2016 Volume 66 Issue 4 Pages 636-645
2016 Volume 66 Issue 4 Pages 636-645
Bacterial blight caused by Xanthomonas oryzae pv. oryzae (Xoo) is a chief factor limiting rice productivity worldwide. XM14, a rice mutant line resistant to Xoo, has been obtained by treating IR24, which is susceptible to six Philippine Xoo races and six Japanese Xoo races, with N-methyl-N-nitrosourea. XM14 showed resistance to six Japanese Xoo races. The F2 population from XM14 × IR24 clearly showed 1 resistant : 3 susceptible segregation, suggesting control of resistance by a recessive gene. The approximate chromosomal location of the resistance gene was determined using 10 plants with shortest lesion length in the F2 population from XM14 × Koshihikari, which is susceptible to Japanese Xoo races. DNA marker-assisted analysis revealed that the gene was located on chromosome 3. IAS16 line carries IR24 genetic background with a Japonica cultivar Asominori segment of chromosome 3, on which the resistance gene locus was thought to be located. The F2 population from IAS16 × XM14 showed a discrete distribution. Linkage analysis indicated that the gene is located around the centromeric region. The resistance gene in XM14 was a new gene, named XA42. This gene is expected to be useful for resistance breeding programs and for genetic analysis of Xoo resistance.
Rice (Oryza sativa L.) is a cereal crop of the family Poaceae. It has been gathered, cultivated, and consumed by many people worldwide. In fact, it is the world’s most important food crop and a primary source of food for more than half the world’s population (Khush 2005). Unfortunately, its production potential is being reduced severely by several rice diseases. Bacterial blight (BB) caused by the Xanthomonas oryzae pv. oryzae (Xoo) pathogen is a chief factor limiting rice productivity worldwide because of its high epidemic potential (Khan et al. 2014, Verdier et al. 2012, Xia et al. 2012).
As a vascular disease that results in systemic infection, BB produces tannish-grey to white lesions along leaf veins (Mew 1987, Mew et al. 1993). Most commonly, plants are affected at the maximum tillering stage. Yields are reduced by 10–20%. Infection at the tillering stage can engender total crop losses (Mew et al. 1993). Actually, BB is prevalent in both tropical and temperate climates. It has been reported in all rice growing regions worldwide except North America (Niño-Liu et al. 2006, Ou 1985).
Developing resistant cultivars is generally regarded as the most effective and economical means of controlling this disease (Khan et al. 2014, Mew et al. 1993, Ogawa and Khush 1989). About 40 genes conferring resistance against various races of Xoo have been identified in both cultivated rice and wild relatives of rice, and have been derived from artificial mutation induction (http://www.shigen.nig.ac.jp/rice/oryzabase/locale/change?lang=en). However, because of the rapid changes in the pathogenicity of Xoo and the emergence of new races, resistance genes break down (Khan et al. 2014, Mew 1987, Xia et al. 2012). To solve this problem, a search for new Xoo-resistance genes has been conducted. Taura et al. (1991b, 1992) identified Xoo-resistant mutant genes xa19 and xa20 using N-methyl-N-Nitrosourea (MNU) mutagen. These genes are resistant to all Japanese and Philippines races. We have identified new mutant named ‘XM14’, which is resistant to all Japanese Xoo races. This study was conducted for identification, and for genetic and linkage analysis of the Xoo resistance gene in XM14.
Xoo are differentiated into many races according to virulence and origin. Races used for this study were six Japanese races: race I (strain T7174), race IIA (strain T7147), race IIB (strain H9387), race III (strain T7133), race IV (strain H75373), and race V (strain H75304). In addition, a race from the Philippines was used: race 5 (strain PXO 112).
The Indica rice cultivar ‘IR24’ was released in 1972 by the International Rice Research Institute (IRRI). IR24 is susceptible to six Philippines Xoo races (race 1 (strain PXO 61), race 2 (strain PXO 86), race 3 (strain PXO 79), race 4 (strain PXO 71), race 5 (strain PXO 112), and race 6 (strain PXO 99) (Taura et al. 1991b, 1992). Moreover, it is susceptible to the six Japanese Xoo races above (Ogawa and Yamamoto 1987). We induced mutation to IR24, as described by Taura et al. (1991a): We soaked rice spikelets of IR24 in 1 mM of MNU solutions for 45 min at 8, 10, and 12, 14, 16, 18 and 20 hr after flowering. M1 plants were selfed to produce M2 generation. We selected a M2 plant resistant to Philippine Xoo race 5 at IRRI, Los Baños, Philippines in 1988. The M3 line derived from the resistant M2 plant was fixed for Xoo resistance. The progeny of the M3 line was named XM14. Thereafter, XM14 was brought to Japan. Then studies of XM14 resistance to Japanese Xoo races were conducted. Preliminary results showed that XM14 is resistant to the six Japanese Xoo races described above. ‘Koshihikari’, a popular Japonica rice cultivar, is cultivated in Japan as well as Australia and the United States. This cultivar is known to be susceptible to all Japanese Xoo races (Noda and Ohuchi 1989).
IAS lines are one of the sets of reciprocal chromosome segment substitution lines (CSSLs) between a Japanese Japonica cultivar ‘Asominori’ and IR24 (Kubo et al. 2002). The graphical genotypes of IAS lines are obtainable at http://www.shigen.nig.ac.jp/rice/oryzabase/strain/recombinant/genotypeIAS. Among them, the IAS16 line carries IR24 genetic background with Asominori chromosomal segment of chromosome 3, on which resistance gene of XM14 was thought to be located from the initial mapping (see Results). Asominori is resistant to Japanese Xoo races I and V while susceptible to races II, III, and IV (Kaku and Kimura 1989). Our preliminary analysis showed that IAS16 is susceptible to the six Japanese Xoo races above.
XM14, IR24, IAS 16, and Koshihikari were tested for their reactions to the six Japanese Xoo races. They had been tested separately before, and were tested under the same condition in 2014. Each Xoo race was inoculated to six plants from each line. XM14 was crossed with IR24 to ascertain the number of gene(s) and dominance involved in Xoo resistance using 216 F2 plants. XM14 was also crossed with Koshihikari. A total of 237 F2 plants from the cross between XM14 and Koshihikari were subjected to preliminary linkage analysis to ascertain the approximate chromosomal location of the resistance gene of XM14. The Results section of this report presents a description that this population showed continuous distribution of LL, probably because of diverse genetic background attributable to the Indica–Japonica cross. To minimize the genetic ‘noise’, the 194 F2 plants from the cross between XM14 and IAS16 were also subjected to linkage analysis because both lines share the IR24 genetic background. During the following season, F3 lines from selected F2 plants from the same cross were grown to confirm the genotypes of the resistance gene in XM14.
F2 plants from the cross between XM14 and IR24 had been planted before, with the preliminary result that XM14 carries a recessive resistance gene. They were planted again in 2014, increasing the number of plants for confirmation for the previous result. F2 plants from the cross between XM14 and Koshihikari were planted in 2012. F2 plants from the cross between XM14 and IAS16 were planted in 2014. F3 lines from the same cross were planted in 2015. Germinated seeds of segregating populations and parental lines were sown in seedling boxes in a greenhouse in May in respective years. About two weeks after sowing, seedlings were transferred out of the greenhouse. About one month after the sowing date, they were transplanted to a paddy field in the experimental farm of the Faculty of Agriculture, Kagoshima University, Kagoshima, Japan. Along with the respective segregating populations, 5 to 10 plants from each parental line were planted.
Inoculation of Xoo and scoringXoo inoculum was prepared and cultured using potato semi-synthetic agar media (Wakimoto 1954) and was incubated at 28°C for 48 hr. The inoculum was then diluted with distilled water. The absorbance was adjusted to A = 0.05 (620 nm) using a spectrophotometer. This value corresponds to the concentration of about 108 colony forming units per milliliter (cfu/ml), which normally provide optimum Xoo infection to the host using the clipping method (Kauffman et al. 1973). Xoo was inoculated using the clipping method during booting to the flowering stage. Resistance of plants to Xoo was scored by the mean lesion length (LL) of three leaves from each plant using a ruler 18 days after Xoo inoculation. Scoring of LL of F3 lines was based on visual observation: LL longer than 3 cm judged by visual observation were scored as susceptible, whereas that shorter than 3 cm was scored as resistant.
DNA markers and linkage analysisPCR-based SSR and Insertion/deletion (Indel) markers were used for linkage analysis. DNA was extracted according to the method described by Dellaporta et al. (1983) with some modifications. Polymerase chain reaction (PCR) mixture (5 μL) consisted of 10 ng genomic DNA, 200 μM dNTPs, 0.2 μM of each primer, 0.25 U of Taq polymerase (AmpliTaq Gold; Applied BioSystems, CA, USA), and 1 × buffer containing MgCl2. PCR conditions were: 95°C for 5 min, 35 cycles 94°C for 30 s, 55°C for 30 s and 72°C for 30 s with subsequent final extension at 72°C for 7 min. After PCR products were separated in 10% polyacrylamide gels, they were stained with ethidium bromide and visualized with ultraviolet light (GelDoc-It® TS Imaging System; UVP, CA, USA).
For initial linkage analysis using the cross between Koshihikari and XM14, we used 113 published SSR markers and Insertion/deletion (Indel) markers, most with primer information derived from reports by Ichitani et al. (2014), IRGSP (2005), McCouch et al. (2002), Panaud et al. (1996), and Rice Genome Research Program (http://rgp.dna.affrc.go.jp/E/publicdata/caps/index.html).
As the linkage analyses progressed, the target regions of the Xoo resistance gene were narrowed. No published DNA markers were present there. Therefore, we developed new PCR-based DNA markers (Table 1). We used Indel information released by Xu et al. (2012) or searched for Indel polymorphism (5–50 bp difference) between a Japonica cultivar ‘Nipponbare’ (IRGSP 2005, Kawahara et al. 2013) and an Indica cultivar ‘93-11’ (Gao et al. 2013, Yu et al. 2002), and/or an Indica cultivar ‘HR12’. Oryza sativa (rice) Nucleotide BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE_TYPE=BlastSearch&PROG_DEF=blastn&BLAST_PROG_DEF=megaBlast&BLAST_SPEC=OGP__4530__9512) was used for Indel information. BLAST searching optimized for highly similar sequences was done using a one thousand to ten thousand base Nipponbare sequence (Os-Nipponbare-Reference-IRGSP-1.0) as the query and 93-11 sequence (GCA_000004655.1) or HR12 sequence (GCA_000725085) as the subject. Uniqueness of the DNA sequences surrounding Indel was confirmed using BLAST with BLASTScope in Oryzabase (Yamazaki et al. 2010, http://www.shigen.nig.ac.jp/rice/oryzabaseV4/blast/search). Primers surrounding Indels were designed using Primer 3 (Untergrasser et al. 2012). Linkage map involving 16 DNA markers on chromosome 3 was constructed using software (AntMap; Iwata and Ninomiya 2006). The Kosambi function was used to estimate the map distances (Kosambi 1944).
| Marker name | Kind of DNA marker | Primer sequence | Location on IRGSP 1.0 pseudomolecule chromosome 3 | |||
|---|---|---|---|---|---|---|
| From | to | Source | ||||
| KGC3_15.36 | INDEL | F | ATTTCCGATGGATAGATAATTGCTCAA | 15369490 | 15369606 | This study |
| R | CTCAGTTGGACAGACAGACGTA | |||||
| KGC3_15.39 | INDEL | F | GCCTGCAAGAATTAACTGCAAAATC | 15392101 | 15392223 | This study |
| R | TCATATTGGCAGATTAAAGCATGCA | |||||
| KGC3_15.57 | INDEL | F | TCAAATAGACTGCTGAGAACCGATC | 15571005 | 15571205 | This study |
| R | GACATGGTGAAGAAATAGCCTCTCC | |||||
| KGC3_15.7 | INDEL | F | CAACGTCAACATCAATACGACACTA | 15729038 | 15729207 | This study |
| R | TGAGAACACGATCTTCAGTAAACAG | |||||
| KGC3_15.9 | INDEL | F | TCGGAGATTGCTATAATAGGGATGA | 15966551 | 15966800 | This study |
| R | AATTTTACCTCATAACCTGTGCTGT | |||||
| KGC3_16.1 | INDEL | F | GTTTAGATATCGCTTTCAGGCATGT | 16117085 | 16117235 | This study |
| R | CGGTTTATAAGGGTAGCCGC | |||||
| KGC3_16.3 | INDEL | F | ATTAGAGTATCCACCAATAAGCCCG | 16323299 | 16323546 | This study |
| R | GAGGTAAGATGAGATCGTGTAGGAG | |||||
| RM15189 | SSR | F | CAGTAAGTGTCTCTGGAAGCTTG | 16699297 | 16699465 | IRGSP 2005, redesigned in this study |
| R | TGCTGAGTAGGTACCTTTCTTAAAAC | |||||
| KGC3_16.7 | INDEL | F | TCGGAGATGTGTATTATCATTCAACT | 16726679 | 16726765 | This study |
| R | GTGGGCGGTTATTCTATATATCAGT | |||||
| RM15191 | SSR | F | CGTCAATCCATCTTGCCGTTAACC | 16747940 | 16748065 | IRGSP 2005, redesigned in this study |
| R | CTCAGCCCGCCTTGTCGAG | |||||
| RM15206 | SSR | F | GAAAGACTCAATAGTAGTACAAAGGAGAG | 16965176 | 16965240 | IRGSP 2005, redesigned in this study |
| R | TCTTCCTGCCAAATATGCAC | |||||
| KGC3_17.02 | INDEL | F | CGGAGAAGCTTGATCGGAGG | 17022626 | 17022820 | This study |
| R | GGAGACCGTATCGACAGTAAATCAA | |||||
| KGC3_17.03 | INDEL | F | GCCCACCTCCTGCACATT | 17034809 | 17034952 | This study |
| R | AGTGCCACCCATGACACG | |||||
| KGC3_17.1 | INDEL | F | ATCATGTCTATCGAGCGTATTTTGG | 17120606 | 17120778 | This study |
| R | CAATCAGCGTGTCGATTTCTTAGTA | |||||
| KGC3_17.2 | INDEL | F | GACAGCCCACACCCATATAGAC | 17213199 | 17213308 | This study |
| R | GAGGATGGCGGAAGGTCG | |||||
| RM3400 | SSR | F | TCTCTCTCCTCTCTCGCTCG | 17266171 | 17266354 | McCouch et al. 2002 |
| R | TAAAACCGAAGTGCTCTCGC | |||||
| RM7642 | SSR | F | ACGAAATATCAGGGCACCTG | 18631946 | 18632139 | McCouch et al. 2002 |
| R | GTTGACTTTGGTCATGAGGG | |||||
| RM16 | SSR | F | CGCTAGGGCAGCATCTAAAA | 23127576 | 23127743 | McCouch et al. 2002 |
| R | AACACAGCAGGTACGCGC | |||||
IR24 was found to be susceptible to the six Japanese Xoo races used for this study, whereas XM14 was resistant to them (Fig. 1, Table 2). The average LL of XM 14 reaction for the Japanese Xoo races was 0.4 cm, whereas that of IR24 reaction was 23.6 cm. The F2 population from the cross between IR24 and XM14 showed a clear bimodal distribution of LL using Xoo race IIA (T7147). We observed a clear gap and classified the 216 F2 plants into 53 resistant plants with LL of 0.1–2 cm and 163 susceptible plants with LL of 7–37 cm (Fig. 2). The ratio 53:163 fitted 1:3, one-gene segregation (χ2 = 0.02, P = 0.88). From the above result and subsequent linkage analysis, it seems readily apparent that XM14 carries a novel Xoo-resistance gene. Therefore, the gene identified in XM14 was named XANTHOMONAS ORYZAE PV. ORYZAE RESISTANCE 42 (XA42) according to the gene nomenclature system for rice (McCouch and CGSNL 2008). Xa42 is a susceptible wild type allele, whereas xa42 is a resistant mutated allele.

Reactions of IR24 and XM14 to six Japanese races of Xoo (races I, IIA, IIB, III, IV and V) 18 days after inoculation.
| Rice accession | Xanthomonas oryzae pv. oryzae race | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| race I (strain T7174) | race IIA (strain T7147) | race IIB (strain H9387) | race III (strain T7133) | race IV (strain H75373) | race V (strain H75304) | |||||||
| Meana | SDb | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| XM14 | 0.3 | 0.2 | 0.2 | 0.1 | 0.7 | 0.2 | 1.2 | 0.6 | 0.3 | 0.1 | 0.3 | 0.1 |
| IR24 | 30.9 | 1.5 | 28.2 | 2.4 | 25.6 | 3.8 | 20.6 | 2.8 | 28.7 | 0.5 | 24.9 | 3.9 |
| Koshihikari | 15.3 | 5.6 | 21.3 | 2.4 | 20.6 | 5.1 | 17.8 | 1.2 | 11.7 | 2.4 | 22.7 | 2.4 |
| IAS16 | 13.6 | 3.6 | 22.3 | 1.1 | 24.1 | 3.1 | 20.6 | 5.3 | 21.9 | 2.9 | 20.5 | 5.1 |

Distribution of lesion length in F2 population from the cross between XM14 and IR24 after Xoo Japanese race IIA (strain T7147) inoculation. Horizontal lines at the top of figure represent the ranges of parental lines. Vertical lines crossing the horizontal line represent the means of parental lines.
The F2 population from the cross between Koshihikari and XM14 showed continuous distribution of LL using Xoo race II with no clear gap (Fig. 3), partly because Koshihikari (Japonica) and XM14 (Indica) have different backgrounds. Large variation in agronomic traits such as the tiller number and plant height caused by Indica–Japonica genetic difference might increase LL variation. Therefore, instead of normal linkage analysis, we adopted the analysis using extreme recessive phenotype proposed by Zhang et al. (1994). Ten F2 plants with the shortest LL (0.1–4 cm) were selected, and DNA was extracted from each plant. Then genotyping was done using 113 SSR and Indel markers covering the whole rice genome (Table 3). If a DNA marker is linked closely to the resistance gene in XM14, then most or all of the ten resistant plants were homozygotes of XM14 allele at the DNA marker locus. Nine plants were homozygotes of XM14 allele at the consecutive four DNA marker loci, RM3400, RM6914, RM1334, and RM5684. Eight plants were homozygotes of XM14 allele at the neighboring DNA marker loci, RM6959, RM3204, RM7642, RM5488, RM411, RM3698, RM3646, RM487, RM7395 and RM6832 on chromosome 3. These results strongly suggest that XA42 is located on chromosome 3. Calculations of the recombination frequency based on extreme recessive phenotype proposed by Zhang et al. (1994) (Table 3) also support that inference.

Distribution of lesion length in in F2 population from the cross between XM14 and Koshihikari after Xoo Japanese race IIA (strain T7147) inoculation. Horizontal lines at the top of figure represent the range of parental lines. The vertical line crossing the horizontal line represents the mean of parental lines.
| Chromosome | DNA markerc | Genotypea | Recombination frequencyd | Chromosome | DNA marker | Genotype | Recombination frequency | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Individualb | Individual | ||||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||||
| 1 | RM1282 | H | X | H | K | K | X | H | X | X | H | 0.40 | 5 | RM249 | H | H | K | K | H | X | H | K | K | X | 0.60 |
| RM220 | K | X | H | K | K | H | H | X | X | K | 0.55 | RM7568 | H | H | K | K | X | X | X | K | K | X | 0.50 | ||
| RM259 | H | X | H | H | H | H | X | X | X | X | 0.25 | E60663 | H | H | K | K | X | X | H | H | K | K | 0.60 | ||
| RM8132 | H | X | H | H | H | H | X | H | X | X | 0.30 | RM6954 | H | K | K | K | H | H | H | H | K | K | 0.75 | ||
| S13623 | H | K | K | H | H | H | H | H | X | X | 0.50 | RM3476 | H | K | H | K | X | K | H | H | K | K | 0.70 | ||
| RM8129 | X | K | K | K | X | H | H | H | X | X | 0.45 | 6 | E30287 | X | X | X | H | X | H | H | X | H | X | 0.20 | |
| RM246 | X | K | K | K | H | H | H | H | X | X | 0.50 | RM253 | H | X | X | X | X | X | H | X | H | X | 0.15 | ||
| RM1297 | X | H | K | K | H | H | K | H | X | H | 0.55 | RM276 | H | X | X | X | H | X | H | X | H | X | 0.20 | ||
| RM212 | K | X | K | K | H | H | K | H | X | H | 0.60 | RM527 | H | X | X | X | H | H | H | X | H | X | 0.25 | ||
| RM5448 | K | X | H | H | H | H | K | H | X | K | 0.55 | RM3 | H | X | X | X | H | H | H | H | K | X | 0.35 | ||
| RM8099 | K | X | H | H | H | H | H | H | H | H | 0.50 | RM3628 | H | H | X | X | X | H | H | H | K | X | 0.35 | ||
| 2 | RM211 | H | K | K | H | H | H | H | H | H | K | 0.65 | RM6782 | H | H | X | X | X | H | H | H | K | K | 0.45 | |
| RM5664 | H | K | K | X | K | H | H | K | H | K | 0.70 | RM58114 | H | K | H | H | X | K | H | H | H | K | 0.60 | ||
| RM6844 | H | H | K | H | K | H | H | H | K | K | 0.70 | 7 | RM481 | H | K | X | H | H | H | H | H | X | X | 0.40 | |
| RM29 | H | H | H | H | X | H | H | H | H | X | 0.40 | S20268 | H | K | X | X | X | H | K | H | H | X | 0.40 | ||
| RM1303 | H | H | H | H | H | X | H | H | H | H | 0.45 | RM1134 | H | K | X | X | H | H | K | H | H | X | 0.45 | ||
| RM3525 | K | H | K | H | H | X | H | X | X | H | 0.45 | C30372 | H | K | X | X | H | H | K | H | H | X | 0.45 | ||
| RM1367 | K | X | K | X | H | X | H | X | X | H | 0.35 | RM3826 | H | K | H | X | H | H | X | X | H | X | 0.35 | ||
| RM240 | H | H | K | X | H | X | H | H | X | X | 0.35 | RM234 | X | H | H | X | H | X | H | X | X | X | 0.20 | ||
| RM6312 | H | H | K | X | H | X | H | H | X | X | 0.35 | RM142 | X | H | H | X | X | X | X | X | X | X | 0.10 | ||
| 3 | RM22 | H | H | H | X | H | H | K | H | K | H | 0.55 | RM1306 | X | X | K | H | X | X | X | K | H | X | 0.30 | |
| E50818 | X | H | X | X | X | X | H | X | H | H | 0.20 | 8 | RM6369 | H | X | H | K | K | X | H | X | X | K | 0.45 | |
| RM6959 | X | X | H | X | X | X | X | X | X | H | 0.10 | RM1376 | H | K | H | H | X | X | H | X | X | K | 0.40 | ||
| RM3204 | X | X | H | X | X | X | X | X | X | H | 0.10 | RM6429 | H | K | H | H | X | H | H | X | X | K | 0.45 | ||
| RM3400 | X | X | H | X | X | X | X | X | X | X | 0.05 | RM6215 | H | K | H | H | X | H | H | H | X | K | 0.50 | ||
| RM6914 | X | X | H | X | X | X | X | X | X | X | 0.05 | RM223 | H | K | K | H | X | H | H | H | X | X | 0.45 | ||
| RM1334 | X | X | H | X | X | X | X | X | X | X | 0.05 | RM7556 | X | K | K | K | X | H | H | H | X | X | 0.45 | ||
| RM5684 | X | X | H | X | X | X | X | X | X | X | 0.05 | E4443 | X | K | K | K | H | H | H | H | X | X | 0.50 | ||
| RM7642 | H | X | H | X | X | X | X | X | X | X | 0.10 | RM3120 | X | K | K | K | H | H | K | H | X | X | 0.55 | ||
| RM5488 | H | X | H | X | X | X | X | X | X | X | 0.10 | 9 | RM219 | X | H | K | K | H | H | H | H | H | X | 0.50 | |
| RM411 | H | X | H | X | X | X | X | X | X | X | 0.10 | RM7038 | X | H | X | K | H | H | X | X | K | X | 0.35 | ||
| RM3698 | H | X | H | X | X | X | X | X | X | X | 0.10 | RM6771 | X | H | X | K | H | H | X | X | K | X | 0.35 | ||
| RM3646 | H | X | H | X | X | X | X | X | X | X | 0.10 | RM7424 | X | H | X | H | H | H | X | X | K | X | 0.30 | ||
| RM487 | H | X | H | X | X | X | X | X | X | X | 0.10 | E61552 | X | H | X | H | X | H | X | X | K | H | 0.30 | ||
| RM7395 | H | X | H | X | X | X | X | X | X | X | 0.10 | RM257 | X | H | X | H | X | H | X | X | K | H | 0.30 | ||
| RM6832 | H | X | H | X | X | X | X | X | X | X | 0.10 | RM6971 | H | K | H | X | X | K | X | X | K | H | 0.45 | ||
| RM15451 | H | X | H | X | H | X | X | H | H | X | 0.25 | E21191 | H | K | H | X | X | K | X | X | K | H | 0.45 | ||
| RM5532 | H | X | H | X | H | X | X | H | H | X | 0.25 | 10 | RM216 | H | H | K | X | X | K | H | X | X | H | 0.40 | |
| RM6266 | H | X | H | X | H | X | X | H | H | X | 0.25 | RM1375 | H | H | K | X | H | K | K | X | X | H | 0.50 | ||
| RM3513 | H | X | H | X | H | X | X | H | H | H | 0.30 | RM258 | H | H | K | X | H | H | H | X | X | H | 0.40 | ||
| RM3436 | H | X | H | X | H | X | H | H | H | H | 0.35 | RM1108 | H | H | K | X | H | H | H | X | X | H | 0.40 | ||
| RM3525 | H | X | H | X | H | X | H | H | X | H | 0.30 | RM5352 | H | K | K | H | H | H | H | X | H | H | 0.55 | ||
| RM3346 | H | H | H | H | H | H | H | H | X | H | 0.45 | RM228 | H | K | K | H | X | H | H | H | H | H | 0.55 | ||
| RM1221 | H | H | H | H | H | H | H | H | X | H | 0.45 | 11 | RM4B | X | H | H | X | H | H | H | H | H | H | 0.40 | |
| 4 | C61009 | X | X | X | X | K | K | H | K | H | K | 0.50 | RM5599 | X | H | X | X | H | K | H | H | K | X | 0.40 | |
| RM7279 | X | X | X | X | X | K | H | K | H | K | 0.40 | S21074 | H | K | X | X | X | K | H | H | K | X | 0.45 | ||
| RM6997 | X | X | H | H | H | H | H | H | H | K | 0.45 | RM5731 | H | K | X | X | X | K | H | H | K | X | 0.45 | ||
| RM303 | H | X | H | H | H | H | H | H | H | K | 0.50 | RM206 | K | K | X | H | H | K | H | H | K | X | 0.60 | ||
| RM252 | H | H | H | H | H | H | H | H | X | K | 0.50 | RM6440 | K | K | X | H | H | K | H | H | X | X | 0.50 | ||
| RM348 | H | K | H | H | K | H | H | H | X | H | 0.55 | RM224 | K | K | X | H | H | K | H | X | X | X | 0.45 | ||
| RM8217 | H | K | H | H | K | K | H | H | X | H | 0.60 | 12 | RM8214 | K | X | H | K | X | X | H | K | K | K | 0.60 | |
| RM6246 | H | K | H | H | K | K | H | H | X | X | 0.55 | RM6296 | K | X | H | K | X | X | H | H | X | H | 0.40 | ||
| 5 | RM7373 | X | H | H | H | H | H | K | X | H | X | 0.40 | RM7102 | K | H | X | K | X | H | H | H | X | H | 0.45 | |
| RM3345 | X | H | H | H | H | H | K | H | H | X | 0.45 | RM1986 | K | H | X | K | H | H | X | H | H | X | 0.45 | ||
| RM7444 | X | H | H | K | K | X | K | H | K | X | 0.55 | RM1103 | X | H | X | X | K | H | X | H | H | X | 0.30 | ||
| RM3777 | X | K | K | K | K | X | K | H | K | X | 0.65 | L714 | X | X | X | X | K | X | H | H | H | X | 0.25 | ||
| C50867 | K | K | K | K | K | X | K | K | K | X | 0.80 | ||||||||||||||
To minimize the genetic ‘noise’ caused by Indica–Japonica crossing and to maximize the usefulness of Indica–Japonica DNA polymorphism for mapping XA42 precisely, we adopted IAS lines, which carry IR24 genetic background with Asominori chromosomal segments (Kubo et al. 2002). Among the eight DNA markers on chromosome 3 used for developing IAS lines, DDBJ accession names of the partial sequence of the six markers, C515, C563, R3156, C1677, R19, and X249, were obtained from http://rgp.dna.affrc.go.jp/E/publicdata/geneticmap2000/chr03.html. C1677 and R19 are located near the 14 SSR markers above. Among IAS lines, only IAS16 carries the Asominori chromosomal segment covering C1677 and R19.
The F2 population from the cross between IAS16 and XM14 using Xoo race IIA showed clear bimodal distribution of LL. Using the LL of 3 cm as the dividing point, the 195 F2 plants were classified into 72 resistant plants with LL of 0.1–2.8 cm and 122 susceptible plants with LL of 4.5–60.1 cm (Fig. 4). The ratio was deviated from to 1:3, one-gene segregation (χ2 = 15.182, P < 0.001). However, the tight linkage between XA42 and DNA markers confirmed one-gene segregation (see below). The reason for the deviation is discussed in the next section.

Distribution of lesion length in F2 population from the cross between the XM14 and IAS16 line after Xoo Japanese race IIA (strain T7147) inoculation. Three classified genotypes were assessed for KGC3_16.3 as indicated: black, homozygous for XM14; white, heterozygous; and gray, homozygous for IAS16. Horizontal lines at the top of figure show the ranges of parental lines. Vertical lines crossing the horizontal line are means of the parental lines.
Linkage analysis of XA42 was performed using 194 F2 plants from the cross between IAS16 and XM14 and polymorphic DNA markers in Table 1. KGC3_15.36 and KGC3_15.39 showed polymorphism between Asominori and IR24, but not between XM14 and IAS16. The other markers in Table 1 showed polymorphism between XM14 and IAS16, in addition to that between Asominori and IR24. These results indicate that the one end of Asominori segment on chromosome 3 in IAS16 is located between KGC3_15.39 and KGC3_15.57.
Fig. 4 presents the frequency distribution of LL separated by the genotype of KGC3_16.3. Homozygotes of XM14 were strongly skewed toward short LL. Heterozygotes and homozygotes of IAS16 were strongly skewed toward long LL. These results demonstrate that XA42 is linked closely with KGC3_16.3. Table 4 shows LL, genotypes of DNA markers surrounding XA42 locus, and results of F3 generation of informative recombinants and non-recombinants. Plant No. 13 showed LL of 2.8 cm, which was close to the tentative dividing point. It was homozygote of XM14 allele for the entire 11 DNA marker loci in Table 4 as was Plant No. 12 with LL of 0.1 cm, and was fixed for resistant plants in F3 generation. This result indicates that Plant No. 13 was a homozygote of XM14 allele for XA42 locus. Plant No. 14 showed LL of 4.5 cm, which was also close to the tentative dividing point. It was a homozygote of IAS16 allele for the entire 11 DNA marker loci in Table 4, as was Plant No. 20 with LL of 32.3 cm, and was fixed for susceptible plants in F3 generation. This result indicates that Plant No. 14 was a homozygote of IAS16 allele for the XA42 locus.
| F2 Individual | Lesion lengthb (cm) | Reactionc | Genotypes of the DNA marker locia | No. of F3 plants | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KGC3_15.57 | KGC3_16.1 | KGC3_16.3 | RM 15189 |
RM 15191 |
RM 15206 |
KGC3_17.02 | KGC3_17.03 | KGC3_17.1 | RM 7,642 |
RM 16 |
Reactiond | ||||
| R | S | ||||||||||||||
| 1 | 57.3 | S | H | H | H | H | H | H | X | X | X | X | X | 7 | 23 |
| 2 | 20.6 | S | H | H | H | H | H | H | X | X | X | X | X | 9 | 21 |
| 3 | 19.6 | S | H | H | H | H | H | H | X | X | X | X | X | 8 | 22 |
| 4 | 0.4 | R | H | H | X | X | X | X | X | X | X | X | X | 17 | 0 |
| 5 | 0.6 | R | X | X | X | X | X | X | X | X | X | X | H | NTe | NT |
| 6 | 0.9 | R | X | X | X | H | H | H | H | H | H | H | H | 30 | 0 |
| 7 | 21.2 | S | H | H | H | H | H | H | H | H | H | H | H | 5 | 11 |
| 8 | 24.6 | S | A | A | A | A | A | H | H | H | H | H | H | 0 | 18 |
| 9 | 31.5 | S | H | H | A | A | A | A | A | A | A | A | A | 0 | 11 |
| 10 | 30.1 | S | H | H | H | H | H | A | A | A | A | A | A | 5 | 17 |
| 11 | 29.6 | S | H | H | H | H | H | A | A | A | A | A | A | 3 | 17 |
| 12 | 0.1 | R | X | X | X | X | X | X | X | X | X | X | X | NT | NT |
| 13 | 2.8 | R | X | X | X | X | X | X | X | X | X | X | X | 15 | 0 |
| 14 | 4.5 | S | A | A | A | A | A | A | A | A | A | A | A | 0 | 15 |
| 15 | 8.6 | S | A | A | A | A | A | A | A | A | A | A | A | NT | NT |
| 16 | 9.3 | S | H | H | H | H | H | H | H | H | H | H | H | NT | NT |
| 17 | 21.2 | S | H | H | H | H | H | H | H | H | H | H | H | NT | NT |
| 18 | 21.4 | S | A | A | A | A | A | A | A | A | A | A | A | NT | NT |
| 19 | 32.1 | S | A | A | A | A | A | A | A | A | A | A | A | NT | NT |
| 20 | 32.3 | S | A | A | A | A | A | A | A | A | A | A | A | NT | NT |
The two recombinant Plant Nos. 4 and 6 were homozygotes of XM14 allele because they showed LL shorter than 1.0 cm, and were fixed for resistant plants in F3 generation. In Plant No. 4, recombination event occurred between KGC3_16.1 and KGC3_16.3. In Plant No. 6, recombination event occurred between KGC3_16.3 and RM15189. XA42 is expected to be located near the loci at which genotypes of the recombinants were homozygotes of XM14 allele. Therefore, XA42 is located close to KGC3_16.3. Results for the other plants in Table 4 all support this idea. Therefore, the dividing point at 3.0 cm clearly classified the F2 plants into resistant homozygous plants of xa42 allele and susceptible plants with the other genotypes (Fig. 4).
Based on the classification, the linkage map surrounding XA42 is shown in Fig. 5. The linkage around XA42 locus was compared with a restriction fragment length polymorphism (RFLP) marker-based high-density linkage map (Harushima et al. 1998), in which some markers have been sequenced. Based on the Nipponbare genome sequence (Os-Nipponbare-Reference-IRGSP-1.0), DNA markers located near each other on Nipponbare pseudomolecules are connected with dotted lines (Fig. 5): XA42 is located around the centromeric region of rice chromosome 3.
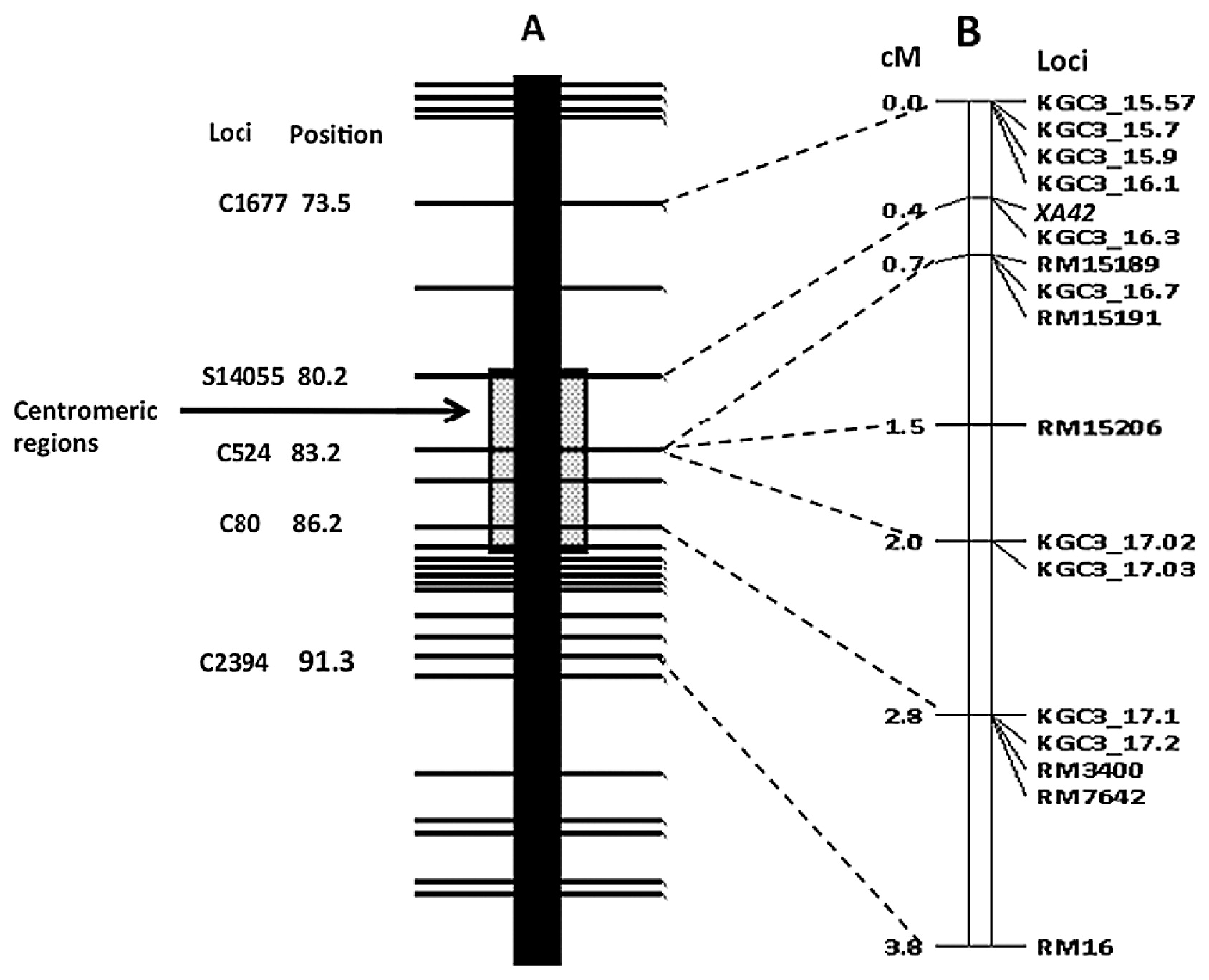
Linkage map showing the location of XA42 gene on chromosome 3: A, RFLP framework map of chromosome 3 modified from Harushima et al. (1998); B, linkage map of XA42 gene constructed from F2 population from XM14 and IAS16 (n = 194). DNA markers located near each other on Nipponbare pseudomolecules are connected by dotted lines.
This study identified a new rice mutant line named XM14. Inoculation tests involving six Japanese Xoo races (I, IIA, IIB, III–V) were conducted, and XM14 exhibited resistance with the average LL of 0.4 cm across all races. Using F2 plants from the cross between XM14 mutant line and its original cultivar IR24 and inoculating Japanese Xoo race IIA (strain T7147), we confirmed that the resistance gene is controlled by a single recessive gene. Linkage analysis showed that this gene was located around the centromeric region of chromosome 3.
Currently about 40 genes conferring host resistance to Xoo have been identified and reported (http://www.shigen.nig.ac.jp/rice/oryzabase/locale/change?lang=en, Hutin et al. 2015, Khan et al. 2014, Kim et al. 2015, Xia et al. 2012). Among all resistance genes, only Xa11 has been reported to be located on chromosome 3, not around the centromeric region but on the long arm (Goto et al. 2009). Located around the centromeric region of chromosome 3, a new gene name xa42 was assigned to this resistant gene in XM14, according to the gene nomenclature system for rice (McCouch and CGSNL 2008). Many resistance genes ‘break down’ when they have been widely used for many years in a large population. Exploitation of new resistance genes is urgently necessary. The new resistant gene xa42 in this study is expected to be useful in resistance breeding programs and genetic analysis of Xoo resistance.
To the six Japanese Xoo races used for this study, XM14 is resistant. xa42 gene in XM14 has been proven to confer resistance against Japanese race IIA (strain T7147). We are not sure that xa42 is resistant to Xoo races other than Japanese race IIA because it is difficult to inoculate plants in segregating populations with many races. Because the probability of identifying Xoo resistant mutant is small (Taura et al. 1991a), the existence of simultaneous plural resistance mutations on one M2 line seems improbable. Therefore, it is plausible that xa42 confers resistance to all races with which XM14 was inoculated. The fact that many of resistant genes reported to date have shown resistance to plural races supports this idea. Future studies using the progeny of resistant plants derived from the F2 population from the cross between XM14 and IR24 will clarify whether the multi-resistance of XM14 is conditioned by xa42 gene only or by a combination of plural genes. To confirm that it is a truly a broad-spectrum gene, it must be isolated, cloned and tested with other Xoo races, especially those from south Asian and African countries where putative new Xoo races have been reported (Gonzalez et al. 2007, Mishra et al. 2013).
XA42 segregation was deviated from the expected ratio of 1:3 in the cross XM14 and IAS16: The xa42 allele located on Indica XM14 chromosome was transmitted more than the Xa42 allele on the Japonica Asominori chromosome. This pattern is the same as that reported by Fukuta et al. (2000): segregation was skewed in favor of Indica over Japonica alleles in chromosome 3 around the centromeric region. Such segregation distortions might have been caused by a reproductive barrier such as gametophyte genes.
Of all 41 identified BB resistance genes, only nine genes have been isolated and characterized (Kim et al. 2015). Among them, xa5, xa13, and xa25 are recessive resistance genes. Actually, xa5 gene encodes a small subunit of transcription factor IIA (TFIIAγ) (Iyer and McCouch 2004). Additionally, xa13 and xa25 genes belong to the MtN3/saliva gene family (Liu et al. 2011, Yang et al. 2006). Using the Rice Annotation Project Database (http://rapdb.dna.affrc.go.jp/, Kawahara et al. 2013, Sakai et al. 2013), we searched for candidate genes of xa42 in the chromosomal region encompassed by KGC3_16.1 and RM15189. Results showed 26 genes coding known proteins and 25 predicted genes coding hypothetical proteins or those for non-protein coding transcript. No genes encode transcription factor or similar proteins or belong to the MtN3/saliva gene family. Spectra of isolated recessive resistance genes to Xoo race are also different: The homozygotes of xa5 is resistant to Japanese races IA, IB, II, IIIA, IIIB, and IV, Philippine races 1–5, susceptible to Philippine race 6 (Ogawa et al. 1991). The homozygotes of xa13 are susceptible to Philippine races 1–5 (Singh et al. 2001), and resistant to Philippine race 6 (Chu et al. 2006), which is virulent to most resistant genes (Ogawa et al. 1991). The homozygotes of xa25 are susceptible to Philippine races 1–8, Japanese races II and IIIA, resistant to a Philippine race 9 (strain PXO339) (Chen et al. 2002). These facts suggest that the cloning of xa42 gene can lead to a new resistance mechanism against Xoo.
Xoo symptoms can be affected by environmental conditions and the rice developmental stage (Mew 1987). The genetic background of IR24 in XM14 has a great benefit for those studying Xoo resistance in rice. Experimental lines of many kinds for Xoo resistance have been constructed under IR24 genetic background: near-isogenic lines carrying single Xoo resistance gene (Ogawa et al. 1991), pyramid lines carrying multiple resistance genes (Huang et al. 1997, Yoshimura et al. 1996), and artificially induced mutant lines (Taura et al. 1991a). Therefore, the effect of newly identified genes such as xa42 on resistance to Xoo can be compared easily with other previously described genes. The effect of pyramiding of xa42 with other genes can also be evaluated easily. Iyer-Pascuzzi and McCouch (2007) reviewed the genetic and molecular resistance mechanism of xa5 and xa13 with special emphasis on their recessive inheritance. Regarding pyramiding, when used in combination with other resistance genes, both xa5 and xa13 provide stronger and broader levels of resistance than when used alone. We are undertaking the pyramiding of xa42 with other resistance genes. In this study, the combination of rough linkage analysis using extreme recessive phenotype using Indica–Japonica cross and precise linkage analysis using CSSLs effectively mapped recessive mutant resistance gene induced in IR24. The same mapping strategy can be applied to other previously identified Xoo resistant mutants with IR24 background such as XM5 (Taura et al. 1991b) and XM6 (Taura et al. 1992), which will also contribute to the study of Xoo resistance in rice.
Selection of Xoo resistant recessive mutant plants in paddy field entails transplanting large number of M2 generation seedlings, Xoo inoculation, and LL measuring, which are very time-consuming. Marker-assisted transfer of this gene to other genetic background requires several iterations of backcrossing. If this gene is cloned and loss of function mutation is the mechanism of resistance, then application of TILLING to the selection of resistant gene carriers in the seedling stage of M2 generation induced by chemicals such as MNU and EMS (Suzuki et al. 2008, Till et al. 2007) can be expected to enhance the development of the resistant gene carrier under diverse genetic backgrounds.
The authors gratefully acknowledge Dr. Atsushi Yoshimura of Kyushu University for the kind provision of IAS lines. The authors are also grateful to Dr. Toyoaki Anai of Saga University for reading the manuscript.