2017 Volume 67 Issue 1 Pages 27-34
2017 Volume 67 Issue 1 Pages 27-34
Sweetpotato [Ipomoea batatas (L.) Lam], which contains high levels of antioxidants such as ascorbate and carotenoids in its storage root, is one of the healthiest foods, as well as one of the best starch crops for growth on marginal lands. In plants, carotenoid pigments are involved in light harvesting for photosynthesis and are also essential for photo-protection against excess light. As dietary antioxidants in humans, these compounds benefit health by alleviating aging-related diseases. The storage root of sweetpotato is a good source of both carotenoids and carbohydrates for human consumption. Therefore, metabolic engineering of sweetpotato to increase the content of useful carotenoids represents an important agricultural goal. This effort has been facilitated by cloning of most of the carotenoid biosynthetic genes, as well as the Orange gene involved in carotenoid accumulation. In this review, we describe our current understanding of the regulation of biosynthesis, accumulation and catabolism of carotenoids in sweetpotato. A deeper understanding of these topics should contribute to development of new sweetpotato cultivars with higher levels of nutritional carotenoids and abiotic stress tolerance.
The sweetpotato ranks seventh in annual production among food crops worldwide. In addition, this crop plant also serves as an alternative source of bioenergy and industrial materials such as natural antioxidants. Moreover, it does not require large amounts of fertilizer and pesticides for its cultivation, and is tolerant to some environmental stresses. Due to its rich nutritional content, in combination with its wide adaptability on marginal land ranging from tropical to temperate zones, it has great potential for preventing malnutrition and increasing food security in developing countries. Because sweetpotato contains high levels of antioxidants, potassium and fiber, the nonprofit Center for Science in the Public Interest (CSPI 2016) recently designated the sweetpotato as one of ten “super foods” that can improve human health. The USDA reported that the sweetpotato can yield two to three times as much carbohydrate as field corn, approaching the amount that sugarcane can produce, in Maryland and Alabama (Ziska et al. 2009). Accordingly, this crop could make a substantial contribution to solving world food and environmental problems. In addition, sweetpotato could serve as an industrial bioreactor to produce various high value–added materials including bioethanol, functional feed and antioxidants including carotenoids.
Carotenoid pigments are widespread in nature. In plants, these molecules play important roles in light harvesting in photosynthetic reaction centers. In addition, in conjunction with chlorophyll in the chloroplast, they protect cells from excessive light by absorbing blue-green wavelengths (Ledford et al. 2004). In humans, carotenoids benefit health by acting as dietary antioxidants and alleviating aging-related diseases. Carotenoids are generated from C40 isoprenoids, which have polyene chains containing 3 to 15 conjugated double bonds. About 700 kinds of carotenoids are synthesized by around 20 biosynthetic enzymes (Britton et al. 2004).
In humans and animals, carotenoids are a dietary source of provitamin A. Thus, because these species are unable to synthesize vitamin A, carotenoids are essential components of their diets. When carotenoids are ingested, they are converted to rhodopsin and retinal, a visual pigment and precursor of retinoid acid, which regulates growth, development and differentiation (Fraser and Bramley 2004). Vitamin A deficiency causes night blindness, skin keratinization, dry eye syndrome, degenerative vision loss, impaired immune function and birth defects (Rao and Rao 2007). In addition, carotenoids directly scavenge reactive oxygen species in biological membranes.
In higher plants, the carotenoid metabolic pathway and the function of the biosynthetic enzymes involved have been well studied. Carotenoids are synthesized by the 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate pathway (MEP/DOXP pathway) of isoprenoid biosynthesis (Fig. 1). Two molecules of geranylgeranyl diphosphate (GGPP) are produced from isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) by geranylgeranyl pyrophosphate synthase (GGPS). Phytoene, the first C40 carotenoid, is formed by condensation of two GGPP molecules, a reaction catalyzed by phytoene synthase (PSY). Phytoene is then processed by a series of enzymes including phytoene desaturase (PDS) and ξ-carotene desaturase (ZDS) to yield lycopene. Lycopene is the substrate for two competing cyclases, lycopene epsilon cyclase (LCY-ɛ) and lycopene beta cyclase (LCY-β). Lycopene is processed by two carotenoid biosynthetic pathways, the α-branch pathway (from α-carotene to lutein) and the β-branch pathway (from β-carotene to neoxanthin through several intermediates), which play distinct and complementary roles in photo-protection (Dall’Osto et al. 2007). Subsequently, α-carotene and β-carotene are modified by hydroxylation, epoxidation, or isomerization to express a variety of structural features. In the α-branch pathway, lutein is synthesized by α-carotene ɛ-ling hydroxylase (CHY-ɛ), whereas, in the β-branch pathway, hydroxylation by β-hydroxylase (CHY-β) converts β-carotene to zeaxanthin, and then zeaxanthin epoxidase (ZEP) mediates the formation of violaxanthin. Violaxanthin is converted to neoxanthin by neoxanthin synthase (NXS).
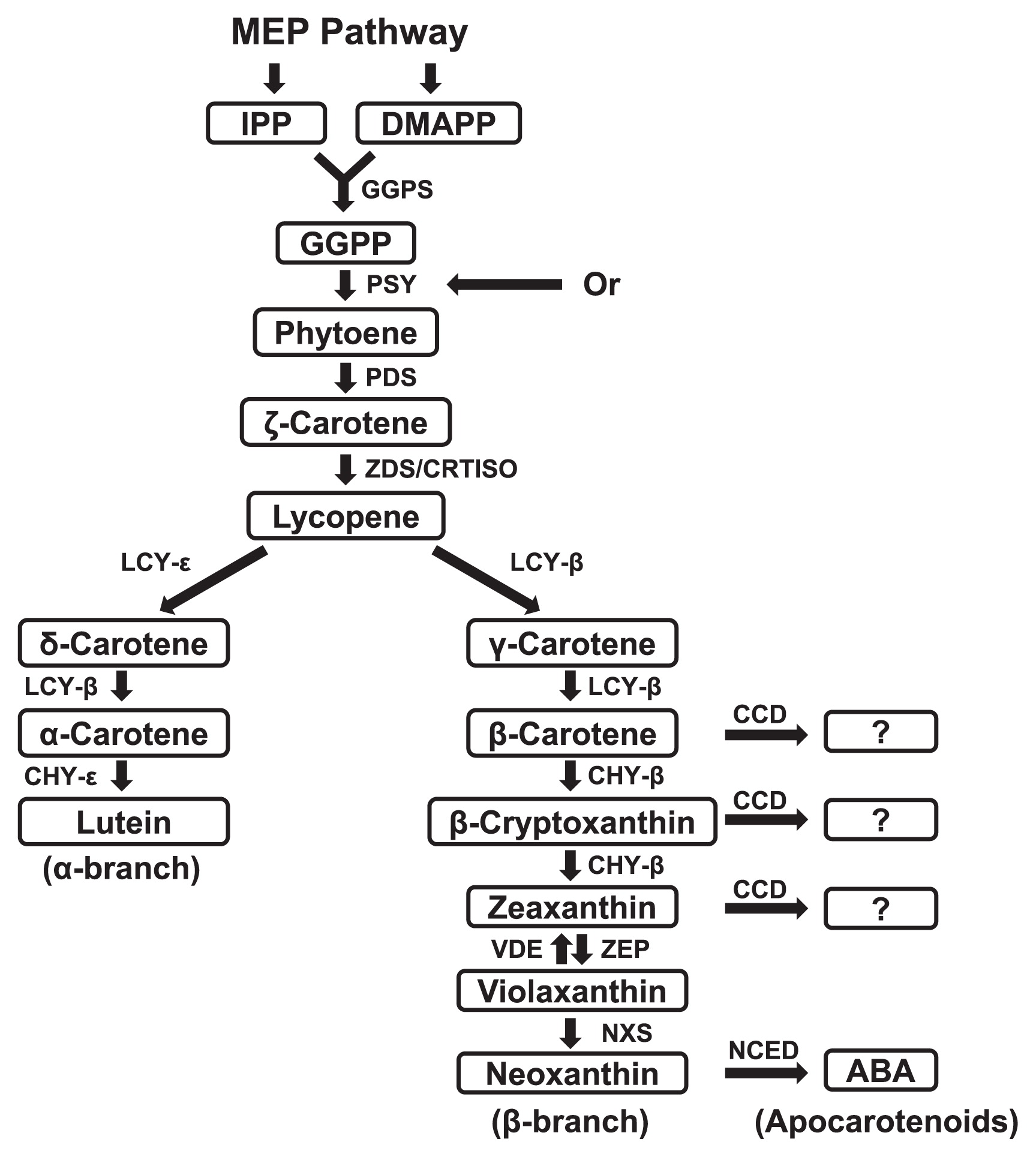
Carotenoid biosynthesis pathway and related enzymes in plants. IPP, isopentenyl diphosphate; DMAPP, dimethylallyl diphosphate; GGPS, geranylgeranyl pyrophosphate synthase; GGPP, geranylgeranyl pyrophosphate; PSY, phytoene synthase; PDS, phytoene desaturase; ZDS, f-carotene desaturase; CRTISO, carotenoid isomerase; LCY-ɛ, lycopene ɛ-cyclase; LCY β, lycopene β-cyclase; CHY-ɛ, ɛ-ring hydroxylase; CHY-β, β-carotene hydroxylase; VDE, violaxanthin de-epoxidase; ZEP, zeaxanthin epoxidase; NXS, neoxanthin synthase; CCD, carotenoid cleavage dioxygenase; NCED, 9-cis-epoxycarotenoids dioxygenase; ABA, abscisic acid; Or, orange.
Carotenoid content is regulated not only by biosynthesis but also by storage and degradation of carotenoids. Carotenoid cleavage dioxygenases (CCDs) lead to the enzymatic turnover of carotenoids into apocarotenoids, including plant hormones and volatiles (Cunningham and Gantt 1998, Giuliano 2014). Therefore, the content and composition of carotenoids can be influenced by CCDs via cleavage-specific carotenoids (Walter and Strack 2011). The five 9-cis-epoxycarotenoids dioxygenases (NCEDs) generate the plant hormone abscisic acid (ABA), whereas strigolactones are synthesized by CCD7 and CCD8 (Auldridge et al. 2006). In addition, apocarotenoids such as pigments, aromatics and flavorants are generated by cleavage of carotenoids by CCD1 and CCD4 (Auldridge et al. 2006, Gonzalez-Jorge et al. 2013). In this review, we summarize our current knowledge of the regulation of biosynthesis, accumulation and catabolism of carotenoids in sweetpotato. Moreover, we discuss the prospects for metabolic engineering of carotenoids in terms of molecular breeding for higher levels of antioxidants and stress tolerance.
All plants contain high levels of carotenoids in their leaves, primarily in thylakoid membranes. Sweetpotato leaves contain four carotenoids: lutein (47.6% of total carotenoids), β-carotene (25.2%), violaxanthin (13.9%) and neoxanthin (9.6%) (Chen and Chen 1993). The carotenoid composition of sweetpotato leaves is very similar to that of other plant chloroplasts (Botella-Pavía and Rodríguez-Concepción 2006).
The variety of carotenoids in flowers and fruits gives them their yellow, orange and red colors. In contrast to other crops, sweetpotato accumulates natural pigments in its storage root. The skin, flesh and storage roots of various sweetpotato cultivars have a wide range of colors, including white, yellow, orange and purple (Bovell-Benjamin 2007). These natural colors are mainly determined by the relative quantities of carotenoids and anthocyanins. The intensity of the yellow or orange flesh color is directly correlated with the carotenoid contents (Takahata et al. 1993). The storage root is an excellent source of carotenoids because its major carotenoid is trans-β-carotene, which has the highest provitamin A activity among the carotenoids (Bovell-Benjamin 2007).
The carotenoids in the storage root of sweetpotato include β-carotene, β-crypthoxanthin, zeaxanthin, violaxanthin and other unknown carotenoids (Ishiguro et al. 2010, Kim et al. 2012, 2013b, 2014). β-carotene, a major component in yellow- and orange-fleshed sweetpotato, has many trans- and cis-forms, including β-carotene itself, 9Z-β-carotene, 13Z-β-carotene, β-carotene 5,8-epoxide, β-carotene 5,8;5′,8′-diepoxide (cis-isomer) and β-carotene 5,8;5′,8′-diepoxide (diastereomer). Recently, Maoka et al. (2007) reported several new carotenoids, ipomoeaxanthins A, B, C1 and C4, isolated from yellow-fleshed sweetpotato (cv. Benimasari). The ipomoeaxanthins have a 5,6-cis-5,6-dihydro-5,6-dihydroxy-β-end group, which might be derived from the corresponding 5,6-epoxy carotenoids by hydrolytic cleavage of an epoxide (Maoka et al. 2007). Ishiguro et al. (2010) analyzed the different colors of eight sweetpotato cultivars or breeding lines, and showed that the main carotenoids in five different yellow-fleshed sweetpotato are β-carotene 5,8;5′,8′-diepoxide (32–51%) and β-cryptoxanthin 5,8-epoxide (11–30%), whereas the content of β-carotene is less than 10%. The main carotenoid in orange-fleshed sweetpotato is β-carotene (80–92%), whereas other carotenoids constitute less than 2% of the total in three different cultivars (Ishiguro et al. 2010).
The carotenoid contents of plants are determined by carotenoid biosynthesis, and the regulation of the pathways involved has been extensively studied. Carotenoid biosynthesis genes such as GGPS, PSY, PDS, ZDS, CRTISO, LCY-ɛ, LCY-β, CHY-β and ZEP have been isolated and characterized in sweetpotato (Table 1) (Chen et al. 2015, Kim et al. 2012, 2013b, 2014).
| Gene name | GenBank accession no. | Cultivar | Reference |
|---|---|---|---|
| GGPS | HQ828090 | Shinhwangmi | Unpublished |
| KC954600 | Nongdafu 14 | Chen et al. (2015) | |
| PSY | JX393305 | Shinhwangmi | Park et al. (2016) |
| KM434312 | Tainung 66 | Unpublished | |
| PDS | HQ828091 | Shinhwangmi | Unpublished |
| ZDS | HQ828088 | Shinhwangmi | Kim et al. (2012) |
| CRTISO | JX393307 | Shinhwangmi | Kim et al. (2013a) |
| LCY-ɛ | HQ828093 | Shinhwangmi | Kim et al. (2013b) |
| Unnotified | Nongdafu 14 | Yu et al. (2013) | |
| LCY-β | JX393306 | Shinhwangmi | Kim et al. (2014) |
| CHY-β | HQ828095 | Shinhwangmi | Kim et al. (2012) |
| ZEP | HQ828089 | Shinhwangmi | Kim et al. (2012) |
| CCD1 | KF730658 | Shinhwangmi | Unpublished |
| KM973213 | Shinzami | Unpublished | |
| CCD4 | JX393308 | Shinhwangmi | Unpublished |
| KM973214 | Shinzami | Unpublished | |
| Or | HQ828087 | Shinhwangmi | Kim et al. (2013a) |
GGPS; geranylgeranyl pyrophosphate synthase, PSY; phytoene synthase, PDS; phytoene desaturase, ZDS; zeta-carotene desaturase, CRTISO; carotenoid isomerase, LCY-ɛ; lycopene epsilon-cyclase, LCY-β; lycopene beta-cyclase, CHY-β; beta-carotene hydroxylase, ZEP; zeaxanthin epoxidase, CCD; carotenoid cleavage dioxygenase, Or; orange.
GGPP is an important metabolic intermediate synthesized by GGPS from IPP and DMAPP. GGPP serves as a precursor of not only carotenoids but also tocopherols, gibberellins, chlorophylls and other diterpenoids (Lange and Ghassemian 2003, Okada et al. 2000). Overexpression of HaGGPS from sunflower increases the total carotenoid contents of Arabidopsis, tobacco and dandelion, as well as stimulating growth (Tata et al. 2016).
Recently, the IbGGPS gene was isolated from storage roots of sweetpotato and transformed into Arabidopsis (Chen et al. 2015). In tobacco, an IbGGPS-GFP fusion protein is localized primarily to leaf chloroplasts. In IbGGPS-overexpressing Arabidopsis, the overall carotenoid content is elevated and the plants are more tolerant than control plants to osmotic stress. In transgenic plants, levels of carotenoids and transcripts of AtPSY1, AtPDS, AtZDS and AtBCH1 are elevated. In particular, α-branch carotenoids such as α-carotene and lutein are significantly upregulated in IbGGPS-overexpressing plants, whereas levels of β-crypthoxanthin and zeaxanthin, which belong to the β-branch, are reduced. The main carotenoid in calli and leaf of Arabidopsis is lutein, which is synthesized from α-carotenoids (Yuan et al. 2015). Because the main carotenoids in sweetpotato are of the β-branch, these data suggest that overexpression of IbGGPS in sweetpotato might increase the levels of α-branch carotenoids.
To increase commercial value or health benefits, specific flavor-related carotenoids have been produced by shifting metabolic flux. Two lycopene cyclases, LCY-β and LCY-ɛ, regulate the branch composition of carotenoids in various plants. Recent studies of the roles of LCY-ɛ and LCY-β in lutein synthesis in tomato, rice and Arabidopsis revealed that relative cyclase activities, as well as the synthesis of cyclic carotenoids, are affected by the expression levels of the two cyclase genes (Giorio et al. 2013, Yu and Beyer 2012). Thus, relative lycopene cyclase activities play a critical role in determining the β-carotene/α-carotene ratio, and thus in controlling pathway flux to carotenes with high provitamin A activity (Harjes et al. 2008).
LCY-ɛ is involved in the first step of the α-branch biosynthetic pathway (Farré et al. 2010). To increase the levels of β-branch–specific carotenoids in sweetpotato, we down-regulated the expression of IbLCY-ɛ in non-embryogenic calli of light orange-fleshed sweetpotato (cv. Yulmi) (Fig. 2A) (Kim et al. 2013b). The resultant transgenic calli exhibited an orange color due to their higher content of β-branch carotenoids such as β-cryptoxanthin, zeaxanthin, violaxanthin and β-carotene. The level of violaxanthin, the main carotenoid in control calli, was increased up to 54-fold in transgenic calli. Furthermore, the levels of β-cryptoxanthin, zeaxanthin and β-carotene were 163-, 27- and 21-fold higher than in control calli, respectively. Lutein was not detected in transgenic calli by HPLC analysis, but was present in control calli at 123 μg/g dry weight (DW) (Kim et al. 2013b). Consistent with this, down-regulation of LCY-ɛ in tobacco and canola results in elevated accumulation of β-branch–specific pathway (Shi et al. 2014, Yu et al. 2008). Interestingly, Yu et al. (2013) reported that overexpression of IbLCY-ɛ in tobacco also increased β-carotene contents. Therefore, to confirm the exact function of IbLCY-ɛ, other α- and β-branch carotenoids should be analyzed in tobacco plants.

Phenotypes of transgenic sweetpotato calli and plants in this review. A, IbLCY-ɛ-RNAi transgenic sweetpotato calli (Kim et al. 2013b). B, IbLCY-β-RNAi transgenic sweetpotato calli (Kim et al. 2014). C, IbCHY-β-RNAi transgenic sweetpotato calli (Kim et al. 2012). D, IbCHY-β-RNAi transgenic sweetpotato storage roots (Kang et al. 2016).
LCY-β is the key enzyme involved in the synthesis of α- and β-branch carotenoids, such as α-carotene and β-carotene, through the cyclization of lycopene. In tomato, down-regulation of LCY-β increases lycopene content without loss of other carotenoids, whereas overexpression of the gene in transgenic tomato causes a significant increase in the β-carotene content of fruit (Rosati et al. 2000). To decrease LCY-β function in sweetpotato, we isolated the LCY-β gene from sweetpotato and transformed an IbLCY-β-RNAi vector into non-embryogenic calli of a light orange-fleshed cultivar (cv. Yulmi) (Fig. 2B) (Kim et al. 2014). Transgenic calli had yellow to orange color, higher antioxidant activity and higher levels of total carotenoids (~2.8-fold) and β-branch carotenoids such as β-crytoxanthin (~8-fold), zeaxanthin (~13-fold), violaxanthin (~1.8-fold) and β-carotene (~2-fold) than control calli. Lycopene was detected in neither transgenic not control calli. Transgenic calli not only had elevated carotenoid content, but also exhibited higher tolerance to abiotic stress such as salt and drought (Kim et al. 2014).
CHY-β, a key regulatory enzyme in the β-branch of carotenoid biosynthesis, catalyzes hydroxylation of β-carotene to β-cryptoxanthin and β-cryptoxanthin to zeaxanthin. To increase the β-carotene content of sweetpotato by inhibiting the further hydroxylation of β-carotene, the effects of silencing IbCHY-β in the carotenoid biosynthetic pathway were evaluated (Kim et al. 2012). Silencing of IbCHY-β resulted in a yellow color and a marked increase (38-fold) in the level of β-carotene, making it the major cellular carotenoid (30% of the total carotenoids) (Fig. 2C). Regarding xanthophylls, the content of β-carotene downstream intermediates zeaxanthin and violaxanthin increased 4.3–15-fold. However, the relative levels of zeaxanthin and violaxanthin as proportions of total carotenoids decreased, from 7% to 2% and 51% to 26%, respectively (Kim et al. 2012). An IbCHY-β-RNAi construct was also introduced into a light orange-fleshed cultivar (cv. Yulmi) by Agrobacterium-mediated transformation. The transgenic plants exhibited an orange flesh color in their storage roots (Fig. 2D). The levels of total carotenoids and β-carotene in the storage roots of IbCHY-β-RNAi transgenic sweetpotato were about 2.4 and 16 times higher than those of control plants, respectively (Kang et al. 2016). Further characterization of IbCHY-β-RNAi sweetpotato, specifically regarding gene expression and abiotic stress tolerance, is underway.
Carotenoids are synthesized in the plastid, and mainly accumulate in the chloroplast and chromoplast (Howitt and Pogson 2006). Carotenoids in the chloroplast form a pigment–protein complex in the photosynthetic membrane along with chlorophyll-binding protein (Farré et al. 2010). However, carotenoids in the chromoplast form a carotenoid-lipoprotein–sequestering structure by combining with polar lipids and carotenoid-associated proteins. Consequently, these organelles maintain high levels of carotenoids (Lu and Li 2008). A study of a splicing mutation in the Orange (Or) gene in cauliflower revealed that carotenoid-sequestering structures are responsible for the accumulation of large amounts of carotenoids (Li et al. 2001, Lopez et al. 2008). Furthermore, in cauliflower the Or gene plays an important role in carotenoid accumulation by inducing chromoplast differentiation (Lopez et al. 2008, Lu et al. 2006, Lu and Li 2008). In fact, plastid fusion/translocation factor (Pftf), which is also involved in chromoplast differentiation, is strongly expressed in the curd of the Or splicing mutant. Introduction of the Or gene into wild-type cauliflower results in generation of a large chromoplast membrane containing elevated levels of β-carotene. In addition, overexpression of the Or gene in Arabidopsis resulted in an orange color with elevated carotenoid content (Lu et al. 2006).
Recently, the sweetpotato Orange (IbOr) gene was isolated from an orange-fleshed cultivar (cv. Shinhwangmi) (Kim et al. 2013a). IbOr is expressed at high levels in the leaves of sweetpotatoes with multiple flesh colors (white, orange and purple), but is highly expressed in storage roots only in orange-fleshed varieties. Two variants of IbOr [encoding IbOr-Wt and IbOr-Ins, which contains seven additional amino acids (KSPNPNL) inserted between residues 131–142 of IbOr-Wt] were transformed into non-embryogenic calli of light orange-fleshed sweetpotato (cv. Yulmi). The average total carotenoid contents in the IbOr-Ins and IbOr-Wt transgenic calli were approximately 13.37 and 3.97 times higher than those in control calli, respectively (Fig. 3A). Levels of all carotenoids were elevated in both IbOr-Wt and IbOr-Ins relative to control, except for lutein in IbOr-Wt. Furthermore, IbOr-Ins was transformed into purple-fleshed sweetpotato to produce both anthocyanin and carotenoids in the same storage root (Fig. 3B) (Park et al. 2015a). IbOr transgenic sweetpotatoes exhibit different color densities in individual transgenic lines. The IbOr-201 line is darker purple than the wild type (WT). IbOr plants have higher carotenoid levels (up to 7-fold) in their storage roots compared to WT plants. Overall, the carotenoid contents of IbOr plants are positively correlated with IbOr transcript levels.

Phenotypes and contents of total carotenoids in transgenic sweetpotato calli and plants overexpressing IbOr genes. A, Overexpression of IbOr-wt and IbOr-Ins in sweetpotato non-embryogenic calli (Kim et al. 2013a). B, Overexpression of IbOr-Ins in purple-fleshed sweetpotato (Park et al. 2015a).
In addition, transgenic alfalfa and potato plants overexpressing IbOr were generated and characterized (Cho et al. 2016, Goo et al. 2015, Wang et al. 2015). Transgenic alfalfa and potato plants exhibit elevated tolerance to various abiotic stresses including oxidative, salt and drought stress, as well as higher carotenoid content (Goo et al. 2015, Wang et al. 2015). Therefore, it is likely that IbOr plays a crucial role in the maintenance of photosynthesis, which confers stress tolerance in plants.
Zhou et al. (2015) reported that the AtOr protein regulates the PSY protein at a post-transcriptional level via direct protein–protein interactions of two proteins in the plastid. In Arabidopsis, the stability of PSY is highly elevated in AtOr-overexpressing lines, whereas in ator/ator-like double mutants the levels of PSY proteins are dramatically reduced without a change in PSY transcription (Zhou et al. 2015). Recently, we confirmed that the IbOr protein also interacts with IbPSY in the chloroplast and increases the stability of IbPSY protein via the holdase chaperone activity of IbOr (Park et al. 2016). Our findings thus suggest a novel mechanism of carotenoid accumulation and stress tolerance that is regulated by the molecular chaperone function of IbOr.
Carotenoids content is not only regulated by biosynthesis, but also by catabolism. CCDs are responsible for enzymatic turnover of carotenoids into apocarotenoids, including the plant hormone ABA and various volatiles (Cunningham and Gantt 1998, Giuliano 2014). Carotenoid accumulation is negatively regulated by CCD1 and CCD4 in chrysanthemum flowers and potato (Campbell et al. 2010, Tanaka and Ohmiya 2008, Zhou et al. 2011). Furthermore, down-regulation of CCD1 or CCD4 results in a significant increase in carotenoid levels in the seeds of Arabidopsis. These results suggest that both enzymes can utilize carotenoids as substrates (Gonzalez-Jorge et al. 2013). Purple-fleshed sweetpotato plants overexpressing IbOr contained high levels of CCD1, CCD4 and NCED transcripts (Park et al. 2015a), which might be involved in regulating carotenoid content homeostasis. The metabolites produced by CCDs in sweetpotato should be elucidated because they represent valuable metabolites and contribute to plant robustness. The regulation of genes involved in carotenoid catabolism in sweetpotato remains to be elucidated in detail. In addition, we expect that rational regulation of carotenoid catabolism-related genes such as IbCCD1 and/or IbCCD4 in sweetpotato might increase the accumulation of carotenoids more than overexpression of IbOr-Ins alone.
Sweetpotato is an attractive bioreactor for global sustainable development, in terms of both food security and human health. Metabolic engineering of useful components, including low–molecular weight antioxidants, has been facilitated by substantial advances in cloning of genes involved in carotenoid biosynthesis and accumulation, as well as techniques for transformation of sweetpotato. Carotenoid levels are among the most important qualities of sweetpotato cultivars because they contribute to stress tolerance in plants and also benefit human health. To date, carotenoid contents of sweetpotato have been increased by conventional breeding and molecular breeding, as well as by the use of transgenic technology to modify the expression of single genes. To further increase carotenoid content by genetic modification and metabolic engineering, however, it may be necessary to control the expression of multiple genes or transcription factor(s). Recently, ripening inhibitor (RIN) and RAP2.2 as transcription factors were reported to regulate PSY and/or PDS expression in tomato and leaves of Arabidopsis (Fujisawa et al. 2013, Welsch et al. 2007). This indicates that transcription factor(s) involved in carotenogenic genes might exist in sweetpotato. Furthermore, synthetic transcription factors and gene activation by genome editing tools, such as CRISPR-Cas9, can modify gene expressions (Kabadi and Gersbach 2014). We speculate that identification of transcription factor(s) and development of synthetic transcription factors in sweetpotato will be a major breakthrough to cope with late limiting carotenoid accumulation in sweetpotato. To this end, ‘omics’ studies such as genomics, transcriptomics, proteomics, metabolomics and phenomics might provide insight into the metabolic networks that control levels of carotenoids, volatiles and isoprenoids. Whole-genome sequencing of sweetpotato is currently being conducted by a consortium of China, Japan and Korea (Lee et al. 2012, Shirasawa et al. 2016).
In addition, transgenic sweetpotato with higher levels of various antioxidants could be generated by ‘gene stacking’. We successfully generated transgenic sweetpotato plants that produce both carotenoids and anthocyanin in the same storage root by overexpressing the IbOr gene, involved in accumulation of carotenoids, or the IbMYB1 gene, a transcription factor involved in the biosynthesis of anthocyanin (Park et al. 2015a, 2015b). Recently, we isolated and characterized five genes, including IbHPPD, for biosynthesis of tocopherols in sweetpotato (Ji et al. 2016). Dehydroascorbate reductase (IbDHAR), which converts inactive oxidized ascorbate to active reduced ascorbate, was isolated from sweetpotato and subsequently used for transformation (Wang et al. 2016). We are currently using a gene stacking approach to generate transgenic sweetpotato varieties that produce various antioxidants such as carotenoids, anthocyanins, ascorbate and tocopherols in the same storage root. Furthermore, a precise understanding of carotenoid biosynthesis and metabolism could contribute to metabolic engineering of sweetpotato for use in sustainable development to solve the global food, energy, health and environmental problems facing the Earth in the 21st century.
This work was supported by grants from the Systems & Synthetic Agrobiotech Center (PJ01106401), the Next Generation BioGreen 21 Project, Rural Development Administration, Korea, and the KRIBB initiative program.