2014 Volume 64 Issue 4 Pages 309-320
2014 Volume 64 Issue 4 Pages 309-320
Hybrid incompatibility plays an important role in establishment of post-zygotic reproductive isolation. To unveil genetic basis of hybrid incompatibilities between diverged species of genus Oryza AA genome species, we conducted genetic dissection of hybrid sterility loci, S22(t), which had been identified in backcross progeny derived from Oryza sativa ssp. japonica (recurrent parent) and South American wild rice O. glumaepatula near the end of the short arm of chromosome 2. The S22(t) region was found to be composed of two loci, designated S22A and S22B, that independently induce F1 pollen sterility. Pollen grains containing either of the sterile alleles (S22A-glums or S22B-glums) were sterile if produced on a heterozygous plant. No transmission of the S22A-glums allele via pollen was observed, whereas a low frequency of transmission of S22B-glums was observed. Cytological analysis showed that the sterile pollen grains caused by S22A could reach the bicellular or tricellular stage, and the nearly-sterile pollen grains caused by S22B could reach the tricellular stage. Our genetic analysis showed repulsion linkage effect is possible to induce strong reproductive barrier by high pollen sterility based on recombination value and transmission ratio of hybrid sterility gene to the progeny was influenced by frequency of competitors on fertilization.
Isolation restricts free gene exchanges between populations or species and leads genetic differentiation of species. Geographical isolation and pre-mating and post-mating-pre-zygotic reproductive isolation act as extrinsic barriers before to fertilization to decrease spatial-temporal opportunity of hybridization between populations. Meanwhile, post-zygotic reproductive isolation provides intrinsic effect after fertilization to restrict gene flow between populations due to hybrid incompatibility such as inviability and sterility in F1 and hybrid breakdown in F2 and consequent generations (Coyne and Orr 2004, Stebbins 1950). So far, some genic phenomena of hybrid incompatibility have been recognized as each single Mendelian factor and these usually accompany segregation distortion at causal loci because inviability or sterility of zygotes (sporophytes) and gametes (gametophytes) carrying incompatible genotypes alter transmission frequency. The t haplotype in mouse and segregation distorter in Drosophila are well-characterized examples that lead transmission ratio distortion (TRD) to their progeny in hybrids (Silver 1993, Temin et al. 1991). It has been inferred that the TRD act as driving force for evolution of reproductive isolation due to so-called “selfish” characteristics as target of selection of gene because gametes carrying selfish gene exclusively fertilize by sterility of gametes carrying opposite alleles in the heterozygotes. Molecular cloning of the genes governing TRD system demonstrated that gene interaction of multiple loci closely linked to each other induced the TRD in mouse (Silver 1993), Drosophila (Temin et al. 1991), wheat (Friebe et al. 2003) and rice (Long et al. 2008) whereas it has been known that interaction of multiple alleles at single locus triggers the TRD in some cases (Chen et al. 2008, Phadnis and Orr 2009). Bateson-Dobzhansky-Muller (BDM) model provided hypothetical evolutionary model in which how neutral condition of alleles causing TRD are maintained in diverged populations. However, driving force of TRD for species divergence cannot be fully elucidated only by gene cloning.
The AA-genome species of genus Oryza are distributed worldwide in six wild and two cultivated species. Among the AA-genome wild species of Asia (O. nivara/O. rufipogon), Oceania (O. meridionalis), Middle and South America (O. glumaepatula), and Africa (O. barthii/O. longistaminata), F1 pollen sterility is one of the most common and important reproductive barriers (Chu et al. 1969, Morishima 1969). Two genetic models of the Mendelian loci controlling F1 gamete sterility have been commonly proposed: single-locus allelic interaction (Ikehashi and Araki 1988, Kitamura 1962) and two-locus interaction involving duplicate gametic-lethal genes (Oka 1974). Molecular cloning of sterility genes has elucidated the molecular basis of allelic interaction (Chen et al. 2008, Long et al. 2008, Yang et al. 2012) and of duplicate gametic-lethal genes as duplication and reciprocal loss of essential genes for pollen development (Mizuta et al. 2010, Win et al. 2011, Yamagata et al. 2010). Gene cloning is providing opportunities to conduct phylogenetic and functional analysis of causal genes to reveal evolutionary pathway of F1 pollen sterility during differentiation of species and molecular mechanism inducing pollen sterility.
O. glumaepatula is a wild species distributed in the South America continents and has been mainly classified into several subgroups (Akimoto et al. 1997). Especially, O. glumaepatula in Amazon basin have similar genetic relationships with O. barthii/O. glaberrima complex in RFLP markers (Doi et al. 2000) and retrotransposon p-SINE1 analysis (Cheng et al. 2002). It has been estimated that origin of wild rice in South America continent was from African continent. However, genetic architectures of F1 pollen sterility between O. sativa and O. glumaepatula and between O. sativa and O. barthii/O. glaberrima have not been comprehensively understood. So far, S3, S18, S19, S20, S21, S29(t), S34(t), S38(t), and S39(t) as pollen killers and S1, S33(t), and S37(t) as gamete eliminators have been identified in hybrids between O. sativa and O. glaberrima (Doi et al. 1998, 1999, Hu et al. 2006, Ren et al. 2005, Sano 1983, 1990, Taguchi et al. 1999, Xu et al. 2014, Zhang et al. 2005). In hybrids between O. sativa and O. glumaepatula, S12, S22, S23, S27, and S28 have been identified (Sano 1994, Sobrizal et al. 2000a, 2000b, 2001, 2002). S29(t) and S21 have been identified in sativa/glaberrima hybrid at distal end of short-arm on chromosome 2 and long-arm on chromosome 7, respectively, and S22(t) and S23(t) identified in sativa/glumaepatula hybrids were mapped around S29(t) and S21 region. The diversity analysis showed that the casual alleles of F1 pollen sterility, S27-glums, were found in O. barthii accessions (Yamagata et al. 2010). Although identities of these genes have not been exactly elucidated, common origin of F1 pollen sterility gene in O. glumaepatula and O. barthii/O. glaberrima could be suggested. Therefore, precise understanding of genetic loci for F1 pollen sterility of AA genome wild species would provide key information for tracing evolutionary process of post-zygotic reproductive isolation during AA genome differentiation. In addition, since hybrid sterility hinders transferring of genes for useful traits from wild species for the improvement of cultivated rice (Oryza sativa), this understanding make it easy for breeders to use the valuable genetic resources of wild species.
Here, we demonstrate genetic dissection of the S22(t) region into S22A and S22B as single Mendelian factors by high-resolution mapping and linkage analysis. Morphological characterization of F1 pollen sterility caused by S22A and S22B during post-meiotic stages was conducted. Finally, we suggested possible idea of genetic model for extremely high degree of pollen sterility in F1 by repulsion phase of the tightly linking alleles causing pollen semi-sterility depending on the recombination values between the two loci using F1 hybrids derived from a cross between S22A nearly-isogenic line (NIL) and S22B NIL as model cases.
Reciprocal crosses between ssp. japonica rice cultivar Taichung 65 (T65) and O. glumaepatula accession IRGC105668 were performed to produce F1 plants. To generate a backcross population for the construction of O. glumaepatula introgression lines (Sobrizal et al. 1999, Yoshimura et al. 2010), repeated backcrossing had been conducted with pollen from T65 (recurrent parent). During this process, 83 BC3F1 plants were genotyped using 106 restriction-fragment-length polymorphism (RFLP) markers distributed evenly throughout the 12 rice chromosomes. The BC4F3 population used for mapping of S22(t) was derived from BC4F1 plants generated by repeated backcrossing with pollen of T65. Plants heterozygous at S22(t) in the BC4F3 population were maintained by repeated self-pollination along with phenotypic selection of plants with semi-sterile pollen and marker-assisted selection of plants heterozygous at S22(t). The BC4F5 population derived from the BC4F4 plant showing pollen semi-sterility were used for the genetic dissection of the S22(t) region.
Evaluation of pollen fertilityPanicles at the flowering stage were collected and stored in 70% ethanol. A few days before anthesis, six anthers from a single spikelet were squashed in 1% I2–KI solution on a slide glass. After the debris was removed, the pollen was observed with a light microscope. Pollen grains with morphology similar to those of T65 were scored as fertile. Pollen grains with abnormal morphology (e.g., empty, unstained, or smaller than normal) were scored as sterile. At least 200 pollen grains were observed to evaluate the pollen fertility of each plant. Hereafter, descriptions of fertility and sterility refer exclusively to the pollen; female fertility was not assessed in this study.
GenotypingFor RFLP analysis, total genomic DNA was extracted from leaf samples frozen in liquid nitrogen by using the CTAB method (Murray and Thompson 1980). The isolated DNA was digested with restriction enzymes ApaI, BamHI, BglII, DraI, EcoRI, EcoRV, HindIII, and KpnI. The digested DNA was separated by 0.8% agarose gel electrophoresis and blotted onto Hybond N+ membrane (GE Healthcare Japan, Tokyo, Japan) by capillary transfer in 0.4 M NaOH solution. The blotted membranes were washed in 2× SSC, dried, and baked at 120°C for 20 min. DNA clones previously mapped by Tsunematsu et al. (1996) and Harushima et al. (1998) were used as probes. Hybridization and signal detection were carried out according to protocols of the ECL Direct Nucleic Acid Labeling and Detection System (GE Healthcare Japan).
For genotyping with simple sequence repeat (SSR) markers, total crude genomic DNA was extracted from freeze-dried samples ground with a Multi-Beads Shocker (Yasui Kikai, Osaka, Japan) (Dellaporta et al. 1983, Wang et al. 1993). Publicly reported SSR markers in rice were used (International Rice Genome Sequencing Project 2005, McCouch et al. 2002, Temnykh et al. 2000). PCR reactions were performed in a 15-μL reaction mixture containing 50 mM KCl, 10 mM Tris·HCl (pH 9.0), 1.5 mM MgCl2, 200 μM each dNTP, 0.2 μM each primer, 0.75 unit of Taq polymerase (Takara, Otsu, Japan), and approximately 25 ng of template DNA in a GeneAmp PCR system 9700 (Applied Biosystems, Foster City, CA, USA). PCR conditions were 95°C for 5 min followed by 35 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s. PCR products were run in 4% agarose gel (Agarose HT, Amresco Inc., Solon, OH, USA) in 0.5× TBE buffer.
Observation of post-meiotic developmentFor observation of post-meiotic pollen development with hematoxylin and I2–KI staining, panicle samples were continually collected from the booting to heading stages and placed in fixative solution containing 4% (w/v) paraformaldehyde, 0.25% (w/v) glutaraldehyde, 0.02% (v/v) Triton X-100, and 100 mM sodium phosphate (pH 7.5) at 4°C for 24 h. After rinsing in 100 mM sodium phosphate buffer, the fixed panicles were stored in 100 mM sodium phosphate buffer containing 0.1% (w/v) sodium azide (NaN3). The hematoxylin staining procedure of Chang and Neuffer (1989) was used with minor modifications. Approximately 100 pollen grains per spikelet were observed to classify the developmental stages of the pollen grains, and the frequency of pollen grains at each stage was calculated as the average of three replicates of florets.
In vitro pollen germinationPollen from plants just beginning to flower under natural conditions was shed by hand-tapping onto medium on a slide glass containing 15% sucrose, 0.01% H3BO3, 0.03% CaCl2, and 0.6% gellan gum (Gelrite, Wako, Osaka). After a few minutes’ incubation at room temperature, photographs were taken under a light microscope.
While developing introgression lines of O. glumaepatula (accession no. IRGC105668) with the genetic background of T65 (Sobrizal et al. 1999, Yoshimura et al. 2010), we mapped the S22(t) locus, which controls gametophytic F1 pollen sterility, on the short arm of chromosome 2 by RFLP markers in a BC4F2 population derived from BC4F1 plants carrying IRGC105668-derived segments of chromosomes 1, 2, 3, 7, and 9 (Sobrizal et al. 2000b). To construct linkage map of S22(t) by SSR marker, we reexamined segregation population of S22(t) in a BC4F3 population derived from BC4F2 plant heterozygous at S22(t). In the BC4F3 population, plants segregated for semi-sterility in a ratio of 83 fertile: 70 semi-sterile (Table 1). Linkage analysis by SSR markers revealed that S22(t) was located between RM12317 and RM279 with genetic map distances of 0.32 cM and 0.99 cM, respectively (Fig. 1A). The complete linkage of S22(t) to RM12329, RM7451, and RM7033 was observed. T65-homozygous and heterozygous plants at RM12329 showed fertile and semi-sterile phenotypes, respectively (Table 1). The IRGC105668 allele at S22(t) (S22(t)-glums) were assumed to be sterile because no homozygous plants for the S22(t)-glums allele were obtained.
| Phenotype | Genotypes at RM12329a | Total | ||
|---|---|---|---|---|
| TT | TG | GG | ||
| Fertile | 83 | 0 | 0 | 83 |
| Semi-sterile | 0 | 70 | 0 | 70 |
| Total | 83 | 70 | 0 | 153 |

Dissection of the S22(t) region. (A) Reexamination of linkage of S22(t) on chromosome 2 in BC4F3 generation derived from hybrids between O. sativa and O. glumaepatula. (B) Graphical genotypes of recombinants between RM12317 and RM279 obtained from a BC4F5 population derived from Taichung 65 (recurrent parent) and O. glumaepatula (n = 753). The recombinants were classified into ten genotypes designated as recombinant type A (RA) through RJ. Numbers in parentheses represent individuals of each recombinant type. F and SS indicate fertile and semi-sterile phenotypes, respectively. Vertical lines indicate locations of the markers used in this experiment. White, hatched, and black boxes indicate T65-homozygous, heterozygous, and IRGC105668-homozygous genotypes, respectively. Grey boxes show regions of recombination. Dotted horizontal lines show candidate genomic regions predicted to contain a hybrid sterility gene.
To determine the precise location of S22(t) on chromosome 2, we genetically dissected the S22(t) region using the 51 plants having recombination between RM12317 and RM279 from among 753 plants in the BC4F5 generation (Fig. 1B). The genotypes of the 51 recombinants were determined for SSR markers RM12329, RM7451, RM12353, and RM7033 (Fig. 1B). Ten genotype combinations were identified and designated recombinant type A (RA) through RJ (Fig. 1B). Comparisons among genotypes RC, RD, and RG provided us critical information regarding the precise location of S22(t). RC was heterozygous at RM12317, RM12329, and RM7451 and T65-homozygous at RM12353, RM7033, and RM279, and the plants were semi-sterile. RD was heterozygous at RM12317, RM12329, and RM7451, and IRGC105668-homozygous at RM12353, RM7033, and RM279; these plants were also semi-sterile. The comparison of RC and RD showed that the gene for pollen semi-sterility was located on the short-arm side of RM12353. On the other hand, the phenotype and genotype of RG seemed to indicate that the genetic factor for pollen semi-sterility was located on the long-arm side of RM12353. These two contradictory interpretations suggested that either S22(t) is located very close to RM12353 or two loci that could each induce gametophytic pollen sterility are located in the S22(t) region. If the latter hypothesis was correct, we would expect the single genetic factor on the short-arm side to be located between RM12317 and RM12353, and the factor on the long-arm side to be between RM12353 and RM279.
Identification of S22A and S22BTo test whether the possible genetic factor on the short-arm side indeed behaved as a single Mendelian factor, we performed linkage analysis using a BC4F6 population derived from RE (Figs. 1B, 2A–2C). Pollen fertility in the BC4F6 population was classified into fertile and semi-sterile classes in a 115 : 112 ratio with 98.0% and 50.4% average pollen fertility, respectively (Fig. 2B). All of the semi-sterile plants were heterozygous and all of the fertile plants were T65-homozygous at RM7451. No IRGC105668-homozygotes were obtained. These results demonstrate that a single gene completely linked to RM7451 controlling gametophytic pollen sterility in this BC4F6 population, as shown by the sterility of pollen grains carrying the IRGC105668 allele. We designated this gene S22A. The linkage analysis using other SSR markers showed that RM12329 and RM12353 are completely linked to S22A and that RM12317 is linked to S22A at a distance of 0.22 cM (Fig. 2C).

Linkage mapping of S22A and S22B. (A, D) Graphical genotypes of parental plants of BC4F6 population for linkage analysis of S22A (A) and S22B (D), respectively. White, hatched, and black boxes indicate T65-homozygous, heterozygous, and IRGC105668-homozygous region, respectively. (B, E) Frequency distribution of pollen fertility in the BC4F6 population for linkage analysis of S22A (B) and S22B (E). White, hatched, and black boxes indicate T65-homozygous, heterozygous, and IRGC105668-homozygous plants at RM7451 (linked to S22A) (b) and RM7033 (linked to S22B) (E), respectively. (C, F) Linkage maps of S22A (C) and S22B (F). White and hatched boxes indicate T65-homozygous and heterozygous genotypes, respectively. Grey boxes show regions of recombination.
To investigate the allele transmission of S22A, we made reciprocal crosses between T65 and semi-sterile plants caused by S22A (designated S22A_SS) (Table 2). When S22A_SS was used as the female parent, segregation of fertile and semi-sterile plants fit a 1 : 1 ratio (fertile : semi-sterile = 54 : 59, χ2 1:1 = 0.22, P = 0.64). When S22A_SS was used as the male parent, only fertile plants were obtained in the F1. This transmission analysis is direct evidence that the pollen semi-sterility was caused by sterility of pollen grains carrying the IRGC105668 allele of S22A.
| Cross combination | Number of plants in F1 | ||
|---|---|---|---|
| Female | Male | Fa | SS |
| S22A_SS | T65 | 54 | 59 |
| T65 | S22A_SS | 84 | 0 |
Next, we mapped the genetic factor on the long-arm side from RM12353 as a single Mendelian factor using a BC4F6 population derived from genotype RG (Figs. 1B, 2D–2F). This population showed a bimodal distribution of pollen fertility, with 54 fertile and 48 semi-sterile plants with 98.6% and 50.6% average pollen fertility, respectively (Fig. 2E). All of the semi-sterile plants were heterozygous, and the fertile plants were either T65-homozygous or IRGC105668-homozygous at RM7033, with the exception of one fertile plant heterozygous at RM7033. The T65-homozygotes, heterozygotes, and IRGC105668-homozygotes segregated in a 45 : 49 : 8 ratio, with segregation distortion from a theoretical ratio of 1 : 2 : 1 (χ2 1:2:1 = 28.7, P < 0.01) at RM7033. These results demonstrate that the segregation of pollen semi-sterility in this BC4F6 population was governed by a single gene that induced sterility of pollen grains from plants carrying the IRGC105668 allele in the heterozygous condition, whereas T65-homozygotes and IRGC105668-homozygotes at RM7033 had normal fertility. This genetic system was considered to fit the typical one-locus model of F1 pollen sterility, and we designated this locus S22B.
To confirm the sterility of pollen grains governed by the IRGC105668 allele at S22B (S22B-glums), we tested allele transmission at S22B using F1 populations derived from reciprocal backcrosses between T65 and the semi-sterile plants caused by S22B (S22B_SS) (Table 3). Pollen semi-sterility was transmitted to the progeny when S22B_SS was used as the female parent, but not when it was used as the male parent. Thus, the semi-sterility of S22B_SS was due to sterility of pollen grains carrying S22B-glums. However, as noted above, in the BC4F6 population for linkage mapping of S22B, eight IRGC105668-homozygous plants were obtained, indicating that S22B-glums was transmitted via pollen at a low frequency and that the pollen sterility induced by S22B-glums was not complete. S22B was mapped between RM7033 and RM279, with a distance of 0.49 cM from each of the two markers (Fig. 2F).
| Cross combination | Number of plants in F1 | ||
|---|---|---|---|
| Female | Male | Fa | SS |
| S22B_SS | T65 | 39 | 34 |
| T65 | S22B_SS | 45 | 0 |
Pollen germination was tested in vitro to characterize the physiological features of the pollen from S22A_SS and S22B_SS plants. We classified pollen grains shed onto germination medium as normal or abnormal on the basis of their diameter compared with pollen grains of T65. As a positive control, T65 pollen germination was observed and found to be normal (Fig. 3A). All of the abnormal pollen grains from the S22A_SS plants and most from the S22B_SS plants failed to germinate, although 1.8% of the latter germinated (Fig. 3B, 3C). This result was consistent with the observation of S22B-glums homozygotes in the progeny of self-pollinated S22B_SS plants due to a low frequency of male transmission of the S22B-glums allele.

In vitro germination test of sterile pollen grains caused by S22A and S22B. (A–C) Pollen just after flowering was shed onto germination medium from T65 (A), S22A_SS (B), and S22B_SS (C). Arrows and black arrowheads indicate normal and abnormal pollen grains, respectively. Occasional germination from abnormal pollen was observed (white arrowhead).
To characterize pollen sterility caused by S22A and S22B, we investigated pollen development at several post-meiotic stages in T65, S22A_SS, and S22B_SS. I2–KI and hematoxylin staining were used to determine the frequency of pollen at different developmental stages in each sample (Figs. 4, 5, 6). Stages of development were assessed on the basis of the development of normal T65 pollen grains. At the unicellular stage, the pollen grains of T65 showed no starch accumulation (Fig. 4A) and the nucleus was located at the side opposite the pollen pore (Fig. 5A). All of the pollen grains in S22A_SS and S22B_SS were likewise at the unicellular stage (Figs. 4A–4C, 5A–5C, 6A–6C).

Observation of post-meiotic pollen development in T65, S22A_SS, and S22B_SS by I2–KI staining. Pollen grains at the unicellular (A–C), early bicellular (D–F), late bicellular (G–I), tricellular (J–L), and mature stages (M–O). Stages were categorized on the basis of development of normal pollen grains in T65. (A, D, G, J, M) Pollen of T65. (B, E, H, K, N) Pollen of S22A_SS. (C, F, I, L, O) Pollen of S22B_SS. Scale bars = 20 μm. Asterisks indicate abnormal pollen grains.
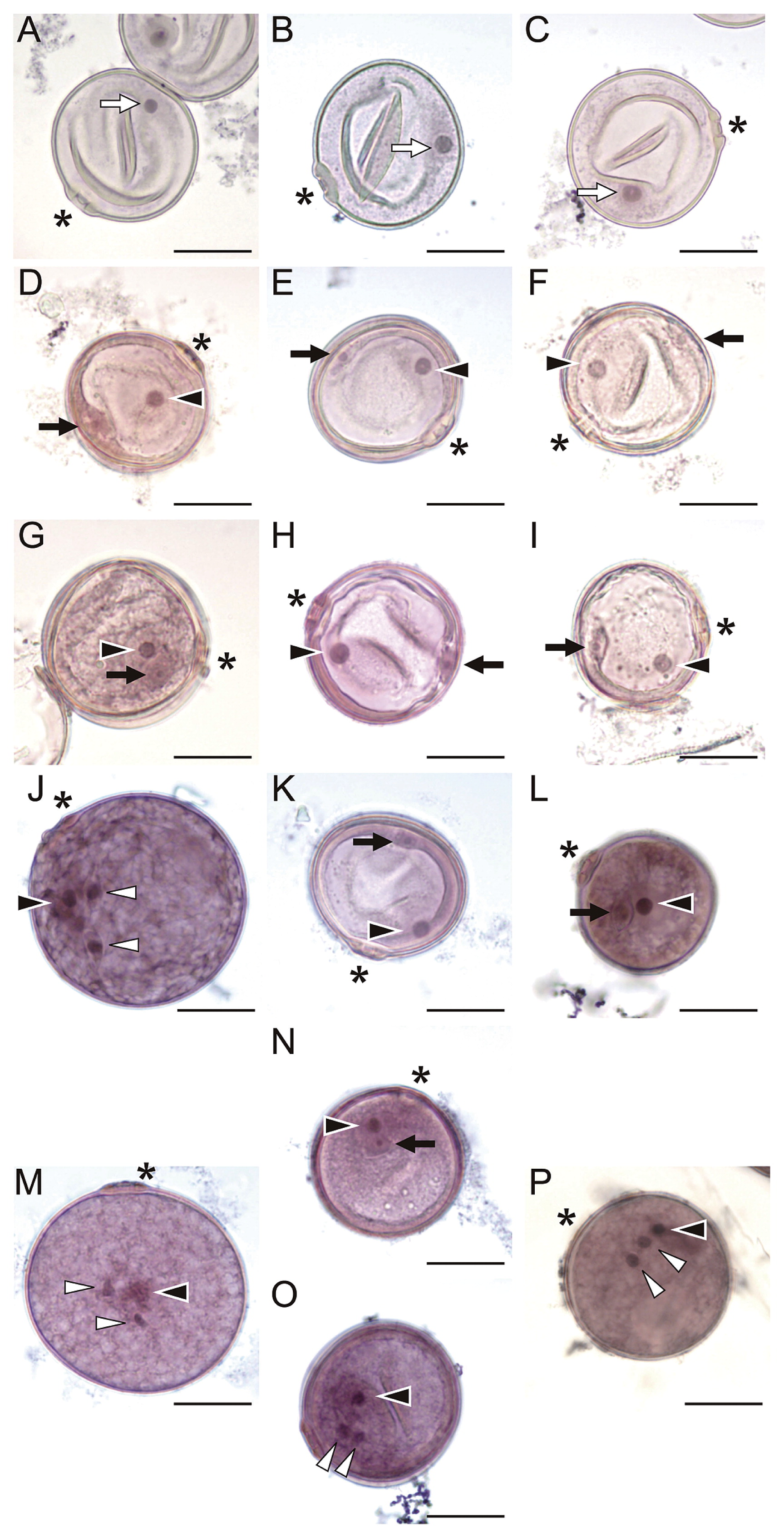
Observation of post-meiotic pollen development in T65, S22A_SS, and S22B_SS by hematoxylin staining. Pollen grains at the unicellular (A–C), early bicellular (D–F), late bicellular (G–I), tricellular (J–L), and mature stages (M–P). Stages were categorized based on development of normal pollen grains in T65. (A, D, G, J, M) Pollen of T65. (B, E, H, K, N, O) Abnormal pollen of S22A_SS. (C, F, I, L, P) Abnormal pollen of S22B_SS. White arrows, black arrows, black arrowheads, and white arrowheads indicate nuclei of unicellular pollen grains, generative cells, nuclei of vegetative cells, and sperm cells, respectively. Scale bars = 20 μm. Asterisks indicate pollen pore.

Frequencies of pollen grains at each development stage. (A, D, G, J, M) Frequency of pollen grain types T65. (B, E, H, K, N) Frequency of pollen grain types in S22A_SS. (C, F, I, L, O) Frequency of pollen grain types in S22B_SS. (A–C) Unicellular stage. (D–F) Early bicellular stage. (G–I) Late bicellular stage. (J–L) Tricellular stage. (M–O) Mature stage. Error bars indicate standard error (n ≥ 200). The frequency of pollen grains at each stage was calculated as the average of three replicates of florets except the average of two replicates for early bicellular stage of the S22B_SS plant (Fig. 6F).
At the early bicellular stage, starch had not accumulated in the pollen grains of T65 (Fig. 4D). The unicellular cell divided into vegetative and generative cells: the nucleus of the vegetative cell had migrated toward the pollen pore, and the generative cell remained at the side opposite the pollen pore (Fig. 5D). The frequency of grains at the early bicellular stage was nearly 100% in each of the three plant types (Figs. 4D–4F, 5D–5F, 6D–6F).
At the late bicellular stage, the pollen grains of T65 had started to accumulate starch (Fig. 4G) and the generative cell had migrated toward the inside relative to the vegetative cells (Fig. 5G). The frequency of pollen grains in the late bicellular stage in T65 was 98.7% (Fig. 6G). A difference between normal and abnormal pollen grains began to become apparent in S22A_SS and S22B_SS (Figs. 4H, 4I, 5H, 5I). Almost half the pollen grains of S22A_SS and S22B_SS had initiated starch accumulation and resembled those of T65 (Fig. 4H, 4I). However, the other half were not stained by I2–KI, and each of those pollen grains had a generative cell that remained at the side opposite the pollen pore (resembling the early bicellular stage) (Fig. 5H, 5I). The frequency of the early bicellular-like stage was 51.2% in S22A_SS and 49.4% in S22B_SS (Fig. 6H, 6I). No morphological differences in the bicellular-like pollen grains were apparent between S22A_SS and S22B_SS with either I2–KI or hematoxylin staining.
At the tricellular stage, the pollen grains of T65 showed more starch accumulation (Fig. 4J), and the generative cell had divided into two sperm cells (Fig. 5J). Both the sperm cells and the vegetative nucleus were rounded. The frequency of pollen grains at the tricellular stage was 98.7% in T65 (Fig. 6J). In S22A_SS, half of the pollen (46.4%) was at the tricellular stage, similar to T65, whereas 45.7% and 7.9% of grains were at early and late bicellular-like stages, respectively (i.e., when starch accumulation starts and the generative cell migrates to the inside relative to the vegetative cells) (Figs. 4K, 5K, 6K). In S22B_SS, the abnormal pollen grains were at either an early (11.3% of total grains) or late bicellular-like stage (40.6%) (Figs. 4L, 5L, 6L). The majority of immature pollen grains were at the early bicellular-like stage in S22A_SS and the late bicellular-like stage in S22B_SS at the time when normal pollen had reached the tricellular stage.
At the mature stage, each pollen grain of T65 showed full accumulation of starch and a mature male germ unit with two slender sperm cells and an obscured vegetative nucleus connected by cytoplasm (Figs. 4M, 5M). The abnormal pollen grains of S22A_SS were incompletely stained (Fig. 4N) and were at the early bicellular-like stage (3.8%), late bicellular-like stage (17.9%), or tricellular-like stage (i.e., pollen grains had a rounded vegetative cell and two sperm cells; 22.1%) (Figs. 5N, 5O, 6N). The abnormal pollen grains in S22B_SS were small and were well-stained by I2–KI (Figs. 4O, 5P). S22B_SS showed pollen grains at mature stage (58.6%), late bicellular-like stage (6.4%), and tricellular-like stage (34.7%) (Fig. 6O). Some small pollen grains of S22B_SS had well-established male germ units (Fig. 5P) and were counted as mature in our observation. Therefore, even though the pollen fertility of S22B_SS as evaluated by I2–KI was 50.6%, the frequency of mature pollen grains as assessed by hematoxylin staining was considered to exceed 50%. This result, along with the observed pollen transmission of the S22B-glums allele and the in vitro germination of small pollen grains presumed to carry S22B-glums, suggested that small pollen grains carrying a mature male germ unit were fertile (Fig. 3).
To summarize these observations, the abnormal pollen development in S22A_SS was first apparent at the late bicellular stage; these abnormal pollen grains could reach the late bicellular and tricellular stages. In S22B_SS, aberrant pollen also started to become apparent at the late bicellular stage, and most of these pollen gains could reach the tricellular stage.
Effect of two lethal factors linked in repulsion phaseSince the sterile alleles S22A-glums and S22B-glums are linked in coupling phase in the IRGC105668 parent, the S22A and S22B loci were thought to be a single Mendelian locus, S22(t), in the initial mapping experiments, which were performed with a relatively small population (n = 153) (Fig. 1). However, it was unknown how TRD pattern change in repulsion phase linkage of the two sterile alleles. Therefore, we investigated the pollen fertility of F1 plants heterozygous at both S22A and S22B with repulsion-phase linkage of S22A-glums and S22B-glums (S22A + S22B_RP plants) (Fig. 7A), which were obtained from a cross between a S22A-glums heterozygous plant derived from self-pollination of the RC lines as the female parent and S22B-glums homozygous plants derived from self-pollination of the RD lines as the male parent (Fig. 1B). The S22A + S22B_RP plants showed high pollen sterility, with only 2.5% ± 0.8% of the pollen grains similar to those of T65 (n = 5, Fig. 7B).

Behavior of two linked F1 pollen sterility loci, S22A and S22B, in repulsion phase. (A) Diagram of zygote carrying pollen sterility loci in repulsion phase, diagram of possible gamete types, expected segregation ratio, fertility of male gametes, and competitive fertilization ratio of gametes produced from S27A + S27B_RP. (B) High pollen sterility in S27A + S27B_RP. A morphologically normal pollen grain is indicated by a white arrowhead.
To explain the genetic basis of this high pollen sterility, we assumed that pollen grains with chromosomes carrying S22A-T65+ and S22B-glums alleles (T+Gs gametes), S22A-T65+ and S22B-T65+ alleles (T+T+ gametes), S22A-glums and S22B-glums alleles (GsGs gametes), and S22A-glums and S22B-T65+ alleles (GsT+ gametes) would be sterile, fertile, sterile, and sterile, respectively, with segregation in a ratio of (1 – r)/2 : r/2 : r/2 : (1 – r)/2, where r represents the recombination value between S22A and S22B (Fig. 7A). Because the frequency of normal size pollen grains (assumed to carry the T+T+ gametes) is r/2, we estimated r/2 as 2.5%, giving a value for r of 5.0%. Indeed, the map distance between the two SSR markers RM7451 and RM7033, which were completely and tightly linked to S22A and S22B, respectively, was 5.4 cM (r = 5.37%) after conversion using the Kosambi’s mapping function (Kosambi 1944) on the rice standard linkage map (http://rgp.dna.affrc.go.jp/publicdata/geneticmap2000/index.html) (Fig. 2C, 2F). This frequency of morphologically fertile pollen appears to correspond to the frequency of segregation of the male T+T+ gametes. This result suggests that normal size pollen grains observed in the S22A + S22B_RP plants were originated from recombination on repulsion-phase linkage of the fertile alleles, S22A-T65+ and S22B-T65+. To confirm the male transmission of the T+T+ gametes to the progeny, we investigated the segregation of genotypes at RM7451 and RM7033 in an F2 population derived from self-pollination of the S22A + S22B_RP plants (Table 4). The IRGC105668-homozygous class at RM7451 was not observed in this F2 population, indicating that the GsT+ and GsGs gametes were not transmitted to the progeny through male gametes, as shown in initial mapping data (Fig. 2B). Meanwhile the male transmission of the T+Gs and T+T+ gametes were observed in the linkage analysis and the in vitro pollen germination test for S22B (Figs. 2E, 3C). Therefore, we described the male transmission ratio of the T+Gs gametes to the T+T+ gametes as (1 – t) : t (Fig. 7A). Frequencies of segregation in female gametes carrying T+Gs, T+T+, GsGs, and GsT+ gametes are expected as (1 – r)/2, r/2, r/2, and (1 – r)/2, respectively. Therefore, theoretical frequencies of zygotic genotypes segregated in the F2 were expected as shown in Table 4. When we assumed r = 0.05, segregation of female and male gametes carrying the T+Gs, T+T+, GsGs, and GsT+ genotypes were expected as 47.5%, 2.5%, 2.5%, and 47.5%. Maximum likelihood estimation (ML) under the condition of r = 0.05 revealed that segregation ratio of and male transmission of the T+Gs, T+T+, GsGs, and GsT+ gametes were 30.5%, 69.5%, 0%, and 0% (t = 0.695), respectively. The observed segregation ratio fit to the estimated ratio by t = 0.695 and r = 0.05 in chi-square test (χ2 = 4.13, df = 5, P = 0.53, Table 4). Interestingly, on the self-pollinated progeny of the S22B_SS plants, we observed segregation of T65-homozygotes, heterozygotes, and IRGC105668-homozygotes genotypes in a 45 : 49 : 8 ratio at RM7033 (Fig. 2E) and transmission frequencies of T+Gs, T+T+, GsGs, and GsT+ gametes are estimated by ML as 15.1%, 84.9%, 0%, and 0%, respectively. In the S22B_SS plants, theoretical segregation ratio of pollen grains carrying T+Gs, T+T+, GsGs, and GsT+ gametes are 50%, 50%, 0%, and 0%, respectively. This data demonstrated that male transmission ratio of the T+Gs gametes changed depending on frequency of competitive normal pollen grains carrying T+T+ genotypes. These experiments suggested that repulsion phase of two sterile alleles tightly linked to each other enforces pollen sterility as a function of recombination value between the two loci and that transmission ratio of leaky allele is influenced by surrounding competitive pollen for fertilization.
| Genotypea | Segregation | ||||
|---|---|---|---|---|---|
| RM7451 (S22A) | RM7033 (S22B) | Theoretical expectationb | Observation | Maximum likelihood estimationc (t = 0.695, r = 0.05) |
χ2 |
| TT | TT | tr/2 | 0 | 2.5 | 2.47 |
| TG | (1 – r)t/2 + r(1 – t)/2 | 50 | 48 | 0.09 | |
| GG | (1 – r)(1 – t)/2 | 19 | 20.6 | 0.12 | |
| TG | TT | (1 – r)t/2 | 47 | 46.9 | 0 |
| TG | rt/2 + (1 – r)(1 – t)/2 | 26 | 23 | 0.38 | |
| GG r(1 – t)/2 | 0 | 1.1 | 1.08 | ||
| GG | TT | – | 0 | – | – |
| TG | – | 0 | – | – | |
| GG | – | 0 | – | – | |
| Total | 1 | 142 | 142 | 4.14 | |
| P | 0.53 | ||||
F1 pollen sterility is a complex trait controlled by multiple loci in rice. In unveiling the genetic mechanism of F1 pollen sterility and its contribution to divergence of AA-genome species, the locations and characteristics of hybrid sterility genes are important and fundamental issues. The dissection analysis of S22(t) suggested two following possibilities of the location using ten genotypes of recombinants. One possibility was that a single S22(t) locus was located near RM12353. The other was that the S22(t) locus actually consisted of two loci, S22A (predicted to be between RM12317 and RM12353) and S22B (predicted to be between RM12353 and RM279). Linkage analysis using two types of BC4F6 populations segregating for genomic regions between these two sets of markers indicated that S22(t) was in fact composed of two loci, S22A and S22B. No epistatic interaction was detected between the two loci because the IRGC105668 chromosomal segment at S22B or S22A was not required for induction of pollen sterility by S22A or S22B, respectively. S22A-glums and S22B-glums were the designations given to the alleles that induced sterility of pollen grains produced on heterozygous plants.
The tight coupling-phase linkage of S22A-glums and S22B-glums on the IRGC105668 chromosome led to our earlier conclusion that S22(t) was a single Mendelian locus for F1 pollen sterility, because the two sterility alleles have similar effects on the segregation of gametes. Hu et al. (2006) reported the presence of S29 in hybrids between O. sativa and O. glaberrima at a position between RM7033 and RM7562 on chromosome 2; thus, S29 and S22(t) might represent the same locus. Our analysis showed that the genomic region of S22B corresponded to that reported for S29. Identification of S22A in O. glaberrima or O. barthii has not been reported. Oryza glumaepatula, which originated in the Amazon basin, is closely related to the O. glaberrima/O. barthii complex according to phylogenetic analysis of AA-genome species using RFLP analysis (Doi et al. 2000). This result suggests that S22A and S22B might also be present in O. glaberrima and O. glumaepatula.
Cytological characterization of sterile pollen grains caused by S22A and S22BDifferent effects of genes inducing abnormal pollen development were shown in S22A and S22B by in vitro pollen germination and morphological evaluation (Figs. 3, 4, 5, 6). Although abnormal pollen development initiated from the late bicellular stage for S22A and S22B, S22A makes abnormal pollen grains retained at bicellular-like or tricellular-like stages at anthesis, but abnormal pollen grains of S22B reached the tricellular-like stages at anthesis and part of those showed germination ability in vitro. These observations provided a cytological basis for non-transmission of S22A-glums and leaky transmission of S22B-glums alleles in this genetic analysis.
Effect of repulsion-phase linkage of S22A-glums and S22-BglumsGene positions within the genome play important roles in reproductive isolation even without functional changes in genes or proteins, as is widely known in Bateson– Dobzhansky–Muller-type incompatibility caused by gene transpositions or by duplication and reciprocal loss of genes located on different chromosomes (Bikard et al. 2009, Masly et al. 2006, Mizuta et al. 2010, Yamagata et al. 2010). Here, we showed positional effects of cis- and trans-linkage relationships of lethal factors of the gametophytic F1 pollen sterility loci S22A-glums and S22B-glums, which alter the degree of pollen sterility predicted by Mendelian genetics. Williams et al. (2001) suggested that zygotic lethal factors linked to each other in repulsion phase can lead to associative overdominance, which maintains the haplotype frequency of heterozygous repulsion genotypes at a high frequency within subpopulations in a conifer. TRD appears to be controlled by a number of zygotic and gametophytic segregation distorters affecting both male and female gametes, and has been analyzed in several F2 populations produced by subspecific hybridization in rice (Harushima et al. 2001). We showed that repulsion-phase linkage of gametophytic lethal factors within a small part of chromosome 2 induces a strong reproductive barrier, and fertile haplotypes are selected owing to the elimination of gametophytic lethal factors through pollen sterility.
Because it is possible that the haplotype distributions of the present accessions are a result of selection involving lethal factors distributed in the genome, identification of the origin of lethal alleles and comparative analysis of present and ancestral types should help to elucidate the intermediate evolutionary pathways of haplotypes leading to the present genome structures. For better understanding of AA-genome divergence in African rice species, investigation of the distributions of accessions containing naturally occurring T+Gs and GsT+ gametes is required to characterize the potential gene pool that may have led to the establishment of reproductive isolation within African species by repulsion-phase linkage. Identification and characterization of S22A and S22B provides basic information from which we can elucidate the origin of sterile alleles and haplotype evolution within species or subspecies in O. glumaepatula and in African Oryza species.
This work was supported by grants from the Ministry of Agriculture, Forestry and Fisheries of Japan (Genomics for Agricultural Innovation, QTL-5002) and from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grant-in-Aid for Scientific Research (A) Grant Number 24248002 and National Bioresource Project (Rice)). This research was partly supported by JST/JICA, Science and Technology Research Partnership for Sustainable Development (SATREPS).