2015 Volume 65 Issue 5 Pages 420-429
2015 Volume 65 Issue 5 Pages 420-429
The rice cultivar ASD7 (Oryza sativa L. ssp. indica) is resistant to the brown planthopper (BPH; Nilaparvata lugens Stål) and the green leafhopper (Nephotettix virescens Distant). Here, we analyzed multiple genetic resistance to BPH and the green rice leafhopper (GRH; Nephotettix cincticeps Uhler). Using two independent F2 populations derived from a cross between ASD7 and Taichung 65 (Oryza sativa ssp. japonica), we detected two QTLs (qBPH6 and qBPH12) for resistance to BPH and one QTL (qGRH5) for resistance to GRH. Linkage analysis in BC2F3 populations revealed that qBPH12 controlled resistance to BPH and co-segregated with SSR markers RM28466 and RM7376 in plants homozygous for the ASD7 allele at qBPH6. Plants homozygous for the ASD7 alleles at both QTLs showed a much faster antibiosis response to BPH than plants homozygous at only one of these QTLs. It revealed that epistatic interaction between qBPH6 and qBPH12 is the basis of resistance to BPH in ASD7. In addition, qGRH5 controlled resistance to GRH and co-segregated with SSR markers RM6082 and RM3381. qGRH5 is identical to GRH1. Thus, we clarified the genetic basis of multiple resistance of ASD7 to BPH and GRH.
Herbivorous insects are one of the factors limiting rice production worldwide. In Asian rice fields, five species of sucking insects are common (Pathak and Khan 1994): the brown planthopper (BPH; Nilaparvata lugens Stål), the small brown planthopper (Laodelphax striatellus Fallén), the green rice leafhopper (GRH; Nephotettix cincticeps Uhler), the green leafhopper (GLH; Nephotettix virescens Distant), and the whitebacked planthopper (Sogatella furcifera Horváth). These species cause yield losses by feeding on rice plants and transmitting viral diseases (Abo and Sy 1997, Pathak and Khan 1994, Sogawa and Cheng 1979). Host plant resistance reduces crop losses caused by insects and viruses. Rice cultivars with resistance to insects and diseases show greater yield stability (Khush 1989).
BPH causes significant yield losses in susceptible rice cultivars in Asia (Sogawa et al. 2003). To date, more than 27 BPH resistance loci have been identified in rice, which are located on chromosomes 2–4, 6, and 9–12 (Fujita et al. 2013). Three of them have been cloned (Du et al. 2009, Ji et al. 2013, Liu et al. 2015, Tamura et al. 2014). Four clusters of loci are located on the short and long arms of chromosome 4, the short arm of chromosome 6, and the long arm of chromosome 12 (Fujita et al. 2013). In 1973, IR26, which carries the BPH1 gene, was the first elite cultivar released with BPH resistance. However, from 1976, the resistance conferred by BPH1 was overcome by a dominant BPH biotype (Khush 1989). Although three elite cultivars (IR32, IR36, and IR42) carrying the BPH2 resistance gene were subsequently released, they also eventually lost resistance in Southeast Asia. BPH collected in Japan is virulent to plants carrying BPH1 and BPH2 (Tanaka and Matsumura 2000). BPH does not overwinter in East Asian paddy fields, because of low temperatures, and migrates annually from northern Vietnam to East Asian countries during the rice cropping season (Otuka 2009, 2013).
GRH causes damage to susceptible rice plants in Japan (Nakasuji and Nomura 1968, Nirei and Nakazato 1975). So far, eight loci for GRH resistance derived from rice landraces and wild relatives of rice have been mapped (Fujita et al. 2013). Near-isogenic lines (NILs) and pyramided lines (PYLs) in the genetic background of the rice cultivar Taichung 65 (T65; Oryza sativa L. ssp. japonica) have been developed for all GRH resistance loci except GRH3 and qGRH9 (Fujita et al. 2010a). GRH1, GRH2, and GRH3 are no longer effective against laboratory GRH biotypes 1, 2, and 3, respectively (Hirae et al. 2007).
The rice cultivar ASD7 (O. sativa L. ssp. indica) has multiple resistance to BPH, GLH (Athwal et al. 1971), and GRH (Fujita et al. 2002). The objectives of this study were (1) to understand the genetic basis of the resistance to BPH, (2) to understand the genetic basis of the resistance to GRH, and (3) to develop NILs carrying each BPH and GRH resistance gene in the same genetic background for future breeding of insect-resistant rice cultivars.
F1 hybrid seeds were obtained from a cross between resistant ASD7 and susceptible T65 (Fig. 1). The F1 plants were self-pollinated to produce two independent F2 populations, which were used for detection of quantitative trait loci (QTLs) conferring resistance to BPH (Fig. 1A) and GRH (Fig. 1B).

Breeding scheme for mapping genes conferring resistance to BPH (A) and GRH (B) derived from the rice cultivar ASD7. The numbers of plants selected for backcrossing or self-pollination and the total plant numbers are indicated in parentheses. MAS, marker-assisted selection; pop., population.
To confirm putative QTLs, the F1 plants were backcrossed to T65 to generate BC1F1 plants, which were backcrossed to T65 to develop BC2F1 plants. Among 49 BC2F1 plants, 10 carrying putative QTLs were selected through marker-assisted selection (MAS) and self-pollinated. Among 156 BC2F2 plants, 7 heterozygous at putative qBPH12 and 20 heterozygous at putative qBPH6 were selected. Finally, four BC2F2 plants were chosen: 105-15, homozygous for the ASD7 allele at qBPH6 and heterozygous at qBPH12; 106-3, homozygous for the T65 allele at qBPH6 and heterozygous at qBPH12; 105-9, heterozygous at qBPH6 and homozygous for the ASD7 allele at qBPH12; and 104-45, heterozygous at qBPH6 and homozygous for the T65 allele at qBPH12. Consequently, four BC2F3 populations derived from the four BC2F2 plants were analyzed.
Preliminary near-isogenic (pre-NIL) and pyramided (pre-PYL) lines were selected from BC2F3 populations derived from self-pollinated progeny of BC2F2 plants. The pre-NIL for qBPH6 was homozygous for the ASD7 allele at qBPH6 and homozygous for the T65 allele at qBPH12; the pre-NIL for qBPH12 was homozygous for the ASD7 allele at qBPH12 and homozygous for the T65 allele at qBPH6; and the pre-PYL was homozygous for the ASD7 alleles at both qBPH6 and qBPH12.
To confirm qGRH5, mapping populations carrying qGRH5 were developed through advanced backcrossing and MAS. Among 156 BC2F2 plants, 3 plants heterozygous in the qGRH5 region were selected. Three plants heterozygous at putative qGRH5 were selected to produce the BC2F3 generation. Three BC2F3 populations derived from the three BC2F2 plants were used. A pre-NIL for qGRH5 was selected in BC2F3 populations derived from the self-pollinated progeny of the BC2F2 plants.
Insect strainsTwo BPH strains were collected in Kanagawa Prefecture in 1966 (designated “1966 BPH”) and in Chikugo in 1989 (designated “1989 BPH”). The BPH strains were maintained by continuous rearing on susceptible cultivar Reiho at 25°C with 16 h light and 8 h dark. The GRH population was collected in Fukuoka Prefecture in 1991 and maintained by continuous rearing on seedlings of the susceptible japonica cultivar Nipponbare. The GRH strain was kept under the same condition.
Evaluation of BPH resistanceStrain 1989 BPH was used for F2 analysis to overcome the resistance controlled by BPH1 (Myint et al. 2009a). Strain 1966 BPH was used for BC2F3 analysis to confirm potential QTLs, because it is avirulent to any of BPH resistance genes (Myint et al. 2009b). An antibiosis test for BPH resistance was conducted according to Myint et al. (2009a) with minor modifications. Each F2 seedling was infested with 10 second-instar 1989 BPH nymphs. Nymph mortality was calculated 5 days after infestation (DAI). We performed the antibiosis test in BC2F3 plants homozygous for T65 alleles at both QTLs: both pre-NILs and the pre-PYL. Five brachypterous 1966 BPH females collected within 24 h after emergence were released onto a single plant 1 month after sowing. Antibiosis was then scored at 2, 3, and 5 DAI. Plants with adult BPH mortality of <40% were categorized as susceptible; plants with BPH mortality of ≥60% were categorized as resistant for mapping of the candidate QTLs. Females with a swollen abdomen were scored at 3 DAI and 5 DAI.
Evaluation of GRH resistanceAn antibiosis test for GRH resistance was conducted according to Kishino and Ando (1978) with modifications. A week after sowing, 98 F2 seedlings, 277 BC2F3 seedlings, and the pre-NIL carrying qGRH5 were infested with 10 first-instar nymphs in test tubes. Nymph mortality was calculated at 3 DAI. For linkage analysis, plants with a nymph mortality of <30% were categorized as susceptible and those with a mortality of ≥40% were categorized as resistant.
Genotyping using SSR markersTotal DNA of each F2 and BC2F3 plant was extracted from freeze-dried leaves by the potassium acetate method (Dellaporta et al. 1983). The genotypes of SSR loci were determined by PCR amplification in a PCR System-9700 (PerkinElmer, Waltham, MA, USA). The PCR reaction mixture (15 μl) contained 50 mM KCl, 10 mM Tris·HCl (pH 9.0), 1.5 mM MgCl2, 200 μM dNTP of each, 0.2 μM primer, 1 unit of Taq polymerase (Takara, Shiga, Japan), and 25 ng of genomic DNA. The thermal cycler was programmed as follows: 5 min at 95°C; then 40 cycles of 95°C for 30 s, 53°C for 30 s, and 72°C for 30 s. The PCR products were separated in 4.5% agarose gels by electrophoresis at 250 V for 1 h in 0.5× TBE buffer. Gels were stained with ethidium bromide and photographed under ultraviolet light.
Construction of linkage maps and QTL analysisGenetic linkage maps of the F2 populations were constructed for BPH resistance (93 plants and 132 SSR markers) (Supplemental Fig. 1) and for GRH resistance (98 plants and 92 SSR markers) (Supplemental Fig. 2); the markers were distributed across all 12 rice chromosomes (International Rice Genome Sequencing Project 2005, McCouch et al. 2005, Temnykh et al. 2001). Mapmaker/Exp 3.0 (Lander et al. 1987) was used to determine the linkage loci and map distances. The phenotypic and genotypic data of F2 plants were used for interval mapping with R/qtl software (Broman and Sen 2009). The critical threshold values of the logarithm of odds (LOD) scores of 3.5 for QTL detection were calculated by conducting 1000 permutation tests at an experiment-wise significance level of 0.05.
Validation of QTLs conferring resistance to BPHTo validate qBPH12 and qBPH6, four BC2F3 populations were analyzed for resistance to strain 1966 BPH: population 1 derived from BC2F2 plant 105-15 (Fig. 3A); population 2 derived from BC2F2 plant 106-3 (Fig. 3B); population 3 derived from BC2F2 plant 105-9 (Fig. 3C); and population 4 derived from BC2F2 plant 104-45 (Fig. 3D). The population 1 and five SSR markers RM3326, RM28466, RM7376, RM28597, and RM6396 on chromosome 12 were used for linkage analysis of qBPH12. Two SSR markers RM8120 and RM8101 on chromosome 6 were used for MAS for qBPH6.
Validation of the QTL conferring resistance to GRHThree BC2F3 populations were used for linkage analysis of qGRH5 and SSR markers. Further detailed linkage analysis was conducted using SSR markers RM6082, RM3381, RM249, RM3437, and RM6024 on chromosome 5.
Seedlings of the parental cultivar ASD7 were highly resistant to strain 1989 BPH (85.0% nymph mortality), whereas those of the other parent, T65, were susceptible (4.4% nymph mortality) (Fig. 2). Nymph mortality on ASD7 × T65 F1 plants was 8.7%. Nymph mortality on 93 F2 plants showed a continuous distribution.
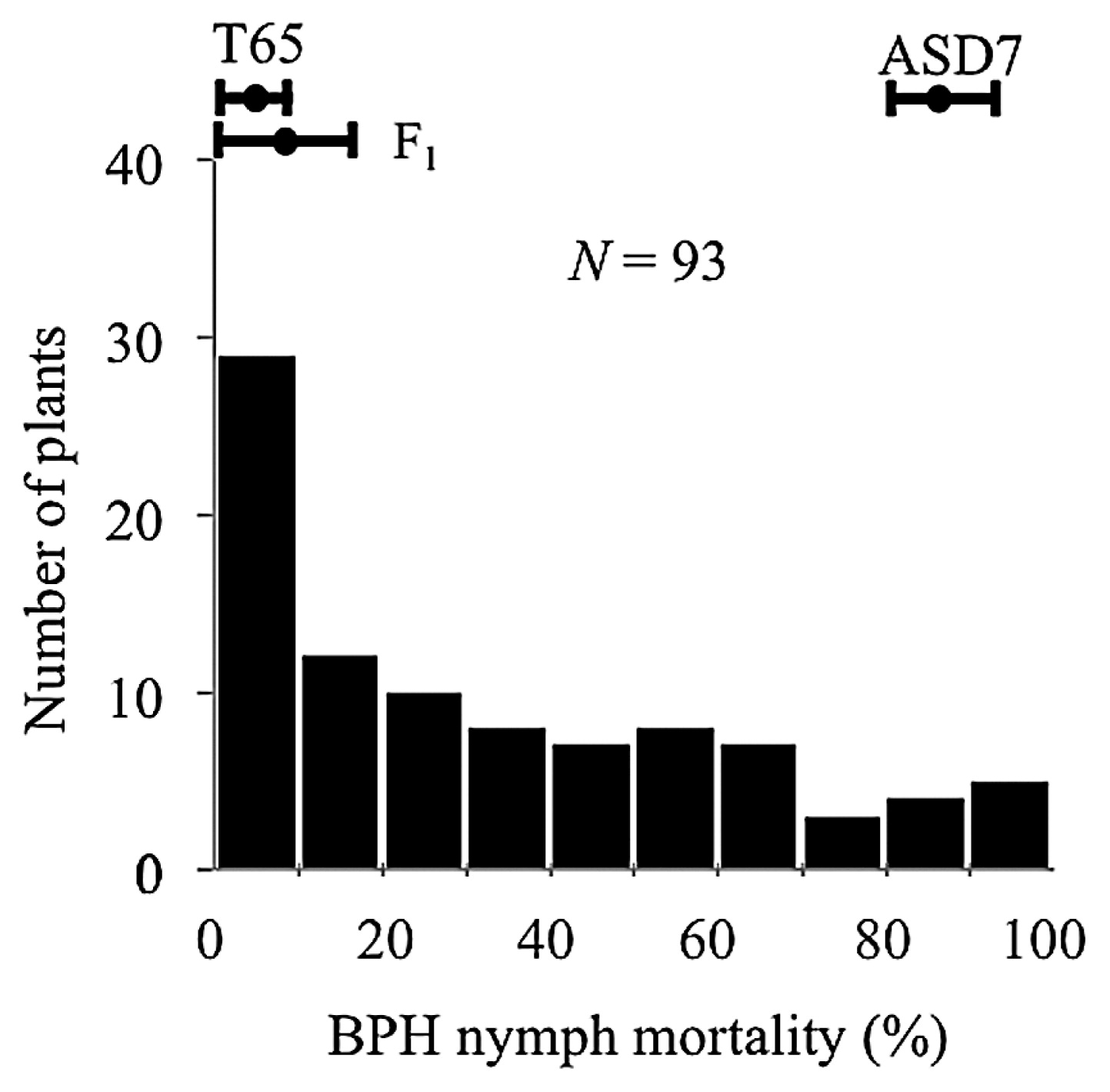
Frequency distribution of BPH nymph mortality on seedlings of an F2 population derived from a cross between ASD7 and Taichung 65. The nymph mortality of resistant cultivar ASD7 was 85.0%, whereas the nymph mortality of susceptible cultivar Taichung 65 was 2.2%. Error bars indicate standard error.
Two QTLs were detected for resistance to BPH that were designated qBPH6 and qBPH12 (Table 1). Interval mapping identified a LOD score peak of 5.3 (P = 0.003) for qBPH12 on the long arm of chromosome 12 between SSR markers RM3326 and S20103 (Table 1). qBPH12 explained 28.8% of the phenotypic variation. A LOD score peak of 3.6 (P = 0.039) for qBPH6 was identified on the distal short arm of chromosome 6 between SSR markers RM8120 and RM8200 (Table 1). qBPH6 explained 19.6% of the phenotypic variation.
| QTL | Chromosome | Marker interval | Position | Peak LODa | PEV (%)b | Additive effectc | Dominance effectd | D/Ae |
|---|---|---|---|---|---|---|---|---|
| qBPH6 | 6 | RM8120–RM8200 | 1.7 | 3.6 | 19.6 | −14.7 | 10.9 | −0.74 |
| qBPH12 | 12 | RM3326–S20103 | 92.3 | 5.3 | 28.8 | −22.1 | 5.3 | −0.24 |
Population 1 showed discrete segregation of adult mortality as well as females with a swollen abdomen at 3 DAI (Fig. 3E, 3I, Supplemental Figs. 4, 5), with 37 plants resulting in high mortality (≥60%) and 6 plants in low mortality (<40%); this distribution fit a 3:1 segregation ratio (χ2 = 2.8). We then conducted linkage analysis using five SSR markers near the qBPH12 locus. The 37 plants that were either homozygous or heterozygous for the ASD7 allele at SSR markers RM28466 and RM7376 gave a high level of adult BPH mortality (≥60%), whereas plants that were homozygous for the T65 allele at these markers have low mortality (<40%). qBPH12 co-segregated with SSR markers RM28466 and RM7376 and flanked by SSR markers RM3326 and RM28597 (Fig. 4). The genetic distance between markers RM3326 to qBPH12 was 11.0 cM, and that between qBPH12 and RM28597 was 5.6 cM (Fig. 4, Supplemental Table 1).
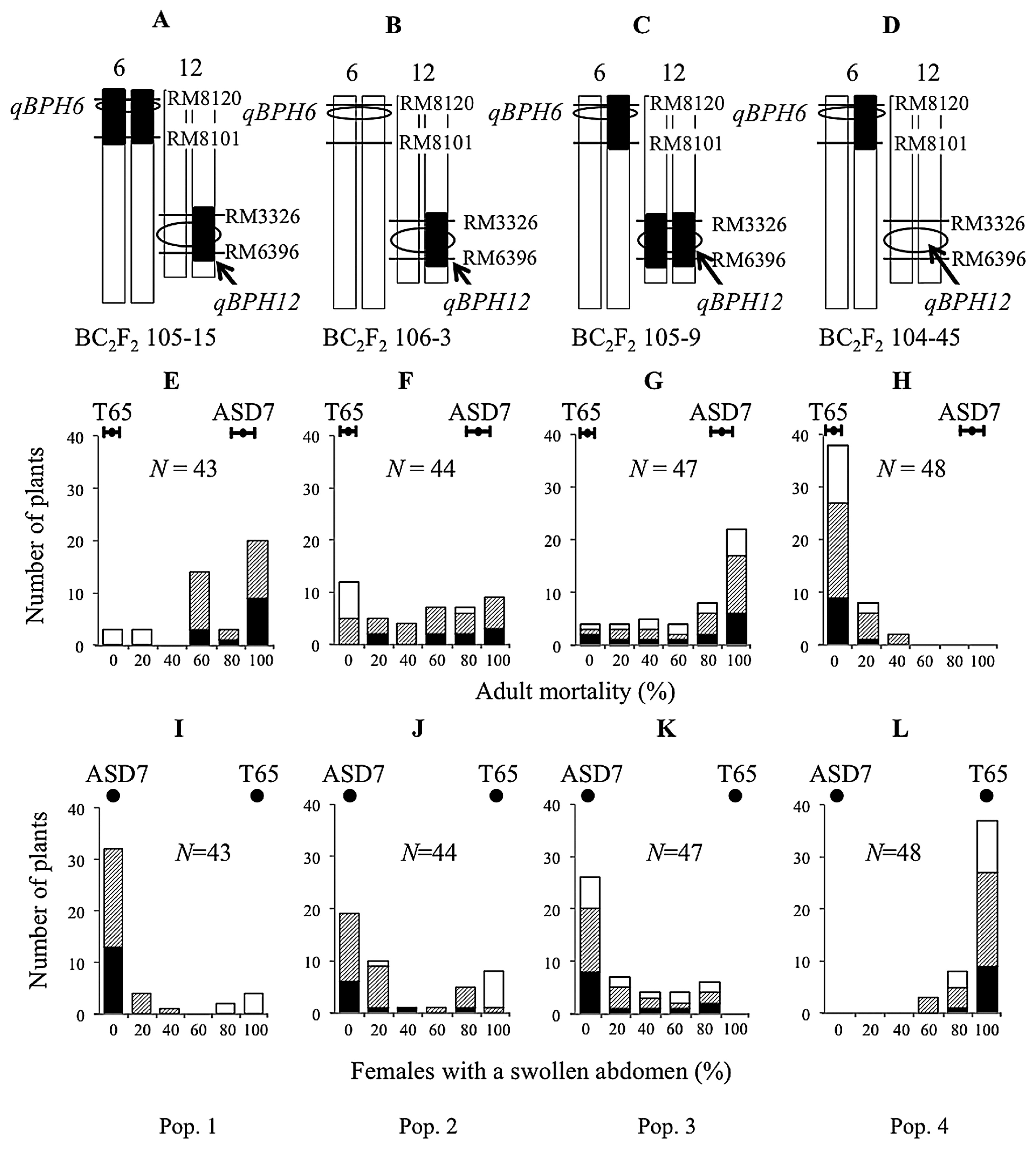
Graphical genotypes and frequency distributions of adult BPH mortality and females with a swollen abdomen on the four BC2F3 populations at 3 days after infestation. (A–D) Graphical genotypes of the four parental BC2F2 plants on chromosomes 6 and 12. Frequency distributions of adult mortality and females with a swollen abdomen are shown in (E) and (I) for population 1, (F) and (J) for population 2, (G) and (K) for population 3, and (H) and (L) for population 4. The adult mortality and females with a swollen abdomen were classified according to the genotypes of host plants at co-segregated SSR markers RM28466 and RM7376 for qBPH12 and the nearest SSR marker RM8120 for qBPH6. pop., population. White, hatched and black bars indicate T65-homozygous, heterozygous and ASD7-homozygous, respectively.

Linkage map indicating the position of qBPH12, a gene for resistance to BPH, on the long arm of chromosome 12. The upper high-density RFLP framework map was quoted from the Rice Genome Research Program (RGP, http://rgp.dna.affrc.go.jp/E/publicdata/geneticmap2000/index.html). Vertical bars in the expanded region represent SSR markers used for linkage analysis. Numbers between vertical bars indicate map distances (cM). N indicates number of individuals.
Discrete segregation of adult BPH mortality and females with a swollen abdomen were not observed in population 2 (Fig. 3F, 3J, Supplemental Figs. 4, 5). Interval mapping in this population identified a LOD score peak of 3.6 (P = 0.0005) for qBPH12 on the long arm of chromosome 12 between SSR markers RM3326 and RM28766 (Supplemental Table 2).
To confirm the genetic effects of qBPH6, two SSR markers RM8120 and RM8101 were used for MAS. Two BC2F3 populations—population 3, derived from the BC2F2 plant 105-9 (Fig. 3C, 3G), and population 4, derived from the BC2F2 plant 104-45 (Fig. 3D, 3H)—were analyzed for resistance to strain 1966 BPH. Population 3 did not show any discrete segregation of adult BPH mortality as well as females with a swollen abdomen at 3 DAI (Fig. 3G, 3K, Supplemental Figs. 4, 5). Association between BPH resistance level and SSR markers in the region of chromosome 6 was not significant. All plants in population 4 showed very low adult BPH mortality and high percentage of females with a swollen abdomen and were categorized as susceptible (Fig. 3H, 3L, Supplemental Figs. 4, 5).
Adult mortality of strain 1966 BPH in pre-NILs and a pre-PYLThe resistance of the pre-NILs and a pre-PYL to BPH is shown in Fig. 5A. Adult BPH mortality on a pre-NIL carrying the ASD7 allele of qBPH12 (40.0%) was significantly lower than that on a pre-PYL carrying the ASD7 alleles of both qBPH6 and qBPH12 (85.5%) and that on ASD7 (80.0%) at 2 DAI. However, mortality on the same pre-NIL (70.0%) and pre-PYL (90.9%) did not differ significantly from that on ASD7 (86.7%) at 3 DAI. Similarly, mortality on the pre-NIL (77.5%) and the pre-PYL (96.4%) did not differ significantly from that on ASD7 (100.0%) at 5 DAI. Adult mortality on the pre-NIL carrying the ASD7 allele of qBPH6 (13.0% at 2 DAI, 13.0% at 3 DAI, and 29.0% at 5 DAI) did not differ significantly from that on the pre-NIL homozygous for T65 alleles at both QTLs (0.0% at 2 DAI, 0.0% at 3 DAI, and 0.0% at 5 DAI) and that on the susceptible control T65 (4.4% at 2 DAI, 4.4% at 3 DAI, and 6.7% at 5 DAI). These data strongly suggest that qBPH6 contributes to a rapid antibiosis response to BPH in the presence of qBPH12. Females with a swollen abdomen on pre-NIL carrying ASD7 allele of qBPH6 were the same as T65 at 3 DAI and 5 DAI. Females with a swollen abdomen of pre-NIL carrying ASD7 allele of qBPH12 and pre-PYL carrying ASD7 alleles of qBPH6 and qBPH12 did not differ significantly from those on ASD7 at 3 DAI and 5 DAI (Fig. 5B).

Adult BPH mortality and females with a swollen abdomen on preliminary near-isogenic lines and a preliminary pyramided line. Plants were used at 1 month after sowing. “T” and “A” indicate homozygous for the T65 and ASD7 alleles at marker loci. Error bars indicate standard error. Means with the same lowercase letter do not differ significantly (P < 0.01, Tukey–Kramer test). DAI, days after infestation.
ASD7 had a high level of GRH resistance (89.0% nymph mortality), whereas T65 was susceptible (1.7% nymph mortality) (Fig. 6A). The F1 plants showed a high level (83.5%) of nymph mortality. Nymph mortality on 98 F2 plants showed a continuous distribution. Interval mapping identified a LOD score peak of 24.3 (P = 0.004) for qGRH5 on the short arm of chromosome 5 between SSR markers RM3322 and RM3437 (Table 2). qGRH5 explained 67.8% of the phenotypic variation.

Genetic basis of the resistance to green rice leafhopper in an indica cultivar ASD7. (A) Frequency distribution of GRH nymph mortality on seedlings of an F2 population derived from a cross between ASD7 and Taichung 65. (B) Frequency distribution of GRH nymph mortality on seedlings of the BC2F3 population, classified by the genotypes of host plants at co-segregated markers RM6082 and RM3381. White, hatched and black bars indicated T65 homozygous, heterozygous and ASD7 homozygous, respectively. (C) Linkage map indicating the position of qGRH5, a gene for resistance to GRH, on the short arm of chromosome 5. The upper high-density RFLP framework map was quoted from the Rice Genome Research Program (RGP, http://rgp.dna.affrc.go.jp/E/publicdata/geneticmap2000/index.html). Vertical bars in the expanded region represent SSR markers used for linkage analysis. Numbers between vertical bars indicate map distances.
| QTL | Chromosome | Marker interval | Position | Peak LODa | PEV (%)b | Additive effectc | Dominance effectd | D/Ae |
|---|---|---|---|---|---|---|---|---|
| qGRH5 | 5 | RM3322–RM3437 | 51.5 | 24.3 | 67.8 | −38.3 | 25.6 | −0.7 |
The three BC2F3 populations derived from these plants were combined and analyzed for GRH resistance using SSR markers in the qGRH5 region on chromosome 5. In total, 83 plants homozygous for T65 alleles at RM6082 and RM3381 were susceptible (nymph mortality of <30%), whereas 194 plants, which were either ASD7-homozygous or -heterozygous at RM6082 and RM3381, were resistant (nymph mortality of ≥40%) (Fig. 6B). This distribution fit a 3:1 segregation ratio (χ2 = 3.6), indicating that a single dominant gene controls GRH resistance in BC2F3 populations.
qGRH5 genotypes unconfirmed in the BC2F3 plants were confirmed using phenotypic evaluation of the BC2F4 generation. qGRH5 co-segregated with RM6082 and RM3381 (Fig. 6C); no recombination was found between RM6082, RM3381, and qGRH5. One recombination event was detected between RM249 and qGRH5. The genetic distances were 0.2 cM between qGRH5 and RM249, 5.3 cM between RM249 and RM3437, and 5.2 cM between RM3437 and RM6024 (Fig. 6C, Supplemental Table 3).
GRH nymph mortality in BC2F3 pre-NILsGRH nymph mortality on the pre-NILs carrying the ASD7 allele of qGRH5 (95.0%) did not differ significantly from that on ASD7 (87.6%) at 3 DAI (Supplemental Fig. 3). Low nymph mortality (2.2%) observed on plants homozygous for the T65 allele at qGRH5 did not differ significantly from that of the susceptible control T65 (1.7%). This demonstrates that qGRH5 is the only QTL that controls the resistance of ASD7 to GRH.
The BPH strains collected in Southeast Asia before 1976 are avirulent to Mudgo (carrying BPH1) or ASD7 (carrying BPH2) (Choi 1979). In Thailand in 1976, the damage score on ASD7 (0.6) was lower than that on IR32 carrying BPH2 (0.9) (Pongprasert and Weerapat 1979), suggesting the presence of other genetic factors responsible for BPH resistance in ASD7 in addition to BPH2.
In this study, two QTLs for resistance to BPH were detected and designated qBPH12, on chromosome 12, and qBPH6, on chromosome 6. qBPH12 was detected as a major QTL, whereas qBPH6 improved resistance to BPH in the presence of the ASD7 allele at qBPH12 (Fig. 3E, 3F). Epistatic interaction between qBPH6 and qBPH12 was observed based on adult mortality at 2 DAI (Fig. 5A). The adult BPH mortality of a pre-PYL homozygous for the ASD7 alleles at both qBPH6 and qBPH12 was significantly higher than that of a pre-NIL homozygous for the ASD7 allele at qBPH12 at 2 DAI. Similarly, epistatic interaction between qBPH6 and qBPH12 was slightly observed for adult mortality and females with a swollen abdomen at 3 DAI and 5 DAI (Fig. 5A, 5B). Responses to the various strains of BPH were definitely different on the pre-NIL for qBPH6 (Present study) and NIL for BPH25 (Myint et al. 2009a). The pre-NIL for qBPH6 was susceptible to all of the BPH strains (1966 BPH, 1989 BPH, and 1999 BPH) (data not shown), while the NIL for BPH25 was resistance to two of three BPH strains (1966 BPH and 1989 BPH), and was susceptible to the remaining 1999 BPH strain. Therefore, qBPH6 identified in this study was not allelic to BPH25.
One dominant QTL, qGRH5, conferring resistance to GRH was detected on chromosome 5 (Table 2). Based on the response to GRH and the location of qGRH5 and GRH1, these results indicate that qGRH5 is identical to GRH1 (Fujita et al. 2010a, Tamura et al. 1999, Yasui and Yoshimura 1999).
Resistance loci at the genomic region of qBPH6 and qBPH12qBPH6 was detected on the distal end of the short arm of chromosome 6, which overlaps BPH25 (Myint et al. 2012), BPH3, BPH4 and qBPH6(t) (Jairin et al. 2007a, 2007b, 2010) and BPH20(t) (Yang et al. 2012) (Fig. 7A). qBPH12 was located on the long arm of chromosome 12, where at least 7 BPH resistance loci have been reported (Fujita et al. 2013). Among them, BPH26, derived from cultivar ADR52 (O. sativa ssp. indica), and BPH18, derived from a wild relative of rice (Oryza australiensis), have been cloned and found to be alleles of the same gene, and flanked by SNP markers DS-72B4 (22.8 Mbp) and DS-173B (22.9 Mbp) (Ji et al. 2013, Tamura et al. 2014). Another resistance gene, BPH1, has been fine-mapped (Cha et al. 2008). As this genomic region contains only BPH26, this strongly suggests that BPH1 is allelic to BPH26. On the other hand, BPH21 must be different from BPH26 because it was fine-mapped to a different location and flanked by the markers B120 (24.1 Mbp) and B122 (24.3 Mbp) (Rahman et al. 2009). Among the remaining three BPH resistance genes, the mapped position of BPH10 (Ishii et al. 1994) and BPH16 (Hirabayashi et al. 2004) overlap with BPH26, but it is difficult to discriminate between these genes because of the low resolution of linkage analysis. BPH9 is located between markers G2140 (19.0 Mbp) and S2545 (21.5 Mbp) (Murata et al. 2000), which is different from the location of BPH26. In the present study, qBPH12 co-segregated with the SSR markers RM28466 (23.0 Mbp), RM7376 (23.5 Mbp) and was mapped between SSR markers RM3326 (21.8 Mbp) and RM28597 (24.7 Mbp) (Fig. 7B). In addition, the BPH26 sequences in ADR52 and ASD7 are identical (Tamura et al. 2014). These data suggested that qBPH12 is allelic to BPH26.
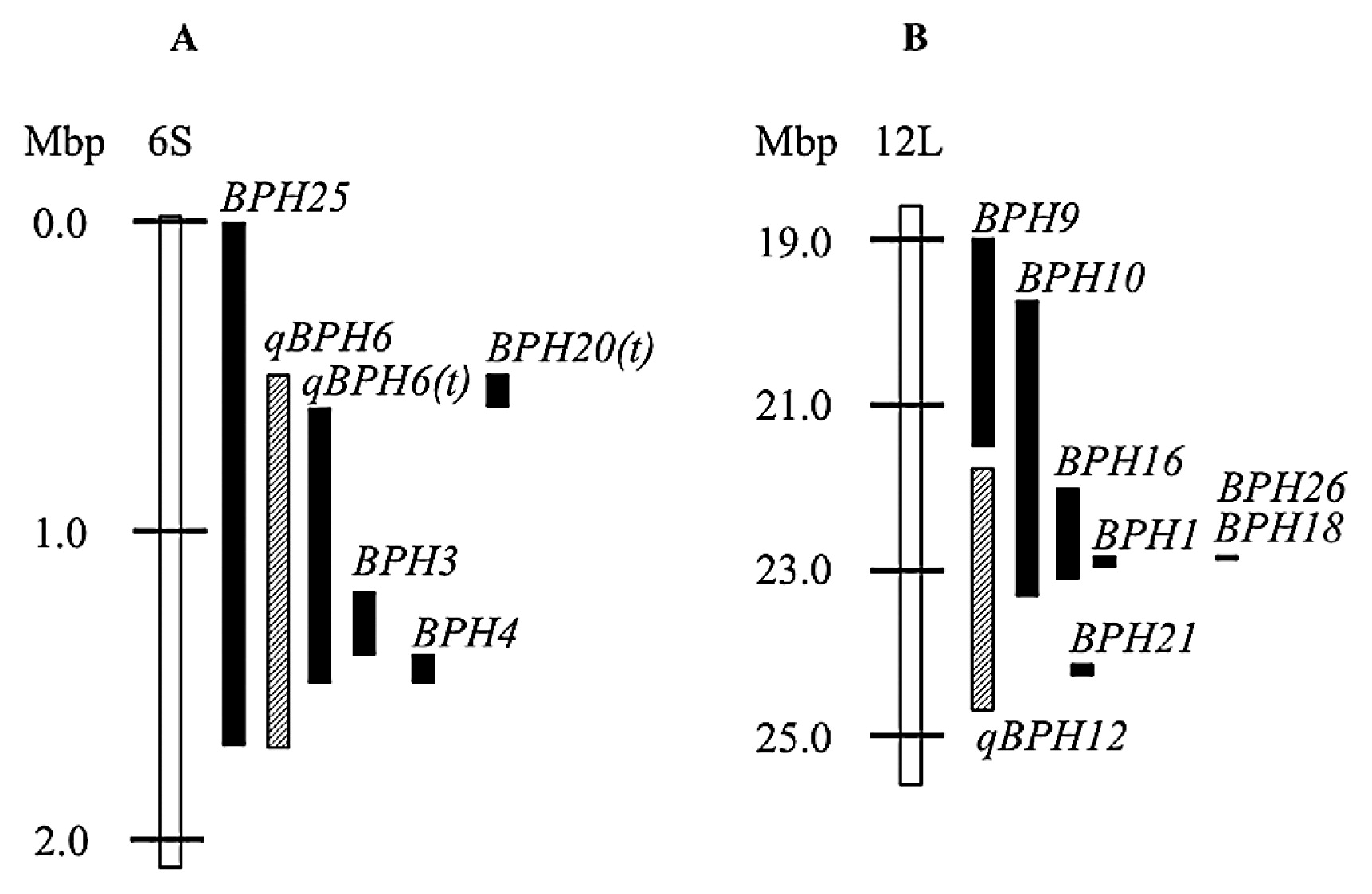
Locations of genes and quantitative trait locus for brown planthopper resistance on (A) the distal end of the short arm of chromosome 6 and (B) the long arm of chromosome 12. Location of genes and QTL is based on physical position of flanking markers. Hatched bars represented qBPH6 and qBPH12 in this study. Black bars represented genes/QTLs in the distal short arm of chromosome 6 and the long arm of chromosome 12 cited from Fujita et al. (2013).
NILs carrying genes that confer resistance to insects are very useful genetic resources for rice improvement. Using these NILs, we may be able to introduce these resistance genes into elite japonica cultivars by MAS and backcrossing without introducing the unfavorable traits of resistant donor cultivars such as strong photosensitivity or tall plant height. The genes related to unfavorable traits were removed during the NIL selection. In previous studies, PYLs showed significantly higher resistance to BPH or GRH than any of the NILs (Fujita et al. 2010a, Myint et al. 2009a, Qiu et al. 2012). These NILs can be used for developing pyramided lines free of any unfavorable traits. Combining different kinds of major resistance genes might contribute to the durability of host plant resistance to insect pests.
Furthermore, cultivation of multiline for specific resistance genes, which are mixtures of NILs carrying GRH or BPH resistance genes, is a useful way to reduce the damage caused by insect pests while monitoring GRH or BPH virulence in the target area of the paddy field. To prevent infection of rice blast at field, the multiline cultivars in the genetic background of elite japonica Sasanishiki and Koshihikari resistant to rice blast have been commercially cultivated for several years without any breakdown of their resistance (Ishizaki et al. 2005, Nakajima et al. 1996). The multiline for rice blast consisted of seeds mixtures of several isogenic lines for rice blast resistance genes and the combination of isogenic lines were altered according to the distribution of the races of the blast pathogens at target region. However, there is no report for multiline of insect resistance. Using by the NILs for GRH or BPH resistance genes, the multiline for insect resistance will be developed in future study. To keep stable utilization of multiline, the monitoring of insect virulence at target region is essential for selecting effective combination of NILs carrying resistance gene in multiline. From the viewpoint of ecological manipulation of diverse alleles at the virulence loci of the insects, multiline cultivars carrying specific genes for resistance to insect pests will be very useful.
We are grateful to the Biotron Application Center, Kyushu University, for the use of the Entomotron in our antibiosis tests. We also thank the staff members of the Research Group for Insect Pest Management, Kyushu Okinawa Agricultural Research Center, for their technical support. This work was supported by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan [Genomics for Agricultural Innovation, (QTL-2001) and Genomics-based Technology for Agricultural Improvement (LTC-0010)] and partly supported by the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (C) (23580009). We also wish to thank Science and Technology Research Partnership for Sustainable Development (SATREPS) by JST / JICA for the doctoral fellowship granted to TVM.