2017 Volume 67 Issue 3 Pages 257-267
2017 Volume 67 Issue 3 Pages 257-267
Drought is a major constraint for sunflower (Helianthus annuus) production worldwide. Drought tolerance traits have been identified in the related wild species Helianthus argophyllus. This study was initiated to develop sunflower drought-tolerant genotypes by crossing cultivated sunflower with this species and analyze drought tolerance traits in the H. annuus and H. argophyllus populations, H. annuus intraspecific hybrids, and H. annuus × H. argophyllus interspecific hybrids along with the commercial hybrid Hysun-33 under three stress regimes: exogenous application of ABA, both by foliar spray and irrigation, and 5% PEG-induced osmotic stress. H. argophyllus populations had a significantly lower leaf area and higher water-use efficiency and leaf cuticular wax content under all treatments, and maintained a higher net photosynthetic rate and stomatal conductance under osmotic stress. Small leaf area and high cuticular waxes content of the wild species were, however, not inherited in interspecific hybrids which suggested for selection in F2 for these traits. Therefore, transgressive plants were selected in the F2 population to establish F3 plant progenies with silver-leafed canopy of H. argophyllus which showed higher achene yield under stress condition. These results are discussed with a view to using H. argophyllus to improve drought tolerance in cultivated sunflower.
Drought is a major yield-limiting factor for sunflower (Helianthus annuus L.) production in semi-aridregions (Rauf 2008, Rauf et al. 2015, Ravishankar et al. 1991). Oil and achene yield losses due to drought stress were reported in various parts of the world (Hussain et al. 2010, Jasinkas 1999, Kakar and Soomro 2001, Woli et al. 2014, Yin et al. 2014). Water shortage induces significant modifications in physiological and biochemical processes involved in biomass production, and modifies dry matter partitioning (Baloğlu et al. 2012, Fernández et al. 2012, Rauf and Sadaqat 2007, 2008a). Breeding for drought tolerance is consequently essential to reduce yield losses in sunflower in drought-prone areas (Adiredjo et al. 2014, Rauf et al. 2015). In order to identify possible sources of drought tolerance, cultivated sunflower breeding lines from diverse sources were evaluated on the basis of relative performance under drought stress (Rauf and Sadaqat 2008b). However, the narrow genetic base of cultivated breeding lines has limited the scope of these studies.
Some wild sunflower species have been reported as drought-tolerant species, and the introgression of traits from these species is expected to increase drought tolerance in cultivated breeding lines (Saucă et al. 2014). Among the related species, Helianthus argophyllus L. was identified as particularly drought-tolerant (Saucă et al. 2014).
Based on this information, we initiated studies to identify possible traits of drought tolerance in H. annuus, H. argophyllus, and their interspecific progenies, by using abscisic acid to induce drought-like symptoms and by irrigating plants with a solution of polyethylene glycol (PEG-6000) to create osmotic stress. Genotypic variations were previously observed in sunflower plants submitted to exogenous application of abscisic acid (ABA) for production of ABA (Ouvrard et al. 1996) and maintenance of relative water content and yield (Hussain et al. 2010), while the use of PEG has proven to be efficient to screen for tolerance to osmotic stress in this crop (Khalil et al. 2016). In the present study, the traits assessed included morphological traits (leaf area, plant height, and biomass), physiological characters (net photosynthetic rate, stomatal conductance, excised-leaf water loss, epicuticular wax content, and leaf silicium content), and plant growth regulator (abscisic acid and zeatin) contents. These traits are relevant to drought tolerance and, therefore, could indicate the type of resistance in sunflower genotypes. Research was carried out with the hypothesis that drought stress induced a significant impact on biomass and leaf gas exchange traits through the production of stress-induced plant growth regulator (abscisic acid). Therefore, application of ABA could induce stress-like symptoms in plants and could be used to evaluate plant responses to drought stress in plant populations differing for drought resistance. The objectives of the study were to explore useful drought-tolerant plant traits in wild species (Helianthus argophyllus L.), a comparison of the wild and cultivated population for traits related to drought, and their transmissibility to interspecific hybrids and derived breeding lines.
Three H. argophyllus accessions (ARG-1805, ARG-1802, and ARG-1806), introduced from the USDA germplasm collection, two cytoplasmic male sterile lines (CMS), two male fertility restorer lines, and a commercial hybrid Hysun-33 of cultivated sunflower (H. annuus) were used in this study. H. argophyllus accessions had drought-resistance traits which could be transferred to the drought-susceptible CMS lines. The two lines (CMS-14 and CMS-20) were crossed with H. argophyllus accessions to develop six (F1) interspecific hybrids (Table 1). Moreover, these lines were also crossed with the two male fertility restorer lines (R-12 and R-18) to yield four intraspecific (F1) hybrids (Table 1). The commercial drought-tolerant hybrid Hysun-33 was used as a control. Details of plant materials are shown in Table 1.
| Parental lines | Origin | Crosses |
|---|---|---|
| argophyllus ARG-1802 | Wild population Texas, United States | CMS-14 × argophyllus 1802 CMS-20 × argophyllus 1802 |
| argophyllus ARG-1805 | Wild population Texas, United States | CMS-14 × argophyllus 1805 CMS-20 × argophyllus 1805 |
| argophyllus ARG-1806 | Wild population Texas, United States | CMS-14 × argophyllus 1806 CMS-20 × argophyllus 1806 |
| CMS-14 | Female breeding line developed by corresponding author | CMS-14 × R-12 CMS-14 × R-18 |
| CMS-20 | Female breeding line developed by corresponding author | CMS-20 × R-12 CMS-20 × R-18 |
| R-12 | Restorer breeding line developed by corresponding author | CMS-14 × R-12 CMS-20 × R-12 |
| R-18 | Restorer breeding line developed by corresponding author | CMS-14 × R-18 CMS-20 × R-18 |
| Hysun-33 | Commercial hybrid | |
| UCA-B11 | F3 selected breeding line | CMS-14 × argophyllus 1802 |
| UCA-B27 | F3 selected breeding line | CMS-14 × argophyllus 1802 |
| UCA-B57 | F3 selected breeding line | CMS-20 × argophyllus 1805 |
| UCA-B58 | F3 selected breeding line | CMS-14 × argophyllus 1806 |
| UCA-B60 | F3 selected breeding line | CMS-20 × argophyllus 1805 |
| UCA-B66 | F3 selected breeding line | CMS-20 × argophyllus 1802 |
The heads (reproductive capitula) of F1 plants were self-pollinated to produce the F2 seed. F2 seeds obtained from 6 interspecific hybrids were germinated in large polythene bags containing 30 kg of soil on 8 August, 2015. There were 150 F2 plants for each hybrid. Plants were moved to the field 45 days after emergence. Single plant selection was carried out in the field on the basis of multiplicity of traits such as silver-leafed canopy color (intense hairiness and smaller leaf), high cuticular waxes, and single-headed growth habit with early to medium maturity. Green-leafed canopy, with broad leafed and single-head plants were also selected to make a comparison for seed yield. Sunflower heads of selected F2 plants were self-pollinated to obtain the F3 seed, which was later used for raising the F3 progenies. To achieve this, the heads were covered with a netting bag to avoid pollen contamination of neighboring plants. Each plant gave rise to a single plant progeny.
Experimental conditions for comparison of interspecific hybrids and parents with standard commercial and experimental hybridsAll the plant material, including parental lines and hybrids, were grown in a growth chamber at the Plant Breeding & Genetics Department, College of Agriculture, University of Sargodha, Pakistan. Two seeds of each genotype were germinated in boxes (15 × 15 × 15 cm) containing 8.5 kg of field soil: sand: silt. Half-strength Hoagland solution (50 ml) after a 7-day interval was used to irrigate all boxes to raise the fertility of the soil and to provide essential plant nutrients. There were 12 boxes per genotype. Temperature was maintained at 25 ± 2°C, while humidity was maintained at 40%, and light intensity at 650 μmol m−2s−1 in all treatments. Photoperiod was maintained for 16 hours by filament bulbs installed within the growth chamber. Treatments included a control (treatment T0) and three stress treatments (Fig. 2). Control plants (treatment T0) were regularly irrigated to field capacity (25% by weight of soil) to maintain optimum moisture contents throughout the growth cycle. Drought-like symptoms were induced through foliar (treatment T1) and irrigational application (treatment T2) of 8 μmol of 50 ml (±) abscisic acid per box (Sigma-Aldrich, USA) from germination (Fig. 2) and at 4-day intervals. Foliar treatment (treatment T1) was applied by spraying 50 ml of 8 μmol (±) abscisic acid per box with a manual hand sprayer at 4-day intervals. Osmotic stress was generated by irrigating plants with a solution containing 50 g L−1 of polyethylene glycol (PEG-6000) according to Khalil et al. (2016) (treatment T3).
Growth conditions for raising F3 plant progeniesSelected F3 lines of sunflower, i.e., silver-leafed canopy (high cuticular waxes, hairiness, and smaller leaves, Fig. 1) and green-leafed canopy (large leaf with low cuticular waxes and hair intensity, Fig. 1) were grown in large polythene bags (50 × 20 cm) containing 30 kg of a soil mixture containing an equal volume of sand and silt. Soil structure was improved by adding 5% farmyard manure. Breeding lines were sown on 15 February, 2016 under controlled conditions (27°C ± 2°C; 16-h photoperiod; PPFD = 650 μmol m−1s−1). Two seeds of each breeding line were sown within each polythene bag and thinned to a single plant after germination. The fertility status of pots was raised by applying di-ammonium phosphate fertilizer (3 g pot−1) after a 30-day interval. No visual signs of disease or insect attack were observed on the plants. The experiment was carried out in a completely randomized design with two factors: (1) breeding lines differing for canopy traits and (2) stress treatment and a control. There were 20 plants treatment−1 and three replications. During the entire crop growth cycle, control plants were irrigated to field capacity to maintain moisture close to the field capacity (FC; 27%, w/w) which was measured through a gravimetric method (Reynolds 1970). However, drought stress was applied by irrigating the plant to 65% of the FC during the entire growth period.

(A) Silver-leafed canopy of Helianthus argophyllus L cv. 1802, (B) Interspecific F1 (Helianthus annuus L. × Helianthus argophyllus), (C) B-58 (F3 progeny) derived from (Helianthus annuus L. × Helianthus argophyllus), (D) B-63 (F3 progeny) derived from (Helianthus annuus L. × Helianthus argophyllus).
Response of genotypes to various treatments of drought. (A) Inbred line R-12, (B) CMS-14 × argophyllus-1802, (C) CMS-20 × argophyllus-1802, (D) CMS-20 × R-12 response to 5% polyethylene glycol (treatment T3), irrigational application of 8 μmol of 50 ml (±) abscisic acid (treatment T1), foliar application of 8 μmol of 50 ml (±) abscisic acid (treatment T1), and control (treatment T0) from left to right.
Leaf area (LA cm2) was assessed using a CI-302 leaf area meter (CID-Bioscience, Camas, USA). Fresh plant biomass (g) (shoot + leaves) (PB) was measured on a digital balance. Achene yield (g) was determined by harvesting the seed of each plant separately and measuring with a digital balance. Days to initiation of flowering was measured by tagging all the plants and regularly observing the formation of buds (R1).
Leaf gas exchange parameters, i.e., net photosynthesis rate (PN, μmol m−2s−1), stomatal conductance (gs, mmol m−2s−1), and transpiration rate (E, μmol m−2s−1) were determined on 26-day-old leaves from the top of the canopy at the 2nd node using a hand-held photosynthesis system, CI-340 (CID-Bioscience Camas, USA). Temperature was maintained at 25 ± 2°C, while humidity was maintained at 40%, and light intensity at 650 μmol m−2s−1 in all treatments. Water-use efficiency (WUE) was the ratio of PN to E (Pn E−1 mmol mol−1).
Excised-leaf water loss (ELWL) was assessed according to Dhanda et al. (1998), 45 days after plant emergence, on fully expanded leaves (14-day-old leaves) at the 2nd node from the top of the canopy. Leaves were removed from the plants and their fresh mass was measured immediately on an analytical balance. Leaves were kept at 25°C for six hours to determine wilting-leaf mass. Finally, leaves were oven dried at 70°C for 24 hours to determine dry mass. Excised-leaf water loss was calculated as (fresh-leaf mass – wilted-leaf mass)/(fresh-leaf mass – dry-leaf mass).
Epicuticular waxes were determined by the method of Ebercon et al. (1977) at 45 days after plant emergence, on expanded (15-day-old) leaves from the top of the canopy. Leaf discs of known size (30 cm2) were dipped in 15 ml pre-distilled chloroform at 25°C for 15 sec. The extract was filtered, chloroform was evaporated, and 5 ml of reagent was added to each sample. The reagent was prepared by dissolving 20 g potassium dichromate in 40 ml distilled water. The solution was mixed and further heated for 30 min in a concentrated 1 liter of H2SO4. Reagent was added to each sample after cooling to room temperature (25°C), and 12 ml of distilled water was added to each sample. The samples were kept at room and color change (various shades of gold to yellow) was awaited. Optical density (590 nm) of the sample was measured using a spectrophotometer (UV 2600, Shimadzu, Japan). The standards were prepared by obtaining cuticular waxes from bulked samples of wild sunflower leaves dipped in chloroform. Bulked waxes were used as standard, which were divided among three replicates (300 mg per sample) with 5 ml reagent (20 g potassium dichromate + 40 ml distilled water + concentrated 1 liter H2SO4) added to each of the replicates. The standards were further prepared by mixing stock solution of 0.1 ml, 0.5 ml, 1 ml, 2 ml, 3 ml, and 5 ml to get final concentrations of 1 μg g−1, 5 μg g−1, 10 μg g−1, 20 μg g−1, 30 μg g−1, and 50 μg g−1, respectively, of standard solution.
Leaf silicium content (μg g−1) was determined according to Dai et al. (2005). Leaf samples of each line and hybrids were oven dried at 60°C for at least 7 days. Dried samples were ground and passed through a sieve of 60-mesh. The samples were again dried at 60°C for 48 hours. 100-mg of sample was poured into polyethylene tubes and 3 ml of 50% NaOH was added to each sample. All tubes containing leaf samples were covered with loose plastic caps. Tubes were vortex and afterward autoclaved at 121°C for 20 min. The volume of the plastic tubes was adjusted to 50 ml through ddH2O. Then, 1 ml sample was added to the volumetric flask to which 30 ml of 20% acetic acid and 10 ml of ammonium molybdate (54 g L−1, pH 7.0) was further added. Next, 5 ml of 20% tartaric acid was added to the tube after a 5-min interval followed by 1 ml reducing solution. Reducing solution was prepared by mixing solution A (2 g of Na2SO3 and 0.4 g of 1-amino-2-naphthol-4-sulfonic acid in 25 ml of ddH2O) and solution B (25 g of NaHSO3 in 200 ml of ddH2O). The volume was adjusted to 250 ml with deionized H2O and stored in a plastic bag in the dark. The volume was adjusted to 50 ml by 20% acetic acid. Measurement was done at 650 nm on a spectrophotometer (UV 2600). Standards were prepared by taking 1 g ultrapure SiO2 and slowly heating it to 1000°C in a muffle furnace. The temperature was stabilized at 1000°C for 1 hour, and 0.1 g of treated SiO2 was further transferred to a nickel crucible and slowly heated to 1000°C after adding 2 g of Na2CO3 to form a lucent melt. The crucible was taken out from the furnace and 5 ml of boiling ddH2O was added to the crucible. The lucent melt was transferred to a plastic bottle which was further dissolved by adding 150 ml of ddH2O. Finally, the volume was raised to 1000 ml in the volumetric flask and the solution was transferred to a plastic bottle. The final concentration of stock solution was 0.1 mg ml−1 of SiO2.
Each plant sample (leaves) (2 g) was ground in a 60-ml solution (methanol: chloroform: 2N ammonium hydroxide, 12:5:3 v/v/v). Obtained extracts were purified and evaporated using Si-C18 columns (Perkin Elmer, Wellesley, MA, USA). Cytokinins (zeatin + zeatin riboside) were determined through the standard ([2H5]Z, [2H5]ZR) (Blagoeva et al. 2004). The method of Dobrev et al. (2005) was followed to determine ABA content through two-dimensional HPLC (Dobrev et al. 2005).
Statistical analysisAll traits were subjected to the analyses of variance under completely randomized design with a factorial arrangement with three replications for the estimation of significance of genotypes, contrasting water stress regimes, and genotypes × water stress regimes. Traits showing significant variation were further used for the comparison of means among genotypes across all treatments. The significance of mean was determined through LSD test. Phenotypic correlations were calculated using computer-based software (Minitab). The significance of the correlations was determined through Pearson correlation critical values for one tailed test at 16 (n-2) degrees of freedom. Percentage increase or decrease in the value was calculated with the following formulas:
where V2 = value in any treatment, V1 = value in control. Drought resistance index was determined according to Fischer and Maurer (1978). The formula for calculation of drought resistance index is:
Drought resistance index = (YS/YN)/(MS/MN); where YS and YN were the seed yield under stress and normal conditions, respectively, and MS and MN are the mean seed yields of all lines under stress and normal conditions, respectively.
Analyses of variance showed that differences (P ≤ 0.05) between the groups (interspecific F1 hybrids, intraspecific F1, parental lines, and commercial hybrids) were a major source of variation when compared to within-group differences. Therefore, values of all the genotypes within-group were shown as averages of all the genotypes within each group.
PB was reduced to 12%, 8%, and 34% in treatments T1, T2, and T3, respectively, compared to T0 (Fig. 3a). Hysun-33 showed the highest plant biomass under T0 (6.81 g) and T3 (5.07 g) and H. argophyllus had the lowest in all treatments. H. argophllus showed a significant (P ≤ 0.05) decrease in PB under stress treatments T1 (0.45 g), T2 (0.93 g), and T3 (0.68 g) and compared to T0 (2.25 g). Interspecific hybrids had similar (P ≥ 0.05) plant biomassas Hysun-33 in T0 (6.36 g), T1 (5.75 g), T2 (5.42 g), and T3 (4.21 g).
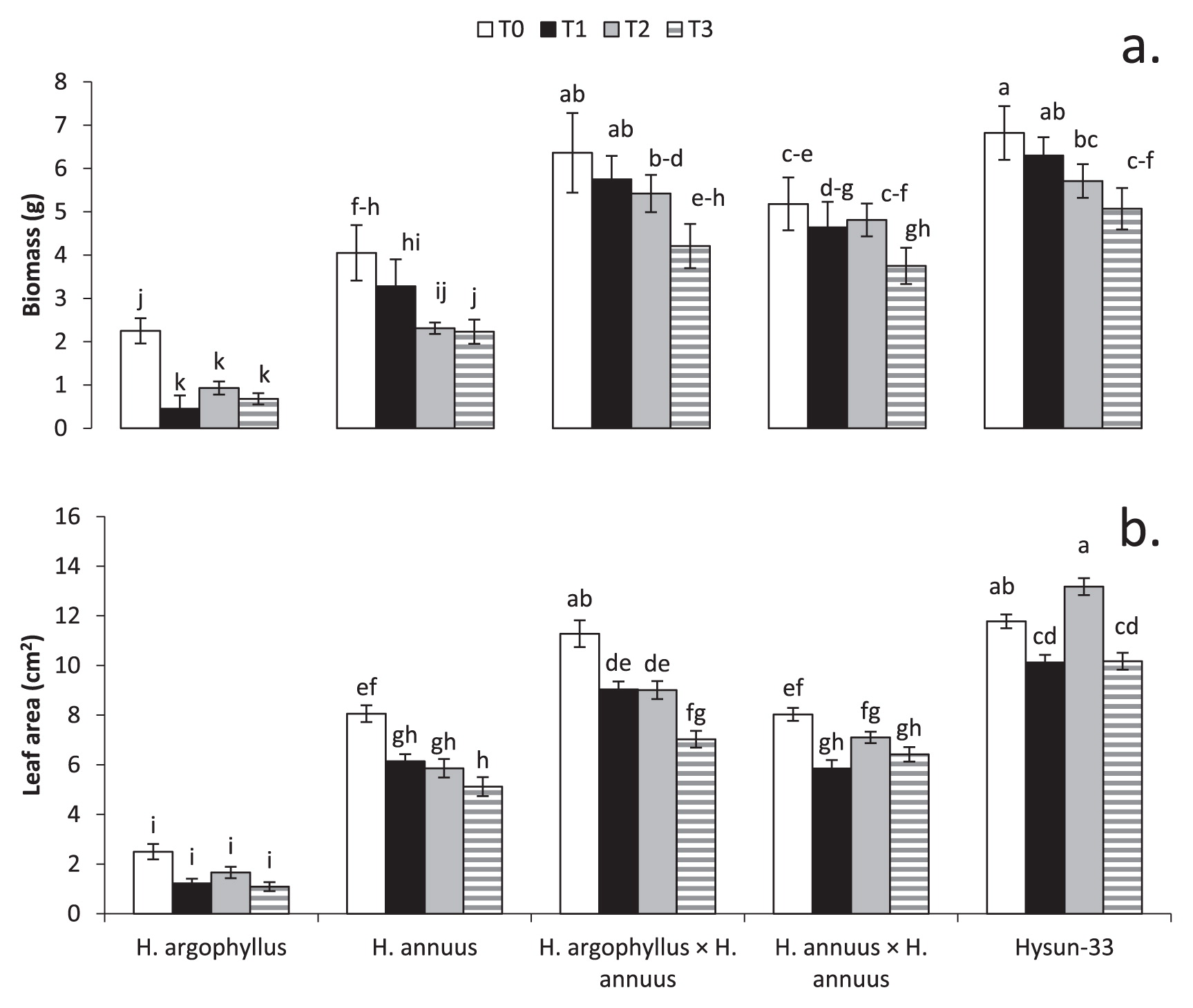
Means of biomass and leaf area (cm2) in the different groups of genotypes with application of 8 μmol of ABA through foliar spraying (T1) or irrigation (T2) under osmotic stress (T3) and control conditions (T0). Means showing similar letters are statistically not significant (P ≥ 0.05). H. argophyllus was the mean of 3 accessions (1802, 1805, and 1806), H. annuus was the mean of four inbred lines (CMS-14, CMS-20, R-12, and R-18), H. annuus × H. argophyllus was the mean of six interspecific hybrids, H. annuus × H. argophyllus was the mean of four intraspecific hybrids, and Hysun-33 was a commercial hybrid.
LA decreased by 24%, 11%, and 24% in T1, T2, and T3, respectively, compared to T0 (Fig. 3b). Hysun-33 showed the highest LA (13.18 cm2) in the T2 treatments which was statistically similar (P ≥ 0.05) to Hysun-33 (11.78 cm2) and interspecific hybrids (11.28 cm2) leaf area in T0. H. argophyllus showed the lowest LA in all treatments (T0 = 2.5 cm2) but showed a non-significant decrease (P ≥ 0.05) in the LA of all treatments.
There was a significant decrease in PN of all genotypes due to stress treatments (Fig. 4a). PN experienced a decrease of 30%, 57%, and 52% in T1, T2, and T3, respectively. Hysun-33 showed the highest PN (6.64 μmol m−2s−1) in T0. PN of this hybrid was drastically (P ≤ 0.05) decreased in T1, T2, and T3. H. argophyllus population tends to maintain PN in all treatments except T2 (1.85 μmol m−2s−1) which showed a significant (P ≤ 0.05) decrease when compared with T0 (2.37 μmol m−2s−1), T1 (2.71 μmol m−2s−1), and T3 (2.46 μmol m−2s−1). Interspecific hybrids showed the highest PN in T3 (3.25 μmol m−2s−1) among stress treatments.
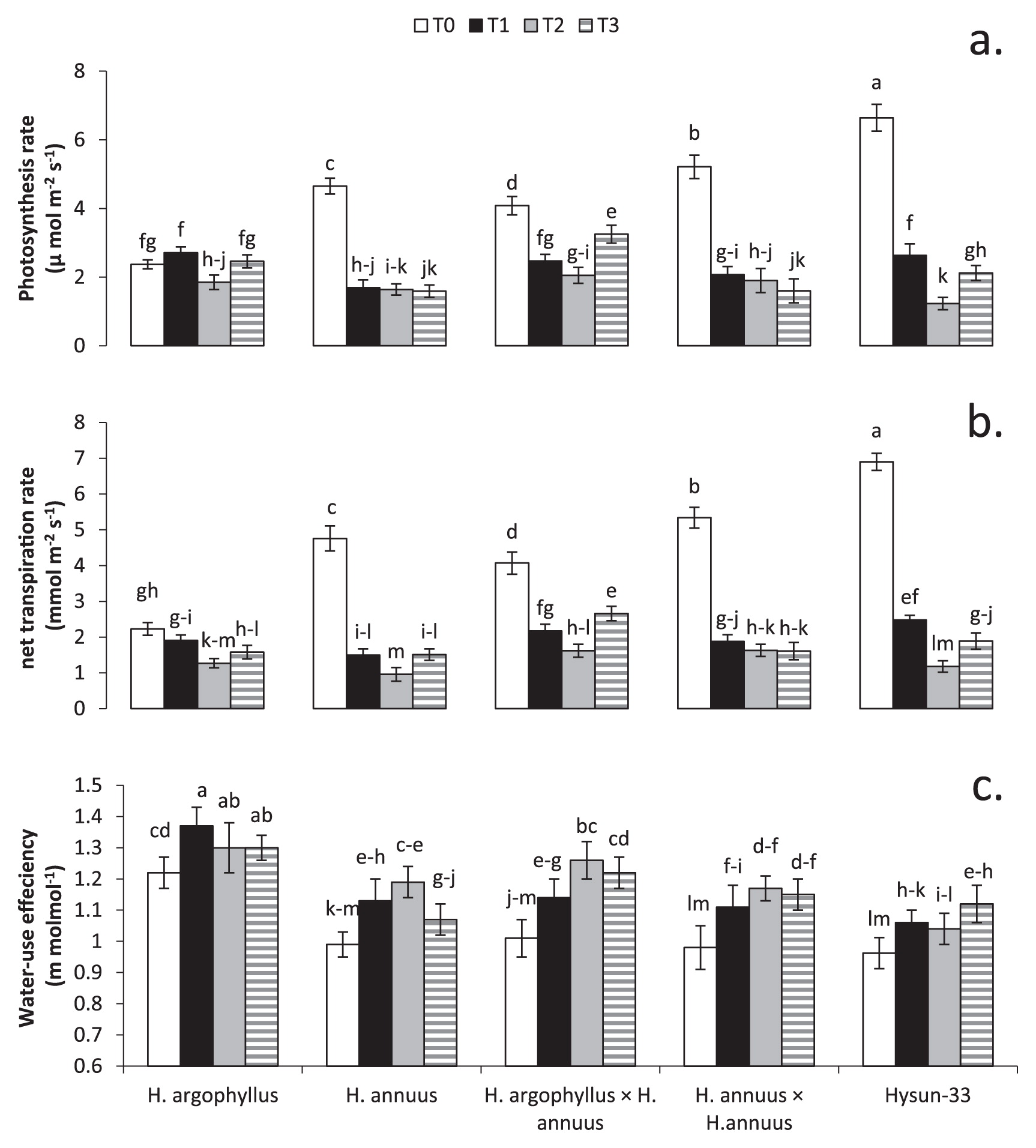
Means of net photosynthesis, transpiration, and water-use efficiency in the different groups of genotypes with application of 8 μmol of ABA through foliar spraying (T1) or irrigation (T2) under osmotic stress (T3) and control conditions (T0). Means showing similar letters are statistically not significant (P ≥ 0.05). H. argophyllus was the mean of 3 accessions (1802, 1805, and 1806), H. annuus was the mean of four inbred lines (CMS-14, CMS-20, R-12, and R-18), H. annuus × H. argophyllus was the mean of six interspecific hybrids, H. annuus × H. argophyllus was the mean of four intraspecific hybrids, and Hysun-33 was a commercial hybrid.
There was decrease of 58%, 69%, and 62% in the transpiration rate (E) due to treatments T1, T2, and T3, respectively (Fig. 4b). Hysun-33 (6.64 μmol m−2s−1) showed the highest E, followed by intraspecific hybrids (5.21 μmol m−2s−1), inbred lines (4.65 μmol m−2s−1), and interspecific hybrids (4.08 μmol m−2s−1) in T0. H. argophyllus populations tend to maintain E in all treatments except T2 (1.27 μmol m−2s−1) which showed a significant decrease when compared with T0 (2.23 μmol m−2s−1). WUE increased by 13%, 17%, and 13% in T1, T2, and T3, respectively (Fig. 4c). H. argophyllus showed the highest WUE in all treatments (T0 = 1.22, T1 = 1.37, T2 = 1.30, and T3 = 1.30). Interspecific hybrids showed WUE efficiency similar (P ≥ 0.05) to H. argophyllus in T2 (1.22).
Silicium content increased by 124%, 90%, and 96% in T1, T2, and T3, respectively, compared to T0 (Fig. 5a). Intraspecific hybrids (755.8 μg g−1) had the highest silicium content in T1. H. argophyllus and interspecific hybrids showed the lowest silicium content in all treatments. Leaf cuticular waxes decreased as a result of the ABA treatments and osmotic stress. There was a decline of 46%, 53%, and 51% in T1, T2, and T3, respectively, when compared to T0 (Fig. 5b). H. argophyllus showed the highest leaf cuticular waxes in all treatments (T0 = 22.92, T1 = 5.67, T2 = 5.65, and T3 = 9.27 μg leaf area−1). All genotypes showed similar (P ≥ 0.05) LW in all treatments except H. argophyllus.
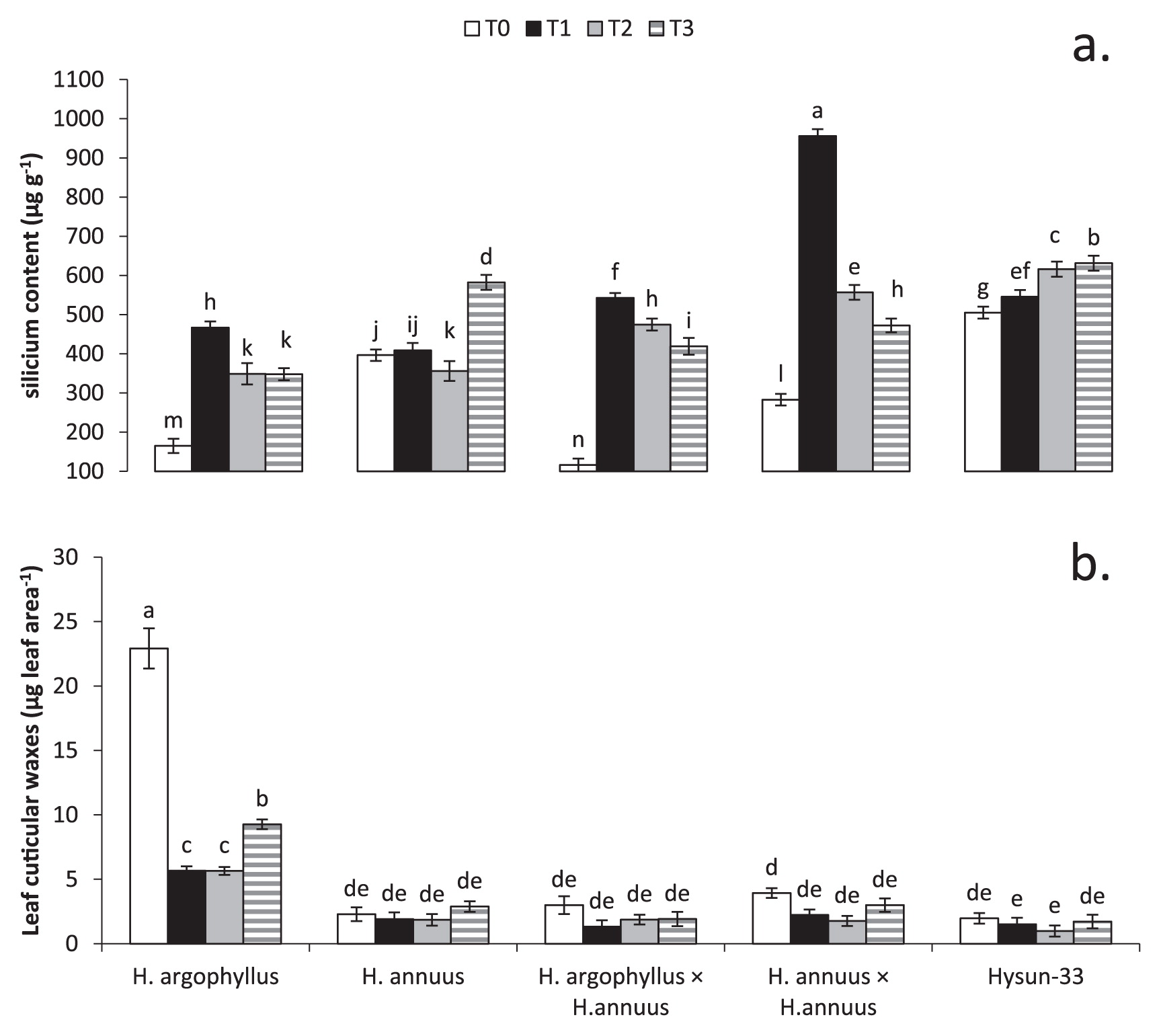
Means of leaf silicon contents and cuticular waxes in the different groups of genotypes, with application of 8 μmol of ABA through foliar spraying (T1) or irrigation (T2) under osmotic stress (T3) and control conditions (T0). Means showing similar letters are statistically not significant (P ≥ 0.05). H. argophyllus was the mean of 3 accessions (1802, 1805, and 1806), H. annuus was the mean of four inbred lines (CMS-14, CMS-20, R-12, and R-18), H. annuus × H. argophyllus was the mean of six interspecific hybrids, H. annuus × H. argophyllus was the mean of four intraspecific hybrids, and Hysun-33 was a commercial hybrid.
Abscisic acid (ABA) content increased by 67%, 133%, and 47% in T1, T2, and T3, respectively, compared to T0 (Fig. 6a). The highest ABA content was noted in interspecific hybrids (620.9 μg g−1), followed by Hysun-33 (606.2 μg g−1) in T2. Interspecific hybrids showed higher ABA contents than intraspecific hybrids in T2.
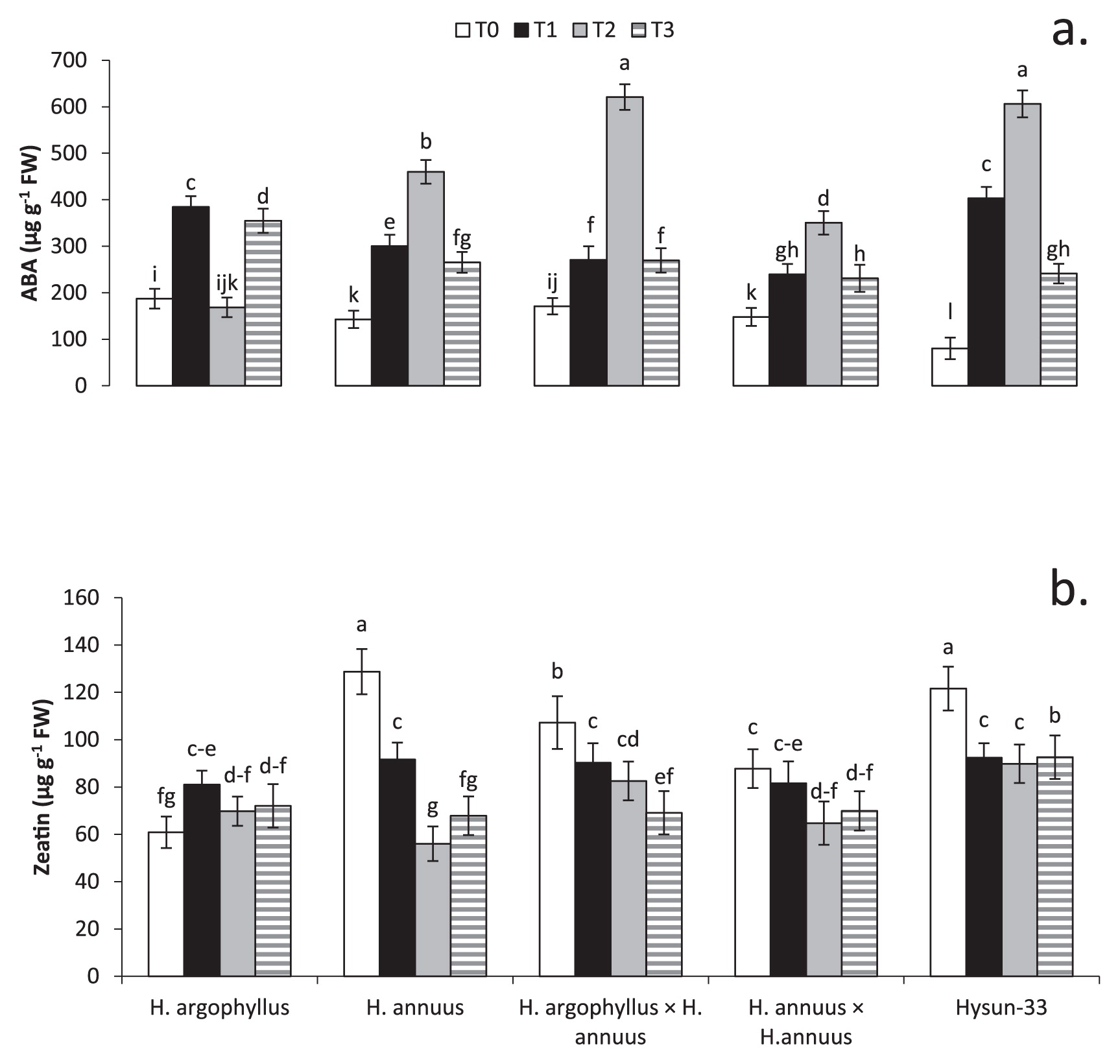
Means of ABA and zeatin contents by fresh weight in the different groups of genotypes with application of 8 μmol of ABA through foliar spraying (T1) or irrigation (T2) under osmotic stress (T3) and control conditions (T0). Means showing similar letters are statistically not significant (P ≥ 0.05). H. argophyllus was the mean of 3 accessions (1802, 1805, and 1806), H. annuus was the mean of four inbred lines (CMS-14, CMS-20, R-12, and R-18), H. annuus × H. argophyllus was the mean of six interspecific hybrids, H. annuus × H. argophyllus was the mean of four intraspecific hybrids, and Hysun-33 was a commercial hybrid.
Zeatin content decreased by 12%, 28%, and 26% in T1, T2, and T3, respectively, compared to T0 (Fig. 6b). Inbred lines (128.76 μg g−1), followed by Hysun-33 (121.6 μg g−1) and interspecific hybrids (107.22 μg g−1), showed the highest zeatin content in T0. All genotypes showed a significant (P ≤ 0.05) decrease in zeatin contents, except T1 (81.58 μg g−1) of inbred lines, when compared with T0 (87.75 μg g−1). However, the zeatin content of H. argophyllus was maintained in all treatments except T1 (81.00 μg g−1) in which it showed a significant (P ≤ 0.05) increase when compared with T0 (60.88 μg g−1).
Mean performance of F3 progenies derived from Helianthus annuus × Helianthus argophyllusMean achene yield of F3 progenies (Helianthus annuus × Helianthus argophyllus) selected for silver-leafed canopy was determined and compared with the parents and progenies selected for green large-leafed canopy (Table 2). Results showed that progenies selected for silver-leafed canopy (high cuticular waxes and intense leaf and stem hairiness) showed higher seed yield under stress condition when compared with parents and F3 progenies with green-leafed canopy (Table 2). Progenies B-66 (green-leafed) and B-57 (silver-leafed) showed the highest seed yield under non-stress condition. Progenies B-58 and B-57 (silver-leafed) showed the highest seed yield under drought condition (Table 2).
| Lines | Seed yield plant−1 (g) | Drought resistance index | Days to flower initiation | ||
|---|---|---|---|---|---|
| Non-stress | Drought | Non-stress | Drought | ||
| UCA-B11 | 22.34 ± 2.19 | 18.32 ± 1.63 | 1.15 | 60.2 ± 1.36 | 58.31 ± 2.13 |
| UCA-B27 | 28.17 ± 3.51 | 21.46 ± 0.83 | 1.07 | 56.37 ± 1.08 | 57.49 ± 2.32 |
| UCA-B57 | 34.39 ± 1.64 | 26.15 ± 1.12 | 1.07 | 58.24 ± 1.16 | 57.10 ± 1.34 |
| UCA-B58 | 30.17 ± 2.19 | 27.15 ± 0.61 | 1.27 | 54.31 ± 2.24 | 54.68 ± 2.12 |
| UCA-B60 | 30.01 ± 3.12 | 19.51 ± 1.19 | 0.92 | 45.34 ± 1.13 | 41.52 ± 1.07 |
| UCA-B66 | 35.32 ± 0.96 | 20.03 ± 0.87 | 0.80 | 41.62 ± 3.16 | 35.12 ± 2.42 |
| CM-14 | 18.16 ± 0.61 | 9.62 ± 1.23 | 0.75 | 36.72 ± 2.34 | 32.23 ± 1.67 |
| CM-20 | 16.41 ± 0.73 | 10.30 ± 0.92 | 0.88 | 39.34 ± 1.82 | 34.55 ± 1.14 |
Silver-leafed canopy with small leaf area (B-11 and B-27 and B-57 and B-58); Green-leafed canopy with large leaf area (B-60 and B-66).
Comparative decrease of achene yield under stress and non-stress condition has been used for the determination of drought resistance index. A line was considered resistant when it has a drought resistance index value greater than unity (Fischer and Maurer 1978). All silver-leafed F3 progenies had a drought resistance index value greater than unity and were characterized as resistant (Table 2).
Selected F3 progenies had higher values than parents for days to initiate flowering. Silver-leafed progenies showed the highest values for days to initiate flowering (Table 2). Therefore, selection pressure may be kept to select “early” lines in advanced generations of silver-leafed canopy.
Correlation analysis of plant traitsExcised-leaf water content (ELWC) was negatively related to Si, PN, and gS, and positively correlated with LW under control condition. It was positively correlated with PN, gS, and E in T2 (Table 3). LA was positively correlated with PB and zeatin content in all treatments except in T1 and the control, respectively, for both traits. It was negatively related to LW and ABA in all treatments except T3 and the control, respectively, and positively related to E in the control and T2. It showed a negative relationship with WUE (Table 3). PB was negatively related to LW and ABA in all treatments except T1 and the control. It was positively related to zeatin in the control and T1 and showed a positive relationship with E in T2 and T3. PB was also negatively correlated with WUE in the control.
| Traits | Treatment | ELWC | LA | PB | Si | LW | PN | gS | ABA | Zeatin | E |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LA | T0 | 0.11NS | |||||||||
| T1 | 0.24NS | ||||||||||
| T2 | 0.29NS | ||||||||||
| T3 | 0.35NS | ||||||||||
| PB | T0 | −0.17NS | 0.52* | ||||||||
| T1 | −0.13NS | 0.26NS | |||||||||
| T2 | 0.28 NS | 0.79* | |||||||||
| T3 | 0.16 NS | 0.69* | |||||||||
| Si | T0 | −0.82** | 0.00 | 0.07NS | |||||||
| T1 | −0.32NS | 0.08NS | −0.06NS | ||||||||
| T2 | −0.26NS | 0.26NS | 0.12NS | ||||||||
| T3 | −0.30NS | −0.15NS | −0.13NS | ||||||||
| LW | T0 | 0.39* | −0.69* | −0.82* | −0.39* | ||||||
| T1 | −0.16NS | −0.56* | −0.30NS | −0.21NS | |||||||
| T2 | −0.29NS | −0.71* | −0.39* | −0.04NS | |||||||
| T3 | −0.20NS | −0.31NS | −0.46* | 0.22NS | |||||||
| PN | T0 | −0.59** | −0.15 NS | −0.35NS | −0.56* | 0.11NS | |||||
| T1 | −0.01NS | 0.24 NS | 0.16NS | 0.03NS | −0.34NS | ||||||
| T2 | 0.39* | 0.16 NS | 0.41* | 0.63* | −0.31NS | ||||||
| T3 | −0.20NS | −0.10NS | 0.43* | −0.04NS | 0.21NS | ||||||
| gs | T0 | −0.55** | 0.03NS | −0.10NS | 0.75* | −0.39* | 0.80** | ||||
| T1 | 0.24NS | −0.24NS | −0.39* | −0.14NS | 0.44* | −0.48* | |||||
| T2 | 0.51* | 0.07NS | 0.00NS | −0.20NS | 0.30NS | 0.50* | |||||
| T3 | −0.16 NS | 0.22NS | −0.10NS | 0.02NS | 0.40* | 0.04NS | |||||
| ABA | T0 | −0.02NS | −0.03NS | −0.14NS | −0.11NS | 0.14NS | 0.07NS | −0.24 NS | |||
| T1 | 0.16NS | −0.41* | −0.56** | −0.42* | 0.40* | −0.46* | −0.66** | ||||
| T2 | 0.18NS | −0.68** | −0.44* | 0.01NS | 0.50* | 0.10NS | −0.18NS | ||||
| T3 | 0.24NS | −0.51** | −0.52* | −0.21NS | 0.25NS | −0.15NS | −0.01NS | ||||
| Zeatin | T0 | 0.17NS | 0.45* | 0.40* | 0.14NS | −0.50* | −0.23NS | 0.10NS | −0.15NS | ||
| T1 | −0.02NS | −0.35NS | 0.42* | 0.03NS | 0.30NS | −0.42* | 0.27NS | 0.448 | |||
| T2 | −0.09NS | 0.48* | 0.14 | −0.01NS | −0.21NS | 0.30NS | −0.02NS | 0.54* | |||
| T3 | −0.01NS | 0.41* | 0.03NS | −0.32NS | −0.28NS | −0.22NS | −0.15NS | −0.40* | 0.14NS | ||
| E | T0 | −0.47* | 0.41* | 0.15NS | 0.40* | −0.50* | 0.47* | 0.62* | −0.16NS | −0.25NS | |
| T1 | −0.14NS | 0.18NS | 0.04NS | −0.39* | 0.26NS | 0.39* | 0.24NS | −0.16NS | 0.17NS | ||
| T2 | 0.63** | 0.39* | 0.53* | −0.16NS | 0.00NS | 0.21NS | 0.39* | −0.39* | 0.25NS | ||
| T3 | −0.10NS | 0.18NS | 0.39* | −0.17NS | −0.55* | 0.05NS | 0.17 NS | −0.40* | 0.35NS | ||
| WUE | T0 | −0.02ns | −0.07NS | −0.56* | 0.05NS | 0.70** | 0.49* | 0.10 NS | 0.21NS | −0.47* | −0.48* |
| T1 | −0.01NS | −0.64* | 0.15NS | 0.13NS | 0.44* | 0.94* | −0.43* | 0.44* | −0.39* | 0.01NS | |
| T2 | −0.24NS | 0.19NS | −0.15NS | −0.20NS | −0.23NS | 0.63* | 0.08 NS | 0.11NS | −0.17NS | −0.58** | |
| T3 | −0.02NS | −0.21NS | −0.23NS | 0.09NS | 0.57* | 0.73* | 0.00 NS | 0.19NS | −0.43* | −0.68** |
Where NS = non-significant when P ≥ 0.05,
Si content was negatively related to LW and PN in the control and ABA, and E in T1. Si was also positively related to gS and E in the control and negatively related to E in T1.
LA was negatively correlated to LW and ABA. Moreover, LW was also negatively correlated to PB (Table 3). However, it showed a positive relationship with WUE (Table 3). LW was negatively related to gS in the control and showed a positive relationship in T1 and T2. LW and ABA had a positive relationship in T1 and T2. Zeatin content had a negative relationship with LW in the control and T3. LW had a positive relationship with WUE in all treatments except in T2. PN and gs had negative relationship with ABA and zeatin in T1 while E was negatively related to ABA in T2 and T3. ABA was positively related to zeatin content in T1 and T2 and negatively related in T3. It was also positively related to WUE in T1. Zeatin was negatively related to WUE in all treatments except T2 (Table 3).
In different crops, ABA signals were reported to reduce leaf area (Thompson et al. 2007), induce stomatal closure, and up-regulatestress-responsive genes (Mizokami et al. 2015, Tuteja 2007). In the present study, application of ABA, both by foliar spray and irrigation, had a significant negative impact on leaf area, stomatal conductance, transpiration, and photosynthesis rate. Moreover, significant negative relationships were noted between ABA content and stomatal conductance, transpiration, and net photosynthesis rates under various treatments. ABA application also decreased cuticular wax content in contradiction with what was reported by Seo et al. (2011) and Macková et al. (2013). It was noted earlier that ABA was a stress plant growth regulator and, thus, signals accumulation of higher cuticular waxes. These ABA-induced changes negatively affected growth and biomass production as previously reported by Sreenivasulu et al. (2012). All groups of genotypes, except H. argophyllus lines, accumulated higher ABA when this growth regulator was applied with irrigation, compared to foliar spray, suggesting a preferential absorption route through the roots from where it was transported to the target site, as previously reported by Wilkinson and Davies (2002).
Osmotic stress strongly affected plant growth, leading to reduced biomass and leaf area. It increased ABA content in the leaves. A similar increase of ABA with osmotic stress has been reported by Robertson et al. (1985). In an earlier study, it was also shown that exogenous ABA treatment reduced the adverse effects of osmotic stress on growth parameters due to continued root growth (Hussain et al. 2010). ABA and cytokinin accumulation are antagonistic (Wilkinson et al. 2012); the increased ABA content is likely to modify the balance of cytokinins, as reported by Bano et al. (1994), in cultivated sunflower. Osmotic stress decreased zeatin content in the leaves, while Škorić (2009) reported an increase in zeatin contentin the capitulum of rewatered plants.
In this study, zeatin and ABA were only negatively related in T3. The negative relationship between both growth regulators suggested that over-production of ABA content resulted in decreased zeatin contents under PEG-induced drought stress.
In exogenous applications of ABA (T1 and T2), both growth regulators were positively related to each other showing that exogenous application of ABA had no effect over endogenous zeatin production. Both growth regulators also had antagonistic effects over WUE. ABA had a positive relationship with WUE, while zeatin had a negative relationship with WUE (Table 2).
Variation among accessions in the response to ABA and osmotic stressesContrasting variation existed among the different groups of genotypes. For instance, H. argophyllus lines had a significantly lower leaf area under all treatments, compared to H. annuus lines, which is expected to reduce evapo-transpiration losses. Moreover, H. argophyllus populations also maintained higher water-use efficiency (in all treatments), net photosynthetic rate, and stomatal conductance in T1 and T3, compared to the rest of the genotypes. Finally, H. argophyllus lines tended to have higher leaf cuticular wax content than the rest of the tested genotypes, suggesting a more effective adaptive mechanism of water retention and protection against UV radiation and bacterial and insect infection (Macková et al. 2013). Cuticular wax is composed of long chains of fatty acid (Jenks et al. 2000), and high cuticular wax content appears as an interesting trait to be introgressed in the cultivated breeding lines as it could protect against leaf water losses and maintain stomatal conductance under stress conditions. The positive relationship between water-use efficiency and leaf cuticular waxes in most of the treatment also suggest its role in avoiding water losses in all environments. Thus, drought tolerance in H. argophyllus was mechanical rather having a biochemical basis.
H. argophyllus was able to maintain zeatin (non-significant, P ≥ 0.05) content under stress treatments. Generally, there was a positive relationship of plant biomass with zeatin in T0 and T1, suggesting the role of zeatin in improving plant biomass. Higher zeatin content production could result in maintaining growth and biomass in stress conditions (Peleg et al. 2011, Reguera et al. 2013).
Transmission of drought tolerance related traits in interspecific hybridsSmall leaf area and high cuticular waxes concentration of H. argophyllus could be considered a strategy to cope with water losses under drought stress and to increase water-use efficiency (WUE). None of these traits was inherited in interspecific hybrids; however, complex chromosomal segregations or crossover products could lead to the selection of lines with desired traits (Niazi et al. 2014). These results suggested to select transgressive segregants in F2 or back cross populations for lower leaf area and high cuticular wax content. Smaller leaves and high cuticular waxes could reduce the transpirational losses. Chen et al. (2003) reported enhanced cuticular wax in Arabidopsis plants transformed with single genes from various sources. This suggested that cuticular wax was a simply inherited trait that could be transferred through simple breeding procedure from wild species into cultivated sunflower.
The smaller leaf area observed in wild sunflower represents a mechanism of drought tolerance that could, however, occur at the expense of yield or biomass since large leaf area has been known to be positively correlated with yield in segregating material of sunflower under heat stress (Kalyar et al. 2013). Higher leaf number could, however, partially compensate for the smaller leaf area (Kalyar et al. 2013).
Encouraging results have been already obtained using H. argophyllus in sunflower breeding programs for drought stress. Belhassen et al. (1996) started breeding for drought tolerance from interspecific hybrids with H. argophyllus. Four cycles of divergent selection using leaf cuticular transpiration as a selection criterion led to the production of two contrasting genotypes, T+ (high level of leaf cuticular transpiration) and T– (low level of leaf cuticular transpiration), the latter showing a better yield tolerance index combined with good potential yield in some locations. These early reports of the presence of drought tolerance in Helianthus argophyllus (Belhassen et al. 1996, Saucă et al. 2014) led to the development of a comprehensive breeding program for determining the basis of drought tolerance in the species and hybrids by comparing with cultivated species (Helianthus annuus L.) and finally concentrating these traits in segregating generations for the development of inbred lines.
The commercial hybrid and the intraspecific hybrids showed a significantly higher accumulation of silicium in all stress environments than H. argophyllus populations and interspecific hybrids. Silicium is not expected to have a significant role in osmotic adjustment, but is a good indicator of transpiration as its absorption and transportation in the plant is passive with water flow.
The present study confirmed the value of H. argophyllus to improve drought tolerance in cultivated sunflower, previously reported by different authors. Additional traits of potential interest were detected in this species such as higher cuticular wax content (which allows reduction of evapo-transpiration losses) and capacity to maintain net photosynthetic rate and ABA and zeatin under osmotic stress. Some of these traits, such as smaller leaves, have to be considered carefully as they could be counter-productive under mild stress or optimal conditions. On the other hand, the effects of growth regulators on final yield under different environmental conditions are not yet fully elucidated. Finally, the inheritance of these traits has to be further investigated. The high excised-leaf water content and leaf cuticular wax content under stress of the wild species was not maintained in the interspecific hybrids.