation inhibitors and block ATP production, which is detrimental for the survival of insect pests. They typically trigger a fast onset, accompanied by debilitating symptoms in treated insect pests and exhibit effectiveness against a wide spectrum across species due to the conserved evolutionary essentiality of ATP production.2) The respiratory inhibitors can be categorized according to their biochemical MoA into three categories; (i) inhibitors of mitochondrial electron transport chain complexes I, II, III and IV, (ii) mitochondrial uncouplers, and (iii) inhibitors of ATP synthesis.1) Especially, those belonging to the first category play an important role in the crop protection field and have been in use globally for decades, thus, there are increasing examples of resistance in many pest insects.2) However, due to the attractiveness of their broad spectrum, novel pesticides are still being developed and launched through various discovery studies based on existing scaffolds and known specific ligands coupled with certain modifications to enhance their physicochemical properties, specificity, and efficacy.3) All these point to the fact that mitochondrial electron transport inhibitors potentially remain as one of the most promising tractable insecticidal MoAs.
Flometoquin (FLO), 2-ethyl-3,7-dimethyl-6-[4-(trifluoromethoxy)phenoxy]quinolin-4-yl methyl carbonate, was developed jointly by Nippon Kayaku Co., Ltd. and Meiji Seika Pharma Co., Ltd. (currently, Mitsui Chemicals Crop & Life Solutions, Inc.). We previously demonstrated that FLO-treated insects exhibited symptoms similar to those induced by respiratory inhibitors (e.g. a quick knock down effect against target insect pests); however, the detailed MoA of FLO remained to be identified.4) In this study, to further elucidate the molecular mechanism of FLO, we examined the effects of FLO and its deacylated metabolite, FloMet (Fig. 1), on the respiratory enzymes in mitochondria from different insect species. We found that FLO itself is not active, but FloMet specifically inhibits the activity of ubiquinol-cytochrome c oxidoreductase (complex III) in mitochondria at the nM level. The reduction kinetics of cytochrome hemes b and c1, and ligand binding assays clearly demonstrated that FloMet inhibits complex III by binding to the Qi site. These results allow us to conclude that FLO is an insecticide acting on the Qi site of mitochondrial complex III and FLO is classified into Group 34 in IRAC.1)

FLO and its deacylated metabolite FloMet were prepared using methods reported previously.5,6) Antimycin A, myxothiazol, azoxystrobin and azoxystrobin analogue, metyltetraprole, were purchased from Sigma-Aldrich Co., LLC and LGC. All other chemicals were of analytical reagent grade and were commercially available.
2. Preparation of the mitochondriaTo conduct biochemical studies using mitochondria, housefly (Musca domestica), western flower thrips (Frankliniella occidentalis), diamondback moth (Plutella xylostella) and honeybee (Apis mellifera) were collected and purchased, and whole insect bodies or the thoraxes were used for preparation of the mitochondria after the materials were frozen in liquid nitrogen and frozen at −80°C. The origin and weight of the materials used for the mitochondria preparation are summarized in Table S1. Mitochondria from housefly, western flower thrips, diamondback moth and honeybee were prepared from frozen insect materials (thoraxes or bodies) according to previously reported method.7) The insect materials were gently homogenized using a glass homogenizer with the buffer containing 250 mM sucrose, 5.0 mM EDTA, and 10 mM Tris-HCl buffer (pH 7.5). The homogenized mixture was filtered through four layers of commercial medical gauze with the help of a vacuum line. Subsequently, the filtrate was re-homogenized in the same buffer and centrifuged twice at 3,000 rpm for 3 min at 4°C in a Hitachi CR20GRIII centrifuge, followed by the centrifugation of the supernatant at 10,000 rpm for 8 min at 4°C. The resulting pellet was washed with the same buffer and centrifuged at 10,000 rpm for 8 min at 4°C. The final pellet was resuspended in the same buffer (pH 7.5) and used as a crude mitochondrial protein preparation. The total protein contents were estimated according to Bradford method using bovine serum albumin as the standard.8)
For the mass-spectrometry based ligand binding assay, submitochondrial particles (an inside-out membrane preparation), derived from green bottle fly (Lucilia sericata), were used as experimental material. The thoraxes from green bottle fly were homogenized with Ultra-Turnax homogenizer (IKA) in the buffer containing 300 mM sucrose, 5 mM HEPES/KOH, 0.12 mM MgCl2, 1 mM EDTA, 2 mM DTT, and protease inhibitors (pH 7.4). After the homogenate was filtered through muslin and the debris were removed by low-speed countrification, the supernatant was then sonicated for 2 min at 20 amplitude, 5 sec on/5 sec off cycle at lower than 10°C using a Qsonica sonicator. The submitochondrial particles were collected by ultracentrifugation at >150,000×g. The resulting pellet was resuspended in the same buffer and kept at −80°C until use.9,10)
3. Measurement of respiratory enzyme activities3.1. Effects of FloMet to respiratory complexesFor the assays of each respiratory complex, insect mitochondria were permeabilized by freeze-thawing to improve the accessibility of substrates, such as NADH and cytochrome c (cyt. c).11–14) NADH oxidase activity (covering complexes I–III–IV) was followed by the oxidation of NADH (340 nm; ε=6.2 mM−1 cm−1) in the buffer (2.0 mL) containing 150 mM KCl, 2.5 mM MgCl2, and 30 mM potassium phosphate (KPi) buffer (pH 7.4) at 30°C using a Shimadzu UV-3000 spectrophotometer. The protein concentration was set to 30 µg/mL, and the reaction was initiated by the addition of NADH (final 150 µM) as an electron donor. NADH-Q1 oxidoreductase activity (covering complex I) was followed by the oxidation of NADH in the same KPi buffer (2.0 mL) containing 0.2 µM antimycin A, 4.0 mM KCN and 50 µM ubiquinone-1 (Q1). The protein concentration was set to 30 µg/mL, and Q1 was used as an electron acceptor. The reaction was initiated by the addition of NADH (final 50 µM) as an electron donor.
Succinate-Q1 oxidoreductase activity (covering complex II) was determined by the coupled reduction of 2,6-dichloroindophenol (DCIP) (600 nm; ε=21.6 mM−1 cm−1) using a Shimadzu UV-3000 spectrophotometer in the buffer (2.0 mL) containing 150 mM KCl, 2.5 mM MgCl2, 0.2 µM antimycin A, 4.0 mM KCN, 30 µM Q1, 50 µM DCIP, and 30 mM KPi (pH 7.4) at 30°C. The protein concentration was set to 30 µg/mL, and the reaction was initiated by the addition of sodium succinate (final 10 mM) as an electron donor.
Succinate-cyt. c oxidoreductase activity (covering complex II-III) was determined by the reduction of cyt. c (550–540 nm; ε=21 mM−1 cm−1) using a Shimadzu UV-3000 spectrophotometer in the buffer (2.0 mL) containing 150 mM KCl, 2.5 mM MgCl2, 1.0 µM rotenone (complex I inhibitor), 2.0 mM KCN, 50 µM cyt. c and 30 mM KPi (pH 7.4) at 30°C. The protein concentration was set to 30 µg/mL, and the reaction was initiated by the addition of sodium succinate (final 10 mM) as an electron donor. IC50 values of FloMet for each insect species were calculated in a probit method with ECOTOX v3 software, based on the inhibition rate at each concentration of FloMet.
3.2. Measurement of reduction of hemes b and c1The redox statuses of cytochromes b (containing hemes bL and bH) and c1 (containing heme c1) were monitored spectrophotometrically at 563–575 nm and 539–553 nm, respectively.15–17) The absorbance change was recorded with a Shimadzu UV-3000 spectrophotometer in a dual wavelength mode. Housefly mitochondria (0.8 mg/mL) were suspended in 2.0 mL reaction buffer containing 150 mM KCl, 2.5 mM MgCl2, 1 µM rotenone, 0.2 µM SF6847 and 2.0 mM KCN, and 30 mM KPi, (pH 7.4) at 30°C. The reaction was initiated by the addition of 5 mM sodium succinate after the equilibration of the mitochondria with an inhibitor(s) for 4 min. The concentration of the inhibitors tested was set to that exhibiting maximum inhibition of complex III. Complete reduction of the hemes was achieved by the addition of excess sodium dithionite (Na2S2O4) in the cuvette.
4. Mass-spectrometry-based ligand binding assayThe binding of FloMet to complex III was quantified by MS-based ligand binding assay,18) which enable saturation and competition experiments using non-labelled reporter ligand. In a typical assay, chemicals (test compounds and/or competitors) were dispensed into a 96-multiwell plate in minimal volume of DMSO (<0.1%) using an automated Tecan liquid dispenser. Then, 900 µL of assay incubation buffer (50 mM Tris-HCl, 120 mM NaCl, 0.1 mM EDTA, pH 7.4) containing 0.1–0.5 mg/mL bacitracin (antibiotics) and 0.05–0.1% n-undecyl β-D-maltoside (detergent) was added into each assay well, followed by the addition of 20–40 µg of the green bottle fly submitochondrial particles. Final assay volume was adjusted to 1 mL using the same assay buffer. After the assay mixture was incubated at room temperature for 1 hr, the mixture was filtrated with the filter mat with the help of a vacuum line.
The filter mat was subsequently washed with the assay buffer or that containing detergent 3–5 times. The corresponding discs containing the bound reporter ligand on the filter mat were cut out, extracted with acetonitrile, transferred onto glass fiber filter plates, and filtered under vacuum into mass spectrometry glass vials. The vials were brought to dryness and the residues were re-suspended in 1 : 1 acetonitrile/water. Finally, the samples were subjected to LC-ESI-MS/MS quantification (Sciex Qtrap5500, running Sciex Analyst 1.7.1) without further sample preparation.
For saturation experiments, submitochondrial particles were incubated with eight different concentrations of reporter ligand in a concentration range of 30 pM–2.5 µM. Non-specific binding was determined in the same way as total binding including both specific and non-specific binding, but in the presence of excess concentration (2.5 µM) of the ligand antimycin A that was found to compete with FloMet. For the competition of ligands for the Qo site, azoxystrobin was used as a reporter ligand at the final concentration of 30 nM, and azoxystrobin analog, metyltetraprole, and FloMet were used as the competitor. For the competition of ligands for the Qi site, FloMet was used as the reporter ligand at the final concentration of 15 nM, and antimycin A and azoxystrobin were used as the competitor.
All results of the binding experiments (Kd, Bmax) are given as the mean±standard error of the mean (SEM; at least three experiments). Specific binding was calculated as difference between total binding and non-specific binding. Data from the binding experiment were analyzed by means of non-linear regression with Prism v7.0 (GraphPad Software, San Diego, California, U.S.A.). For saturation experiments the One site—specific binding non-linear regression equation was used to obtain saturation isotherms with calculated Kd (equilibrium dissociation constants) and Bmax (maximum density of binding sites) values. For competition experiments, the One Site—Fit LogKi (inhibition constant of the test compound) non-linear regression equation was used to obtain sigmoidal competition curves. The top level (total binding in absence of the competitor antimycin A) was set to 100% and the bottom level (non-specific binding) was set to 0% where appropriate. Ki values were calculated from the concentration at which a test compound inhibited 50% of specific analyte binding.
As the insects treated with FLO exhibited symptoms analogous to those induced by respiratory inhibitors, FLO is suggested to target to any of mitochondrial respiratory chain enzymes. In addition, the deacylated metabolite, FloMet, was also observed to slightly elicit insecticidal activity against the diamondback moth.19) Therefore, using the housefly mitochondria, we examined the effects of FLO and the metabolite FloMet on NADH oxidase activity (covering complexes I, III, and IV). As illustrated in Fig. 2, FLO and FloMet were added to the mitochondria during NADH oxidation at the final concentration of 40 nM each. In contrast to FLO, which showed almost no inhibition, FloMet exhibited remarkable inhibition against NADH oxidase activity. These results clearly indicate that FLO itself is not active, but its metabolite FloMet is active as an inhibitor targeting either respiratory complex I (NADH-ubiquinone oxidoreductase), III (ubiquinol-cyt. c oxidoreductase), or IV (cyt. c oxidase). Although the mechanism of converting FLO to FloMet has yet to be elucidated in the target pest, a similar activation mechanism is known in the case of many pro-acaricides including acequinocyl.20,21)
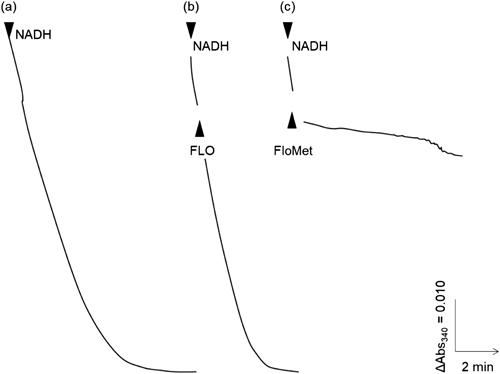
Next, we examined the effects of FloMet on individual respiratory enzymes in the housefly mitochondria. Figure. 3 shows the activity of complex I using NADH and ubiquinone-1 (Q1) as electron donor and acceptor (NADH-Q1 oxidoreductase activity), respectively. FloMet, at the concentration exhibiting significant inhibition of NADH oxidase activity, did not exhibit inhibition, while a typical complex I inhibitor fenpyroximate elicited significant inhibition. FloMet also exhibited no discernible effects on the activity of complex II (succinate-ubiquinone oxidoreductase, Fig. 4). These results suggest that the target of FloMet neither includes complexes I nor II. On the other hand, FloMet exhibited a concentration-dependent inhibitory effect against complex II–III; succinate-cyt. c oxidoreductase activity (Fig. 5). These results demonstrated that complex III is the molecular target for FloMet.



We compared the effects of FloMet on respiratory complex III between target and nontarget insects using the mitochondria isolated from western flower thrips, diamondback moth, and honeybee. As summarized in Table 1 referring to Fig. S1A–C, the effects of FloMet on succinate-cyt. c oxidoreductase activities are presented as IC50 values (the molar concentration needed to reduce the control activity by 50%). FloMet exhibited potent inhibition against mitochondria from the western flower thrips, the diamondback moth, and the housefly, with the IC50 values of 2.9, 18, and 5.0 nM, respectively. In contrast, it exhibited a relatively lower inhibitory effect on the honeybee mitochondria, with an IC50 value of 298 nM. That alone does not explain the selective toxicity between target pests and honeybee. However, these findings were consistent with in vivo acute toxicity of FLO against the honeybee4) and indicate a notable specificity towards target insect pests rather than nontarget beneficial insects. The mechanism underlying selectivity among insect species will be explored in the future investigations.
| F. occidentalis | M. domestica | P. xylostella | A. mellifera | |
|---|---|---|---|---|
| IC50value | 2.9 nM | 5.0 nM | 18 nM | 298 nM |
There are two distinct reaction sites for ubiquinone/ubiquinol in complex III: one site is in close proximal to the matrix side (Qi site), facilitating the transfer of electrons from cytochrome b (containing heme bL and bH) to ubiquinone. The other site, proximal to the intermembrane space (Qo site), enables the transfer of electrons from ubiquinol to the Rieske iron-sulfur cluster, followed by the reduction of cytochrome c1 (containing heme c1). Complex III inhibitors are divided into two groups known as “Qi site” and “Qo site” inhibitors, typically represented by antimycin A and myxothiazol, respectively. To identify the binding site of FloMet in complex III, we examined the effects of FloMet on the reduction kinetics of cytochromes b and c1 hemes using the housefly mitochondria.
It is well-established that cytochrome b hemes are immediately reduced by the addition of respiratory substrate (succinate) in the presence of Qi or Qo site inhibitor (Fig. 6A, trace f, g). Consistent with previous studies, only the simultaneous addition of Qi and Qo site inhibitors (antimycin A and myxothiazol, respectively) resulted in the suppression of the reduction of b hemes (trace b). The addition of FloMet alone did not affect the reduction level of the b hemes (trace e), and the combination of antimycin A and FloMet did not suppress the reduction of b hemes (trace d). These results indicated that FloMet is unlikely to act as a Qo site inhibitor; however, the simultaneous addition of FloMet and myxothiazol did not significantly suppress the reduction of b hemes (trace c). This is similar to the behavior observed in the study of UK-2A, another Qi site inhibitor. The effects of UK-2A on the reduction of b hemes differ slightly from those of antimycin A.16)

To clarify the role of FloMet as a complex III inhibitor, we further investigated the effects of inhibitors on the c1 heme reduction. Antimycin A did not affect the reduction level of the c1 heme, whereas myxothiazol significantly reduced the reduction level (Fig. 6B, traces a–c). The profile of the c1 heme reduction by FloMet was comparable with that by antimycin A. Combining all these results in the measurement of the reduction of cytochrome b and c1 hemes, we concluded that FloMet binds to the Qi site in complex III, albeit in a slightly different manner from antimycin A.
3. Ligand binding assaysReduction kinetics of cytochrome b and c1 hemes in the presence of FloMet leads to the speculation that FloMet binds the Qi site, a position where antimycin A binds, but in a slightly different manner. Therefore, ligand binding studies were performed to further corroborate that FloMet specifically acts on the Qi site. Using in-house established protocols, the saturation binding of FloMet was determined using submitochondrial particles derived from the green bottle fly. The binding isotherm of FloMet showed saturation at high concentration and the data fitted to One-site binding kinetics equation, indicating specificity of the binding (Fig. 7)

Subsequently, we tested the binding competition of FloMet with two established reference tool compounds: antimycin A (Qi site inhibitor) and azoxystrobin (Qo site inhibitor). FloMet could not displace azoxystrobin out of its binding site apart from partial displacement at very high concentration, micromolar range (Fig. 8A). The reverse assay to displace FloMet using azoxystrobin confirmed that there is no exclusive binding of the two entities indicating that FloMet is unlikely to bind to the Qo site. On the other hand, antimycin A displaced FloMet at low nano molar range confirming exclusive binding and providing further evidence that FloMet binds to the Qi site of complex III (Fig. 8B). However, considering the difference between FloMet and antimycin A on the reduction kinetics of cytochromes b and c1 hemes, the binding site(s) of these two inhibitors are not identical; they may share a common binding domain in the Qi site with partially overlapping sites. Further studies will be needed to characterize their binding modes at the Qi site comprehensively.

We investigated the effect of FloMet, the biologically-active form of FLO, on insect respiratory chain complexes; FloMet potently inhibited only complex III without any observed effects on complexes I and II. Furthermore, the target site was identified to be the Qi site of complex III using orthogonal techniques; the measurement of the reduction of cytochrome hemes b and c1 (functional) and the competitive ligand binding assays (binding). FLO is the first insecticide to inhibit the Qi site of mitochondrial respiratory chain complex III and is the only compound classified in Group 34, a new group in the IRAC classification, to-date.
Currently, FLO is registered in Japan, Korea, and other countries, and is used as one of the excellent control agents for sucking pests such as thrips and whiteflies. We hope that FLO will continue to support farmers’ efforts in controlling such sucking pest spectrum for several decades to come via suitable rotation sprays.
The authors are grateful to Prof. Hideto Miyoshi, Kyoto University, for his helpful discussion and experimental advice.
The authors declare no conflicts of interest associated with this manuscript.
The online version of this article contains supplementary materials (Supplemental Table S1, Fig. S1A–C), which are available at https://www.jstage.jst.go.jp/browse/jpestics/.