2018 Volume 68 Issue 1 Pages 35-52
2018 Volume 68 Issue 1 Pages 35-52
Lilies and tulips (Liliaceae family) are economically very important ornamental bulbous plants. Here, we summarize major breeding goals, the role of an integrated method of cut-style pollination and fertilization followed by embryo rescue and mitotic and meiotic polyploidization involved in new assortment development. Both crops have been subjected to extensive interspecific hybridization followed by selection. Additionally, spontaneous polyploidization has played a role in their evolution. In lilies, there is a tendency to replace diploids with polyploid cultivars, whereas in tulip a majority of the cultivars that exist today are still diploid except for triploid Darwin hybrid tulips. The introduction of molecular cytogenetic techniques such as genomic in situ hybridization (GISH) permitted the detailed studies of genome composition in lily and tulip interspecific hybrids and to follow the chromosome inheritance in interspecific crosses. In addition, this review presents the latest information on phylogenetic relationship in lily and tulip and recent developments in molecular mapping using different DNA molecular techniques.
Lilies and tulips (Liliaceae family) are economically very important bulbous ornamental crops. Cultivars of these two crops are used as cut-flowers and garden plants, and are now indispensable for horticultural use. In Japan, tulips were introduced in the early 20th century, and thereafter the cultivation system of tulip bulbs was established in Niigata and Toyama prefectures. Approximately 100 cultivars have been developed thus far, mostly via both intra- and inter-specific crosses. Since many Lilium species are native to Japan, bulbs that were dug in native habitats had been exported to foreign countries in the early 20th century. During those years, Lilium species became domesticated and are currently produced in Japan, especially in Kagoshima, Niigata and Toyama prefectures. The Okinoerabu island of Kagoshima is the major producer of L. longiflorum bulbs. At present, about one hundred and fifty million cut flowers are produced on ca. 80 hectares in Japan. The main production areas are Hokkaido, Saitama, Niigata, and Kochi.
Nine lily species are indigenous to Japan. Some species grow in isolated island regions or mountain areas, and others are widely distributed throughout Japan (Supplemental Fig. 1). L. longiflorum is indigenous to the Ryukyu islands. L. dauricum, L. lancifolium and L. medeoloides, etc., are widely distributed from the Eurasian Continent to Japan. L. auratum, L. speciosum, L. japonicum, L. rubellum, L. alexandrae and L. nobilissimum are grown in Honshu, Shikoku, Kyushu, and Ryukyu Islands. Therefore, since antiquity, Japanese have collected lily bulbs in native habitats and enjoyed gardening by planting them in their private gardens. Consequently, in the Edo era, many cultivars of “Sukashi Yuri (Thunberg lily)” were developed by intraspecific crossing of L. dauricum and L. maculatum (Shimizu 1987). L. dauricum is reported to be involved in “Sukashi Yuri” breeding, which is supported by the sequence analysis of the LhMYB12 gene regulating tepal anthocyanin pigmentation (Yamagishi and Nakatsuka 2017). After Von Siebold introduced some species of Japanese lilies into Europe from Japan at the end of 19th century, the breeding of lilies was started in Europe. Relatively recently, Lilium species endemic to Japan have played an important role in the development of commercial lily cultivars as follows. Cultivars of Easter Lily are variants derived from the intraspecific crosses of L. longiflorum. Asiatic hybrid lilies are derived from interspecific hybridization among L. dauricum, L. lacifolium and L. maculatum and so on. L. auratum, L. speciosum, L. japonicum. L. rubellum, L. alexandrae and L. nobilissimum contributed to produce Oriental hybrid lilies. The production of these hybrid lilies is one of flower breeding’s greatest achievements. The Oriental hybrid ‘Star Gazer’ is the most important historical cultivar in this group because this is the first cultivar with an upright flower direction in Oriental hybrid lilies (Van Tuyl et al. 2011). Modern lily breeding aimed at inter-sectional hybridization and combining the three distinctive hybrid groups: Longiflorum (L), Oriental (O) and Asiatic (A) hybrids.
Tulip has a relatively long history of breeding in comparison with lily breeding. It is thought that garden tulips originally were developed in the Ottoman Empire around the 16th century. De Busbecq, who was the Austrian Ambassador to Turkey, sent tulip bulbs to Europe in the 16th century. European horticulturists were enthusiastic about growing tulips, and consequently, tulips became popular throughout Europe. Thus, T. gesneriana L. is the primary species distributed to consumer. More than 3,000 cultivars have been released from Holland (Van Scheepen 1996). Darwin hybrid tulips were spontaneously developed by the interspecific cross of T. gesneriana and T. fosteriana Hoog ex W. Irving (Bryan 2002, Doorenbos 1954, Lefeber 1960, Marasek et al. 2006, Van Tuyl and Van Creij 2005). Most of the Darwin hybrid tulips are triploids, possessing large flowers, sturdy stems, and an increased big plant size, due to hybrid vigor and triploidy. Their appearance was a revolution in the tulip market (Kho and Baer 1971, Kroon and Van Eijk 1997).
In the breeding of both tulips and lilies, interspecific hybridization plays an important role. Therefore, in this review, we shall first describe the phylogeny among the species in both lilies and tulips, because this enables the development of crossing plans effective for interspecific breeding. Then, we review the research regarding interspecific incongruity that exists in both genera, and the techniques developed for overcoming such incongruity. We then give detailed information regarding polyploidy and its relevance to chromosome inheritance in interspecific crosses.
Lilies (the species of genus Lilium) are distributed throughout the cold and temperate regions of the Northern Hemisphere. At present, there are approximately 100 known species. Several authorities classified lilies based on morphological characters (Baker 1871, Endlicher 1836, Wilson 1925). Comber (1949), based on 15 morphological characters, classified the genus Lilium, and is considered to be the most authoritative. He classified the genus into seven sections by each lectotype; section Martagon, typed by L. martagon; section Pseudolirium, by L. philadelphicum; section Liriotypus, by L. candidum; section Archelirion, by L. auratum; section Sinomartagon, by L. davidii; section Leucolirion, by L. longiflorum; and section Daurolirion, by L. dauricum. Sections Pseudolirium, Sinomartagon, and Leucolirion were divided into four (2a–2d), three (5a–5c), and two (6a and 6b) subsections, respectively. However, there is some dispute regarding Comber’s classification (Haw 1986), and molecular phylogenetic estimations have provided more precise relationships among Lilium species. The first comprehensive molecular phylogeny was reported by Nishikawa et al. (1999), who inferred phylogenetic relationships among 55 species of Lilium and two relatives from nucleotide sequence variations in the internal transcribed spacer (ITS) regions of 18S–25S nuclear ribosomal DNA (Supplemental Fig. 2).
Molecular phylogeny has clarified several issues of Lilium classification. In section Leucolirion, subsection 6b (L. longiflorum and four other species) has different phylogenetic background from subsection 6a (L. regale and three other species). Section Leucolirion is characterized by a trumpet-shaped flower and consists of two subsections, 6a and 6b, and sections Leucolirion and Archelirion were regarded as a monophyletic group (Comber 1949, Lighty 1968). However, subsection 6b was excluded from the clade comprising of subsection 6a and Archelirion, and subsection 6b was more closely related to a lectotype (L. davidii) clade of Sinomartagon. This relationship is supported by the evidence that the subsection 6b species are more cross-compatible to the species involved in Sinomartagon (Asano 1981, 1982b, Asano and Myodo 1977a).
Although Comber (1949) classified section Daurolirion as a sole group, section Daurolirion is an ingroup of Sinomartagon including the lectotype species. Sinomartagon is closely related to Daurolirion because the cross compatibility between the two sections is high (Shimizu 1987). Furthermore, they are basic to Asiatic Hybrids, which is one of the major cultivar groups in lilies (Leslie 1982). The molecular phylogenetic tree supports this relationship.
L. henryi of section Sinomartagon is not included in the lectotype clade of Sinomartagon, and shows a sister relationship to subsection 6a of section Leucolirion. Fertility data and cytological study have supported the relationship. L. henryi was well hybridized with the species of this subsection 6a and the horticultural cultivars obtained are known as “Aurelian hybrids” (McRae 1998). In a cytological study, the C-band patterns for this species matched with those of L. regale and L. sulphureum (Smyth et al. 1989).
L. bulbiferum of section Liriotypus was not included in the clade of other Liriotypus, and belonged in that of Daurolirion. The distribution of section Liriotypus is Europe, West Asia and the Himalayas. Comber (1949) interpreted that L. bulbiferum fitted into this section well. However, this species is well hybridized with Daurolirion and Sinomartagon species (McRae 1998).
Hayashi and Kawano (2000) showed the phylogenetic relationship among sections using the matK region of chloroplast DNA. Their phylogenetic estimation clarified that genera Lilium and Nomocharis were separated into three groups; (1) section Pseudolirium (subsection 2d), Archelirion, Sinomartagon (L. duchartrei and subsection 5c), Leucolirion (subsection 6a) and genus Nomocharis; (2) section Martagon, Liriotypus, Sinomartagon (rest of subsection 5a and all 5b), and Durolirion; (3) section Pseudolirium (subsection 2a, 2b and 2c). The classification into these three groups were supported by analyses using both the matK region (Nishikawa et al. 2000, Nishikawa 2007) and combined regions of ITS, matK and rpl16 (Rønsted et al. 2005).
L. philadelphicum is the lectotype of section Pseudolirium of Comber (1949). However, only this species was separated from the clade of the rest of the species of this section. In addition, monophyletic relationships between L. philadelphicum and the other species of Pseudolirium were weakly supported in an ITS tree (Nishikawa et al. 1999, 2001). The section is the only taxon distributed in North America, but monophyly of the section remains an open question.
Section Sinomartagon consists of three subsections, 5a, 5b and 5c. The section is a very complex section because some rather disparate species were grouped together in this section (Jefferson-Brown and Howland 1995). Nishikawa et al. (2001) estimated the comprehensive phylogenetic relationship of this complex section using 21 Sinomartagon species based on the sequence variation of ITS. The results showed that all 5c and three 5a (L. henryi, L. lankongense and L. duchartrei) were phylogenetically excluded from the clade of Sinomartagon, comprising of all 5b and the rest of 5a including L. davidii, the lectotype of this section.
Nomocharis-like species were included in subsection 5c by Comber (1949). Haw (1986) classified all the Nomocharis-like species into section Lophophorum, and considered that they were clearly related to the species of the genus Nomocharis. Austin (1999) accepted such a classification. Moreover Withers (1997) agreed, distinguishing between hybrids derived from Nomocharis-like Lilium and those from other members of the section Sinomartagon. In a cytological study, the C-band pattern of L. macklinae (subsection 5c) did not appear to correspond to any species in section Sinomartagon or, indeed, any other species banded (Smyth et al. 1989). All molecular phylogenetic trees reported to date have supported the relationship (Hayashi and Kawano 2000, Nishikawa et al. 1999, 2000, 2001, Nishikawa 2007). Therefore, Nomocharis-like species are appropriately classified as a different section from Sinomartagon, like section Lophophorum.
L. lankongense and L. duchartrei of section Sinomartagon are closely related (Jefferson-Brown and Howland 1995, McRae 1998), and Haw (1986) considered that L. lankongense was a synonym of L. duchartrei. Moreover, L. lankongense and L. lancifolium showed a very limited C-band similarity (Smyth et al. 1989). These results support that L. lankongense and L. duchartrei should be regarded as a different section from Sinomartagon including L. lancifolium.
Section Archelirion consists of six species mainly native to Japan (Supplemental Fig. 1). This section has been used as breeding material for Oriental hybrids, which is one of the major groups in lily cultivars. This section was monophyletic in trees based on both morphological characters (Asano 1986) and molecular biological analyses (Dubouzet and Shinoda 1999, Haruki et al. 1997, Nishikawa et al. 1999). However, using the matK gene of the chloroplast genome, Hayashi and Kawano (2000) reported a polyphyletic relationship of this section.
Nishikawa et al. (2002) postulated the phylogenetic relationships of section Archelirion based on the nucleotide sequence variations of three spacer regions in chloroplast DNA, trnT-trnL, trnL-trnF and atpB-rbcL (Supplemental Fig. 3). As a result, section Archelirion was divided into two major clades, one consisting of L. auratum var. auratum and L. rubellum, and the other consisting of the rest of the taxa in Archelirion. Each taxon was monophyletic, but two varieties of L. auratum, var. auratum and var. platyphyllum, were classified into a different clade, so that the phylogeny of L. auratum var. auratum is different from that of L. auratum var. platyphyllum, and it has a sister-relationship with L. rubellum. The relationship of the two varieties of L. auratum is supported by simple sequence repeat (SSR) analysis (Yamamoto et al. 2017). However, monophyly of section Archelirion is doubtless because the section forms a reliable clade in ITS trees of the nuclear genome (Nishikawa et al. 1999, 2001) and high cross compatibility within the section was reported (Shimizu 1971).
Ikinci et al. (2006) estimated the phylogenetic relationship within 17 species of section Liriotypus based on the sequence variation of ITS. They confirmed the previous result that section Liriotypus is monophyletic excluding only L. bulbiferum. The Liriotypus clade can therefore be divided into two groups, one comprising northeast Turkish-Caucasian species and another the European species in addition to L. candidum and the two Turkish endemics L. ciliatum and L. akkusianum.
Interspecific hybridization of liliesAs mentioned above, the genus Lilium contains ca.100 species and is divided into 7 sections. Various important Lilium species including L. longiflorum, L. auratum and L. speciosum are native to Japan. Those Lilium species had been introduced to Europe and the USA at the end of 19th Century. After their introduction, lily breeders have started to produce horticultural cultivars. In the beginning of lily breeding, interspecific hybridization using closely related species, i.e., intra-sectional hybridization was conducted, and then cross breeding using remotely related species has been attempted (Supplemental Fig. 4). C. M. Hovey developed some hybrids from crossing L. auratum × L. speciosum in 1864. In the reciprocal cross, E. Parkman developed a hybrid in 1869 (Shimizu 1971). Thereafter, a tremendous number of crosses and the selection of prominent cultivars have led to the success story of lily breeding. The parental species have both desirable and undesirable traits. Interspecific hybridization has been attempted to incorporate the desirable traits of both parental species into one plant (Jefferson-Brown 1988). For example, L. speciosum has some undesirable traits such as late-flowering and pending flower shape, but these characteristics were replaced in the progeny by the contributions of L. rubellum (early-flowering) and L. nobilissimum (upright flower) (Supplemental Fig. 4). Thus, in the beginning of Oriental hybrid breeding, the cultivars have been selected for upright flower shape and cut-flower production. Flower colors are also an important trait, so yellow-flowered OT hybrids have been successfully developed from the crossing between Oriental hybrids and Trumpet hybrids.
Incompatibility in the interspecific hybridization of lilies can be caused by the pre-fertilization barrier, whereby the elongation of the pollen tube arrests halfway in the stylar canals causing incompatibility. Such a pre-fertilization barrier is efficiently overcome by the cut-style pollination technique (Asano and Myodo 1977a, Watts 1967). Another cause of limited success of hybridization is due to the post-fertilization barrier that is caused by degradation of hybrid embryos and/or endosperm in incongruous crossings. For overcoming the post-fertilization barrier, the embryo rescue technique is very useful. The successful production of interspecific hybrids using embryo rescue has resulted in a broadening of genetic variation. The first report of hybrid lily production via embryo rescue was written by Nakajima (1940), who produced a hybrid from the crossing between L. speciosum and L. auratum. Thereafter, Skirm (1942) rescued hybrids in the cross between L. henryi and L. regale. Pre- and post-fertilization barriers occur at the same time in the cross between two remotely related species, such as L. longiflorum × Asiatic hybrid lilies. Therefore, the combined application of cut-style pollination and embryo rescue is general practice in order to overcome incongruity in the crosses of remotely related species (Asano and Myodo 1977a, 1977b) (Fig. 1). As a result, hybrid lilies such as LA (L. longiflorum × Asiatic hybrids) and LO (L. longiflorum × Oriental hybrids) hybrids have been released into the commercial market.
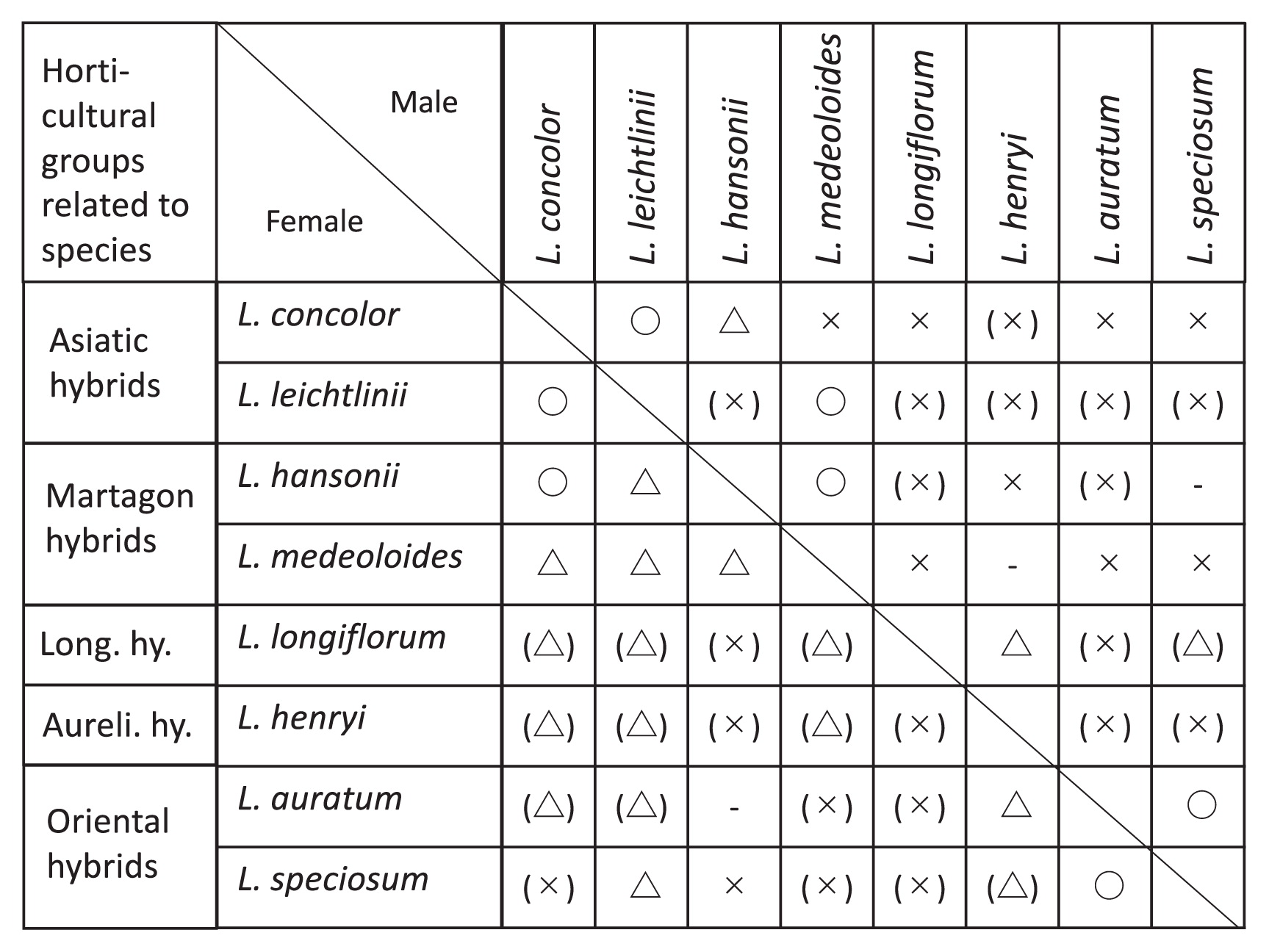
Interspecific crosses of Lilium species cited with modification from Asano (1987). ○, hybrids easily obtained in normal crossing; △, hybrids obtained in normal crossing followed by embryo rescue; (△), hybrids obtained by cut-style pollination followed by embryo rescue; × and (×), no hybrid obtained in normal crossing and cut-style pollination, respectively.
For successful embryo rescue in interspecific hybridization, various factors are considered. It is necessary to first determine the crossing direction, which one of the two species is used as the female because unilateral incompatibility is often observed. In lilies, successful crosses between remotely related species were mostly made in one direction (Asano 1980) (Fig. 1). Second, the level of crossability is determined on a case by case basis, depending on the parental species/cultivars used as crossing materials. Therefore, various genotypes have been used in interspecific breeding program of many crops (Asano and Myodo 1977a). Third, optimal isolation time of immature hybrid embryos (days after pollination) should be determined. The timing of isolating hybrid embryos depends upon the crossing combination. For example, 35–40 days after pollination in the cross using L. longiflorum as female (DAP), 40–60 DAP in the cross of Asiatic Hybrids × L. concolor, and 50–60 DAP in the crosses among species of section Archelirion (Oriental hybrids) have given successful results. Finally, various methods of embryo rescue technique including embryo, ovule and ovary culture are available for the production of hybrids in lilies (Asano and Myodo 1977b, Emsweller et al. 1962, Hayashi et al. 1986, Kanoh et al. 1988, North and Wills 1969, Okazaki et al. 1992, 1994, Ronald and Ascher 1976, Van Tuyl et al. 1991). As for culture condition, Asano and Myodo (1977b) showed that Murashige and Skoog (1962) medium is suitable for the culture of immature embryos when it is adjusted to pH 5.0 and supplemented with 20–40 g/L sucrose with 10−4–10−2 mg/L NAA. Okazaki et al. (1994) reported that the relatively small hybrid embryos require high sugar content in culture medium. Especially, hybrid embryos obtained in the crosses among species of section Archelirion (Oriental hybrids) require high osmoticum medium, even in the larger size of hybrid embryos. It is noted that the optimum sucrose concentration of 6% sucrose, or 3% sucrose plus 2% mannitol should be used for embryo culture of lilies (Okazaki et al. 1994).
Methods of polyploidization of liliesIn bulbous crops, there is a tendency to replace diploids with polyploid cultivars (Ramanna at al. 2012). Polyploids, especially triploids and tetraploids, are highly welcome in breeding programs, due to their desirable traits such as vigorous growth, thick leaves, and big flowers. In the case of interspecific crosses, most inter-sectional F1 hybrids are sterile in both male and female gametes. To accomplish further backcrossing of sterile F1 hybrids with the parental species, it is useful to produce amphidiploids by somatic auto-polyploidization of F1 hybrids (allodiploids). There are two manners to produce polyploids, i.e., mitotic and meiotic polyploidization. Mitotic chromosome doubling using antimitotic agents such as oryzalin, colchicines, and surflan, inhibits mitosis during metaphase by interfering with the function of microtubules, and leads to the production of amphidiploids. In Lilium, there are many studies on the optimal concentration of different chemicals and time of treatment used to induce polyploids (Liu et al. 2009, Ming et al. 2012, Wu et al. 2010). Mitotic chromosome doubling to restore F1 fertility has also been used e.g., in L. henryi × L. candidum, L. longiflorum × Asiatic hybrids, and L. longiflorum × L. candidum. Using these tetraploids, backcrossing was performed with Asiatic and Oriental hybrids (Van Tuyl et al. 1997). Inter-sectional hybrids between Oriental hybrids and Asiatic hybrids were successfully treated with colchicine (0.5%) for 24 h to obtain tetraploid plants (Wu et al. 2010). According to Ming et al. (2012), oryzalin is more efficient than colchicine to produce tetraploids in the lily species.
An alternative to somatic chromosome doubling is the use of 2n gametes obtained via meiotic polyploidization. 2n gamete producers that naturally produce 2n gamete have been reported, but the rate of 2n gamete production in those plants is low. There have been many attempts to increase their production; e.g., by applying different environmental conditions or caffeine injection in small flower buds of different sterile OA hybrids, but none of these attempts has shown to be significantly efficient (Lim et al. 2005, Lokker et al. 2004). The formation of 2n gametes can be induced by applying flower buds of lilies with nitrous oxide (N2O) gas to the flower buds of lilies (Akutsu et al. 2007, Barba-Gonzalez et al. 2006a). N2O inhibits the chromosome segregation in metaphase I during meiosis of pollen mother cells (PMCs), and consequently, the nucleus restitutes forming 2n gametes (Akutsu et al. 2007). Kitamura et al. (2009) demonstrated that N2O treatment induces depolymerization of the microtubules of PMCs so that bivalents are held at the equatorial plane during meiosis followed by asymmetrical cell plate formation without delay, resulting in 2n gametes formation. Similarly, when interspecific hybrids are partially fertile with moderate bivalent formation at metaphase I, the treatment at prophase-metaphase I is also effective in Oriental Trumpet hybrids (Luo et al. 2016). In contrast, for the N2O treatment at metaphase I, in hybrids derived from the cross between distantly related species, the N2O treatment at metaphase I is not effective because those allopolyploids reveal scattering of non-paring chromosomes at metaphase I of PMCs, and subsequently, the cell plate unequally divides the scattered chromosomes to produce aneuploid daughter cells (Okazaki et al. 2012). This happens both with and without the N2O treatment. In such a sterile hybrid (allopolyploid), e.g., Oriental Trumpet hybrid ‘Yelloween’, the N2O treatment can be optimally applied to anthers in 1–6 mm buds that contain the proliferating PMCs (Nukui et al. 2011). The N2O treatment at this timing induced tetraploidization of the proliferating PMCs, resulting in the formation of amphidiploid in PMCs, followed by the formation of 2n gametes during the meiosis. Thus, it can be noted that there are two optimal timings of N2O treatment to flower buds; one is buds containing PMCs undergoing anaphase I and the other is buds containing the proliferation stage of PMCs.
Nitrous oxide has been originally applied to zygotes as a polyploidizing agent in various crops. Similarly, N2O was applied to flowers of Asiatic hybrid lilies 10–13 days after pollination where the first cell division takes place in the fertilized eggs of lilies, and consequently, polyploids are effectively obtained (Okazaki et al. 2012, Sato et al. 2010).
Polyploidy in liliesTetraploid Easter lily induced by colchicine was reported to have larger but fewer flowers and thicker leaves, and bloom later than the diploid counterpart (Emsweller and Lumsden 1943, Emsweller and Uhring 1960). A chimeric Asiatic hybrid ‘Kiyotsubeni’ (4x/2x) was reported to become more tolerant to leaf scorch than the original diploid form (Okazaki and Hane 2005). A triploid form of Lilium tigrinum, a native lily, has been vegetatively propagated across a wide region of Japan due to its vigorous growth. Accordingly, a large majority of lily cultivars (>80%) are polyploid including triploid (2n = 3x = 36), tetraploid (2n = 4x = 48) and a few aneuploid cultivars (Zhou 2007, Zhou et al. 2008b). Zhou and Van Tuyl (2011–2012) reported that crosses within a section (intra-sectional hybridization) can be made relatively easily, resulting in the popular types of triploid lily cultivars such as Longiflorum (LLL), Asiatic (AAA), Oriental (OOO) where LLL, etc. means that the cultivar contains three genomes of the respective cultivars. At present, tetraploid Asiatic hybrid lilies (AAAA) are commonly distributed in the commercial market.
On the other hand, inter-sectional F1 hybrids of Lilium species are difficult to produce, due to pre- and post-fertilization barriers. However, they can be rescued by the combined use of cut-style pollination and embryo rescue, as previously mentioned. The resulting diploid inter-sectional F1 hybrids showed intermediate morphology between the parental species. Since this intermediate morphology is esthetically undesirable, further backcrossing of diploid F1 hybrids with the parental species has been conducted (Fig. 2). Triploid LAA or ALA hybrids were obtained from backcrossing Longiflorum × Asiatic hybrids (LA) with Asiatic hybrids as either the female or male parent; LAA and ALA are derived from the reciprocal crosses, LA × AA and AA × LA, respectively. Similarly, Oriental × Trumpet hybrids (OT) were used as male or female parents for backcrossing with Oriental cultivars (i.e., OO × OT, OT × OO). Thus, triploid commercial cultivars of LA and OT hybrids usually result from backcrossing diploid F1 hybrids with diploid parental cultivars (AA or OO). In contrast, commercial LLO cultivars seem to be derived from the cross of diploid Longiflorum (LL) × tetraploid hybrids (LLOO) (Zhang et al. 2012). As a result, triploid LAA (ALA) and OOT (OTO) hybrids presently occupy more than 45% of the acreage used for lily production. Thus the importance of interspecific sesquiploid hybrids in lily breeding should be noted.

Interspecific hybrids and their parent. (A) L. longiflorum, (C) L. Asiatic Hybrids ‘Connecticut King’, (B) their hybrid, (D) a Longflorum hybrid ‘Rote horn’ derived from the cross between L. longflorum and L. Asiatic Hybrids ‘Utagoe’, with a perfect trumpet shaped flower due to asymmetrical genome composition, (E) a hybrid of L. × formolongi × an Asiatic Hybrid, with a well-balanced flower shape as well.
Naturally occurring 2n gamete formation arising from aberrant meiosis is important for lily breeding. Many intersectional hybrids producing 2n pollen were selected and used extensively to produce BC1 progeny in different backcross combinations to diploid cultivars (AA × OA; OA × AA; OO × OA; LA × AA; OO × LA; AA × LA; OO × OT; OT × OO and Martagon × Asiatic (MA) hybrid × AA) and to tetraploid cultivars in the combination OAOA × OA (Barba-Gonzalez et al. 2005a, 2005b, 2006b, Chung et al. 2013, Lim et al. 2001, 2003a, 2003b, Luo et al. 2012, Zhou 2007). Some of these results are summarized in Supplemental Table 1. It is known that the two types of spontaneous 2n gamete formation via nuclear restitution, i.e., FDR (first division restitution) and SDR (second division restitution), occurs in interspecific hybrids (Bretagnolle and Thompson 1995). SDR 2n gametes result from the omission of the second meiotic division after normal anaphase I, whereas FDR 2n gametes result from the omission of the first meiotic division followed by a direct equational division of univalent chromosomes as in a mitotic division (Supplemental Fig. 5). Asano and Myodo (1977a, 1977b) and Asano (1980, 1981) produced a lot of interspecific hybrid derived from crosses between distantly related species. One of the hybrids, resulting from the cross of L. auratum ssp. platyphyllum × L. henryi (Auratum Henryi hybrid), showed high pollen fertility (83.6%) despite its restricted bivalent formation due to the distant relationship of these two species (Asano 1984). He reported that the fertile pollen of Auratum Henryi hybrid was produced via restitution nuclei formation during the meiotic process, in which the formation rate of dyad was 91.2%. Although he did not use the terminology of FDR/SDR, his observation clearly showed that the 2n gametes were produced via not only FDR in completely asynaptic PMCs containing 24 univalents, but also a unique meiotic process in PMCs with chromosome parings (Supplemental Fig. 6). In the latter PMC, paired chromosomes (bivalents) separated reductionally, while univalent chromosomes divided equationally at the equatorial zone and the sister chromatids moved to each pole. Both FDR and the unique meiotic process produced 2n gamete in the Auratum Henryi hybrid. Similarly, Lim et al. (2001) observed this type of meiotic process in LA hybrid lilies, analyzed more details regarding the chromosome assortment of this meiotic process using Genomic in situ hybridization (GISH), and designated it Indeterminate Meiotic Restitution (IMR) because this meiotic polypoidization contains both reductional and equational separations in anaphase I of PMCs (Fig. 3).

Schematic diagram of indeterminate meiotic restitution (IMR) in microsporogeneis in interspecific hybrid lilies. For clarity only three pairs of homoeologous chromosomes of the respective species (back and gray) and an example of the chromosome assortment are shown.
After the identification of IMR, GISH analyses more clearly identified mechanisms leading to 2n gamete formation in the subsequent progenies of F1 hybrids. FDR and IMR have been identified in Longiflorum × Asiatic (LA), Auratum × Henryi (AuH) and Longiflorurm × Henryi (LHe) hybrids (Chung et al. 2013, Lim et al. 2003a). Barba-Gonzales et al. (2005a) found that some F1 of OA hybrids produced 2n gametes via both FDR and IMR mechanisms. Luo et al. (2012) confirmed 2n eggs formation due to first division restitution (FDR) in F1 Oriental × Trumpet (OT) Lilium hybrids. In triploid BC1 created from the backcrossing of interspecific F1 hybrids of Longiflorum × Asiatic and Oriental × Asiatic hybrids to Asiatic parents, GISH analyses showed that 2n gametes were mostly produced through FDR (Khan et al. 2010). The occurrence of SDR was seldom recorded in the interspecific hybrids of lilies except for the cross of Lilium hybrids ‘Enchantment’ × L. pumilum (Lim et al. 2004). The SDR mechanism is thought to occur only in hybrids that are developed from a cross between closely related species, because SDR requires normal meiosis I, i.e., a normal pairing of bivalents in metaphase I, followed by their disjoining in anaphase I. Overall, in the 2n gamete producer of hybrid lilies, the 2n gametes are mostly formed through FDR, and in a few cases through IMR, because the chromosomal composition of FDR gametes might be more balanced than that of IMR gametes (Zhou et al. 2008a).
Chromosome recombination in hybrid liliesSomatic chromosome doubling of a diploid interspecific hybrid produces an allotetraploid (amphydiploid) containing two diploid genomes from different species (Asano 1982b). In allotetraploids (Fig. 4), homologous chromosome pairs of one parental species during meiosis leads to normal meiosis, and consequently fertile gamete production (Asano 1982a, 1982b, Lim et al. 2000). Though such 2n gametes have played a predominant role in the origins of sesquploid hybrids of lilies, the preferential homologous chromosome pairing which takes place in the amphidiploids has the drawback of inhibiting meiotic recombination between genomes coming from different progenitors. As a result, the resulting fertile gametes possess only a few genetic variations so that the progenies of allotetraploids exhibit fixed heterozygosity (Barba-Gonzalez et al. 2008, Fukai et al. 2005, Lim et al. 2001, Xie et al. 2010). In other words, somatic chromosome doubling is not important for introgression breeding due to the absence of intergenomic recombination in the progenies.

Genome composition of an amphydiploid derived from crossing L. formolongi ‘Augusta’ (LL) with 2n gamete of L. Asiatic Hybrids ‘Regata’ (AA). Genomic in situ hybridization to somatic metaphase chromosome complement shows 24 chromosomes of L genome (green fluorescence) and 24 chromosomes of A genome (blue DAPI fluorescence), indicating that ‘Augusta’ provided spontaneous 2n female gamete. Bar = 10 μm.
On the other hand, meiotic polyploidization occurring in hybrid lilies highly induced intergenomic recombination, which is favorable for introgression breeding (Barba-Gonzalez et al. 2004, 2006a, 2006c, Chung et al. 2013, Khan et al. 2009a, 2009b, 2010, Lim et al. 2003a, Luo et al. 2012, Xi et al. 2015, Xie et al. 2010, Zhou et al. 2008a, 2008c). Some of these results are summarized in Supplemental Table 1. Khan et al. (2010) demonstrated a large number of intergenomic recombinants in the BC1 progenies of both LA (Longiflorum × Asiatic) and OAOA (Oriental × Asiatic) hybrids obtained after unilateral sexual polyploidization. However, there were differences in the rate of recombination between male and female parents. Lower numbers of recombinant chromosomes were obtained in BC1 progenies of LA hybrids when these F1 interspecific hybrids were used as male parents, compared to the reciprocal cross where LA hybrids were used as female parents for backcrossing to diploid Asiatic hybrids (Khan et al. 2010). Lim et al. (2001) reported that the chromosomal recombination tended to occur at the proximal site rather than at interstitial and distal regions. Barba-Gonzalez et al. (2005a) reported that when OA hybrids were backcrossed to the parental Asiatic hybrids, the crossovers were unevenly distributed within the genome, and the larger chromosomes had fewer crossovers than the smaller chromosomes. Khan et al. (2009a) constructed chromosomal recombination maps where 248 chromosomal recombination sites identified in BC1 plants obtained by backcrossing LA hybrids with Asiatic hybrids (AA) were mapped to 12 chromosomes. Similarly, 116 recombinant sites identified in BC1 plants obtained by backcrossing AA with OA hybrids were also mapped. From those results, Khan et al. (2009a) also revealed an unequal distribution of chromosomal recombination site on 12 chromosomes in the BC1 progenies.
Another important aspect of recombinant chromosomes is that if the recombination occurs in 2n gametes when backcrossing F1 hybrid lilies to their parental cultivars, two types of meiotic products are expected, the reciprocal and non-reciprocal product of a recombinant event (Supplemental Fig. 7). When a gamete with the non-reciprocal product fuses with a recessive allele containing gamete of the backcross parent containing recessive alleles, the resulting progenies reveal a nulliplex allele, which might express the phenotype of recessive genes (Barba-Gonzalez et al. 2005a). In fact, huge variants of flower colors were obtained when the triploid BC1 plants (AA × OA) were produced through the fertilization of 2n gametes (Barba-Gonzalez et al. 2008).
Aneuploidy in hybrid liliesKhan et al. (2009b) reported that when diploid LA hybrids were backcrossed with parental Asiatic hybrids, they produced diploids, triploids and aneuploids (2x − 1, 2x + 2, and 2x + 3). Allotriploid lilies can also produce aneuploid or euploid functional female gametes, which can be used as the maternal parents in lily introgression breeding. All of the BC2 progenies of triploid OT hybrids (OOT) were aneuploids with chromosome numbers that varied from 25 to 29 (Luo et al. 2012) (Supplemental Table 1), and 25 to 33 chromosomes (Zhou et al. 2014). Additionally, Chung et al. (2013) reported that when the BC1 plants (OOT) derived from the cross of the Oriental × Auratum Henryi hybrids were used as the female, subsequent BC2 progeny was obtained, whereas no BC2 plants were obtained in the reciprocal cross. Crossing allotriploid BC1 progeny (AOA) with 2n gamete-producing OA hybrids resulted in aneuploid (3x + 2) and pentaploid (2n = 5x = 60) progenies (Barba-Gonzalez et al. 2006b). Xi et al. (2015) reported that allotriploid LO hybrid cultivar (LLO) always produced aneuploid functional female gametes, so that all of the progenies derived from crossing LLO either with allotetraploid Oriental × Trupmet (OTOT) or autotetraploid Trumpet (TTTT) were aneuploids. Barba-Gonzalez et al. (2006b) observed the transmission of recombinant chromosomes from BC1 allotriploid AOA to the progenies in variable frequencies. Luo et al. (2012) reported that GISH analysis revealed extensive intergenomic recombination in the BC1 progenies of OT and MA hybrids. A large number of Trumpet or Martagon chromosome segments were transmitted to the BC1 progenies from the F1 OT or MA hybrids. However, very few Trumpet chromosome segments were transmitted from the triploid BC1 parent to the BC2 progenies (Supplemental Table 1). The aneuploid and diploid lines in the subsequent backcrosses can produce lines with added or substituted alien chromosomes/segments, which are very useful resources for developing introgressed lines at the diploid level (Khan et al. 2009b).
Linkage maps of liliesMolecular linkage maps are important for quantitative and qualitative trait analysis. In Lilium, several molecular marker technologies have been applied to construct linkage maps, these include randomly amplified polymorphic DNA (RAPD), inter-simple sequence repeat (ISSR), amplified fragment length polymorphism (AFLP), diversity array technology (DArT) markers, and nucleotide binding site (NBS) profiling (Abe et al. 2002, Shahin et al. 2011, Van Heusden et al. 2002). Chen et al. (2015) utilized sequence-related amplified polymorphism (SRAP) in the construction of a genetic map of lily using a recombinant inbred line (RIL) population of 180 individuals. In total, 78 markers were used for mapping. The total map length was 2135.5 cM consisting of 16 linkage groups. The number of markers in the linkage groups varied from 1 to 12. The current published linkage maps are relatively low density marker maps, but recently several transcriptome profiling data are available in Lilium species (Lang et al. 2015, Shahin et al. 2012, Villacorta-Martin et al. 2015), which will be useful for development of EST markers and the construction of linkage maps.
These marker techniques were used for the mapping of either ornamental traits like flower color, flower pigmentation and flower longevity (Abe et al. 2002, Van der Meulen-Muisers et al. 1995), spotless (Abe et al. 2002, Shahin et al. 2011), carotenoid color of tepals (Nakano et al. 2005), stem color (Shahin et al. 2011), antherless (Shahin et al. 2011) and flower direction and/or disease resistance traits (Shahin et al. 2011, Straathof et al. 1996, Tang et al. 2015, Van Heusden et al. 2002). Abe et al. (2002) constructed a map including 95 RAPD and 119 ISSR markers in a population derived from a cross between the two Asiatic lily cultivars ‘Montreux’ and ‘Connecticut King’, to elucidate the genetics of floral anthocyanin pigmentation. Van Heusden et al. (2002) used 251 AFLP markers in an Asiatic backcross population (AA population) comprising 100 descendants to map disease resistance against two diseases: Fusarium oxysporum and LMoV (Lily mottle virus). Four putative quantitative trait loci (QTL) for Fusarium resistance were identified whereas a single LMoV resistance locus was found. The map density proposed by Van Heusden et al. (2002) was increased by Shahin et al. (2011) by application on the same Asiatic backcross population next to the previously used AFLP markers, NBS-profiling and DArT markers. For Fusarium resistance, six putative quantitative trait loci (QTL) were identified in the AA population. Resistance to LMoV was mapped as a locus on LG AA10. In addition to the QTL analyses, the functional characterization of genes controlling agronomic traits is also useful for development of marker assisted selection. In Asiatic and Oriental hybrid lilies, the MYB12 transcription factor regulates anthocyanin biosynthesis and its allelic variations determine flower color (Yamagishi 2011, Yamagishi et al. 2012, 2014, Yamagishi and Nakatsuka 2017). On the other hand, it is known that deposition of carotenoid pigments is regulated by differential expression of the carotenoid cleavage dioxygenase 4 (LbCCD4) gene (Hai et al. 2012) and capsanthin-capsorubin synthase (CCS) gene (Jeknić et al. 2012). The polymorphic sites of these genes might be available for marker assisted selection in flower color of lilies.
Tulipa species are widely distributed across geographic regions that have a cold to temperate climate with a dry summer, from Southwestern Europe and North Africa to Northwestern China and the Western Himalayas (Wilford 2006). Wild species have been collected from these regions and hybridized in Turkey since the 16th century. As a result, different tulips have been selected for various traits of flower shapes. The commonly called Istanbul tulips, tulips with thin pointed tepals, became popular in Turkey. Additionally, both early and late flowering tulips were already available in Istanbul (Mordak 1990 cited in Christenhusz et al. 2013). De Busbecq, who was the Austrian Ambassador to Turkey, sent tulip bulbs to Europe in the 16th century. Then, the French botanist, Clusius transplanted tulip bulbs into the botanic garden of Leiden University, and distributed the seeds and bulbs to Europe; consequently, tulips became popular throughout Europe. In the meantime, Conrad Gesner saw tulips in Herwart’s garden in Augsburg in 1559. Gesner’s “Tulip in the Garden of Johann Heinrich Herwart” was later published as a wood-block print in his 1561 book, De Hortis Germaniae Liber Recens. The tulip depicted by Gesner seems to be T. schrenkii (syn. T. suaveolens), based on its morphology, which is an early flowering species and has a short stem (Christenhusz et al. 2013). Linnaeus (1753) gave a single name, T. gesneriana, to a large number of tulips cultivars derived from the complex crossing of wild species whose lectotype specimen was one of tall garden tulips, different from T. schrenkii (Christenhusz et al. 2013). Thus, it is not clear which wild species contributed to develop the cultivated tulips found in Turkey and Europe, and T. gesneriana L. is still used as a collective name to represent a large number of cultivars.
The classification of Tulipa species has been conducted by several authors. This history is well reviewed by Wilford (2006) and Christenhusz et al. (2013). The infrageneric classification of Tulipa spp. is listed in Table 1. Hall (1940) identified approximately 100 species in the genus Tulipa (Supplemental Fig. 8). Since there are a lot of synonymous species, Christenhusz et al. (2013) revised the species identification of the genus Tulipa as to comprise 76 species by the identification of lectotypes of each species in the genus Tulipa. Generally, the classification of tulips has been conducted using key characters that show large variations among Tulipa spp., such as hairiness of stem, leaf shape, tepal shape, and bulb tunic as well as flowering time, plant height, color of anthers, and appearance of a back blotch in the flower (Supplemental Fig. 9). As a result, the genus Tulipa is divided into two subgenera; Eriostemones and Tulipa (syn. Leiostemones). The subgenus Eriostemones is classified into three sections; Australes, Saxatiles and Biflores (Van Raamsdonk and De Vries 1992). The subgenus Tulipa was divided into five sections where Oculussolis, designated by Hall (1940), is renamed as Tulipanum, and the remaining four sections are the same as those reported by Hall (1940) (Van Raamsdonk and De Vries 1995a). Zonneveld (2009) and Veldkamp and Zonneveld (2012) modified the previous classification based on nuclear genome sizes (DNA 2C value), morphology, and geographical distributions of tulip species. As a result, they established the new subgenus Clusianae, because the species of this group contain smaller nuclear DNA content than other tulips (Zonneveld 2009) and have a distinct karyotype and C-banding pattern (Blakey and Vosa 1982). Thus, there is a general consensus concerning the primary division of the genus Tulipa based on morphological characters, while infrageneric classifications of the genus Tulipa have been undertaken using molecular analysis of plastid and genome sequences.
| Hall (1940) | Van Raamsdonk and Vries (1992, 1995a) | Veldkamp and Zonneveld (2012) | ||||
|---|---|---|---|---|---|---|
| subgenus | section | subsection | subgenus | section | subgenus | section |
| Leiostemones | Gesneriana | Tulipa | Tulipa | Tulipa | Tulipa | |
| Kolpalowskianae | Kolpalowskianae | Kolpalowskianae | ||||
| Eichleres | Eichleres | Lanatae | ||||
| Oculus-solis | Tulipanum | Tulipanum | ||||
| Spriranthera | ||||||
| Multiflorae | ||||||
| Clusianae | Clusianae | Clusianae | ||||
| Eriostemones | Australes | Eriostemones | Australes | Eriostemones | Australes | |
| Biflores | Biflores | Biflores | ||||
| Saxatiles | Saxatiles | Saxatiles | ||||
| Orithyia (Section) | Orithyia | Orithyia | ||||
Refer to more details (Christenhusz et al. 2013) for historical overview of infrageneric classification of Tulipa spp.
Yanagisawa et al. (2012) constructed a molecular phylogeny based on the plastid DNA sequences of tulips including 32 Tulipa species, by using Amana edulis as an outgroup. The coding regions of trnL and matK, and the intergenic spacer (IGS) region of trnT-L in chloroplast were sequenced and used for constructing a phylogeneic tree (Supplemental Fig. 10). They reported that in the analysis of combined matK and trnT-L sequences, the Clusianae clade was separated from the Tulipa and Eriostemones clade, which agrees with the classification of Veldkamp and Zonneveld (2012). In addition, the Eichleres subsection, except for T. praestans and T. tubergeniana is monophyletic and closely related to the species of Tulipa subgenus. The phylogenic position of those two species remains to be determined. Especially, the position of T. tubergeniana should be re-examined because the sample used in this study is a variant of T. tubergeniana, ‘Keukenhof’, and its trnT-L sequence is identical to one group of T. gesneriana and T. schrenkii. This data indicates that T. tubergeniana, ‘Keukenhof’ might be a hybrid derived from a cross of T. gesneriana. T. ingens, morphologically related to T. tubergeniana was included in the Eichleres clade of the matK tree (Yanagisawa et al. 2012), indicating that T. ingens is a member in the section Eichleres. Christenhusz et al. (2013) made a more comprehensive phylogenic tree of wild tulips using the five plastid sequences (trnL intron and trnL–trnF spacer, rpl16 intron, rps12–rpl20 intergenic spacer and matK) and the internal transcribed spacer (ITS) region of rDNA. They identified four clades which corresponds to the subgenera suggested by the previous report (Veldkamp and Zonneveld 2012), but the combination of plastid and ITS sequence data did not dissect the species relationship within subgenus Tulipa.
Apart from the phylogeny of wild species, which species contributes to the large variability of garden tulips has been an issue of considerable dispute. Yanagisawa et al. (2012) reported that the garden tulips were divided into the three groups by combined plastid DNA sequences of trnT-L and matK (Supplemental Fig. 11). Group A included T. tubergeniana ‘Keukenhof’, T. schrenkii (syn. T. suaveolens) and early flowering cultivars including ‘Couleur Cardinal’ and ‘Murillo tulip’ registered in 1815 and 1860, respectively (Van Scheepen 1996), indicates that T. schrenkii may have contributed to the production of early flowering tulips such as ‘Duc van Tol’ tulips. This assumption is supported by the phenotypic observation that T. schrenkii and ‘Duc van Tol’ tulips share similar morphological traits and bloom at the same time of early spring. Group C included 10 cultivars such as ‘Faust’ and ‘Zulu’ which were recorded in the 19th century, indicating that they originated from a very old common progenitor of the ancestral cultivar and species. Group B included 13 cultivars of T. gesneriana, T. acuminata, T. marjoletti and T. praecox. It is known that T. acuminata does not correspond to a wild species and normally is compatible with T. gesneriana (Fig. 5), indicating a hybrid origin of T. acuminata from its close relatives, such as T. gesneriana. T. marjoletti is thought to be a naturalized species in Southern Europe, so called neo-tulipae. Therefore, it is reasonable that T. acuminate, T. marjoletti and garden tulips consist of one clade. Tang et al. (2013), in their study of genetic diversity of SNP markers in the 49 accessions of T. gesneriana, reported that early and late flowering cultivars were separately plotted in the principal coordinate analysis; even so, the level of the genetic diversity of T. gesneriana estimated by polymorphic SNP is similar to that of T. fosteriana, indicating a relatively small variation of SNPs in T. gesneriana. Taken together, T. gesneriana is thought to be generated from the crosses among the closely related species, including T. schrenkii. However, which species contributed to the large variability of late flowering tulips cultivars including T. acuminata and T. marjoletti has not been uncovered. More studies using nuclear coding genes as well as including more taxa in phylogenetic analysis are required to clarify the phylogeny of garden tulips.

Interspecific crosses of Tulipa species cited with modification from Hagiya (1971). a Cultivar classification according to Classified list and international register of tulip names (1958); SE, Single Early; M, Mendel; T, Triumph; D, Darwin; B, Breeder; C, Cottage; L, Lily-flowered; P, Parrot; F, Fosteriana; K, Kaufmanniana; G, Greigii. b ◎, several successful attempts, effectivity high (compatible); ○, one successful attempt, effectivity high; △, several successful attempts, effectivity low; ×, no seed obtained; –, not determined.
As mentioned above, the genus Tulipa, includes approximately 80 species, is divided in two sections of Eriostemones and Tulipa (syn. Leiostemones), and was recently reclassified into four subgenera (Christenhusz et al. 2013). In general, productivity, disease resistance, flower color, flower shapes, strong stem, fragrance, and vase life are important in tulip breeding. It is worthwhile for commercial tulips to broaden variation by incorporating useful agronomic traits, e.g., disease resistance, of wild species via interspecific hybridization (Straathof and Eikelboom 1997, Van Tuyl and Van Creij 2006). Interspecific incongruity is separately designated as pre- and post-fertilization barriers, dependent upon the timing of cessation of the fertilization process as described previously for lily. For the pre-fertilization barrier of tulips, pollen tubes arrest at the different positions within the pistils. Van Creij et al. (1997a) reported that pollen tubes arrested in the transmitting tissue and the ovary wall of pistils in the cross of T. gesneriana with the distantly related species, whereas when T. gesneriana was crossed with the closely related species such as T. fosteriana, T. kaufmanniana, and T. didieri, most pollen tubes reached the ovule and normally penetrated the micropyles. In the cross of T. gesneriana with T. praestans and T. agenensis, the penetration percentages of ovules were lower than that of the compatible cross (T. gesneriana × T. gesneriana). In the cross of T. gesneriana × T. agenensis, even after pollen tubes grew to the embryo sac, they stopped; either failing to penetrate the micropyle or they entered the embryo sac but did not discharge after entering into the embryo sac. Additionally, Kho and Baer (1971) observed that pollen tubes continued to grow inside the embryo sac, and coiled up in the embryo sac without fertilization. Generally, in interspecific crossing, pollen tubes remain shorter and the number of pollen tubes is lower, compared to those in a compatible cross (Van Creij et al. 1997a).
Seed set data in crosses among Tulipa species also showed various levels of interspecific barrier (Hagiya 1971, Van Creij et al. 1999, Van Eijk et al. 1991, Van Raamsdonk et al. 1995b). No hybrids were reported to produce seeds in the crosses of T. gesneriana with the distantly related species belonging to the sections Kolpakowskianae and Clusianae, and the subgenus Eriostemones, except for the result reported by Hagiya (1971), who obtained some hybrid seeds in these cross combinations (Fig. 5). In the crosses between such a distantly related species, pollen tube growth was arrested at the stigmatic surface or inside pistils, causing no fertilization (Van Creij et al. 1997a). Therefore, further researches are needed to confirm the results published by Hagiya (1971). On the other hand, Van Creij et al. (1997a, 1997b) reported that when T. gesneriana was crossed with T. agenensis (syn. T. oculus-solis, T. praecox), the percentage of pollen tube penetration into ovules was high. Accordingly, some authors reported that T. gesneriana produced hybrids when crossed with species of the section Tulipanum (T. agenensis and T. stapfii) (Hagiya 1971, Van Eijk et al. 1991, Van Raamsdonk et al. 1995b). In addition, hybrids have been more effectively obtained from the cross of T. gesneriana × T. agenensis (T. oculus-solis) via embryo rescue techniques (Van Creij et al. 1999). These crossing data suggest that T. gesneriana and the species of section Tulipanum including T. stapfi and T. agenensis are closely related in the phylogeny, which is supported in the phylogenic analysis reported by Yanagisawa et al. (2012) where section Tulipanum is closely related to T. gesneriana.
Section Eichleres, which relates more closely to T. gesneriana, is subdivided into eight series, some of which were used for the crossing experiment in the studies of Hagiya (1971) and Van Raamsdonk et al. (1995b, 1997). They made both directions of cross between section Tulipa and section Eichleres, and reported that hybrid seed set was high in the cross with T. gesneriana as female, but low in the reciprocal cross, suggesting so-called unilateral incompatibility (Fig. 5). The unilateral incompatibility of inter-species crosses is likely to occur commonly in Tulipa spp. Hagiya (1971) got hybrid seeds from the crosses between T. gesneriana and section Eichleres, except for the cross of T. gesneriana × T. ingens. Van Eijk et al. (1991) made interspecific crosses between T. gesneriana cultivars and 10 species belonging to the Eichleres section, using T, gesneriana as female parents, and they successfully obtained hybrids, except for the crosses with T. micheliana, T. kaufmanniana and T. praestans. In these interspecific crosses, the cross of T. gesneriana L. with T. fosteriana is most important, because it has resulted in Darwin Hybrids tulips, consisting of more than 50 original cultivars released to commercial market.
Embryo rescue techniques in tulipsIn tulips, the post-fertilization barrier occurs as a lack of endosperm and the formation of a jelly-like structure formation that hampers embryo growth. To develop embryo rescue techniques, various culture conditions influencing culture performance were analyzed using embryos obtained from intra- and interspecific crosses of tulips. Custers et al. (1992, 1995), using non-abortive embryos collected from flowers obtained 3 to 9 weeks after pollination in the intraspecific crosses of T. gesneriana × T. gesneriana, examined various culture conditions for the ovule culture of tulips. As a result, half strength MS medium containing 6% sucrose is suitable for increasing bulblet weight. Use of Petri dish containing a thin layer (6 mm in depth) of MS medium revealed a higher rate of bulblet formation, compared to culture tubes with the thick medium. Culture in continuous darkness was superior to 16h light in bulblet formation. The optimal growing temperature is 12–15°C. According to this result, the following sequence of culture condition has been used for ovule culture of tulips (Custers et al. 1995); (1) cultures are planted for 12–15 weeks at 15°C, (2) 12 weeks at 5°C to induce germination, (3) 12–18 weeks at 15°C to permit seedling growth and bulblet formation, (4) 6 weeks at 15°C for ripening. At the same time, Custers et al. (1992, 1995) compared embryo culture and ovule culture methods, and demonstrated that ovule culture could be applicable for rescuing small embryos (0.3–0.7 mm), whereas an embryo size of 3 mm in length was necessary to have a reasonable result in isolated embryo culture. These results indicate that ovule culture is superior to embryo culture for rescuing immature embryos of tulips.
In contrast, Okazaki (2005) reported that embryo culture method was applicable for rescuing immature embryos (0.2–0.6 mm). In his study, when the ovules and embryos of infraspecific cross (Demeter × Christmas Dream) were excised for culture from ovaries 7 weeks after pollination where the size of embryos was ca. 0.6 mm in length, the rate of bulblet production was 80.7% in the embryo culture, and 78.3% in the ovule culture, indicating the same effectiveness in both methods. The embryo size that can be rescued is inconsistent in the two studies of Custers et al. (1995) and Okazaki (2005). This might be due to a difference in the culture conditions of embryo culture as follows. Okazaki (2005) performed embryo culture in accordance with the method of Custers et al. (1992) but with a minor modification; embryos (ovules) were incubated at 20°C for 5–6 weeks, and transferred to new MS medium, and then were incubated at 5°C during 8 weeks from July to September. After that, the cultures were incubated for 12–15 weeks at 15°C darkness to induce bulblet formation. In his study, the cultured ovules formed bulblets via normal germination (Fig. 6A), whereas in the embryo culture, some embryos normally grew and reached mature embryos size 5 weeks after culture, others expanded in width, while still others became crescent shape instead of the normal spindle shape. After cold treatment (8 weeks at 5°C), normal spindle shape embryos tended to produce bulblets in a germinative manner, and malformed embryos tended to form a bulblet via adventitious bud formation (Fig. 6B, 6C).

Bulblet formation via culture in ovules and embryos excised after 7 weeks after pollination of the intraspecific cross of T. gesneriana; ‘Christmas Dream’ × ‘Ile de France’. (A) a bulblet via ovule culture, (B) and (C) bulblets via germinative and adventitious growth in embryo culture, respectively.
On the other hand, Van Creij et al. (2000) developed another type of embryo rescue technique that is ovary-slice culture followed by ovule culture, by which the germination percentage of hybrid seeds was mostly comparable to direct ovule culture. They cut an ovary into eight pieces and the ovary slices were cultured under the same culture condition as that of Custers et al. (1995). The period of ovary-slice culture (7–11 weeks) does not affect the germination rate in the subsequent ovule culture. Since ovary-slice culture is not time consuming at the start of culture, this two-step culture method has an advantage for flexibly scheduling the subsequent ovule culture.
Application of embryo rescue to interspecific crossesVan Creij et al. (1999) applied ovary-slice culture to interspecific crosses of T. gesneriana × T. praentans and T. gesneriana × T. agenesis, which had never been reported to produce true hybrids in the normal cross. As a result, they obtained unique hybrids obtained from those cross combinations, indicating a new approach to broaden genetic variation in T. gesneriana. On the other hand, embryo culture techniques are also applicable for improving hybrid recovery rates in the case where only a few seedlings are obtained in normal interspecific crosses. Custers et al. (1995) applied direct ovule culture to the interspecific cross where T. gesneriana Christmas Marvel (CM) and Gander (GA) were used as female parents, and T. kaufmanniana as the male parent. In the normal cross of this combination, CM and GA produced 1.6–7.0 and 0.1–0.8 seeds per pod, respectively, while the hybrid formation rate of ovule culture was 2–13 time higher than that of a normal cross. This result indicates that ovule culture successfully improves organogenesis from normally abortive interspecific hybrid embryos. Similarly, Okazaki (2005) applied embryo culture to rescue abortive embryos in the cross of T. gesneriana with several species of section Eichleres. As a result, hybrids were effectively produced with relatively high bulblet formation rates per cultured embryo (18.0 to 37.8%), while a fewer normal seedlings with a low germination rate (0.6 to 20%) were obtained without using embryo rescue techniques. Using a flow cytometer, ploidy levels were analyzed in not only the seedlings germinated in soil, but also in the bulblets regenerated by embryo culture. Only three plants (0.01%) were triploid among 267 hybrid plants via embryo culture, whereas 14 triploids (16.1%) predominantly appeared among 87 hybrid seedlings germinated in soil, indicating that the triploid condition promotes normal seed formation, leading to a high rate of germination in such cross combinations. This result might explain the reason why triploid forms of Darwin Hybrid tulips have effectively been produced in T. gesneriana × T. fosteriana.
Polyploidy in tulipsIn contrast to modern lily cultivars such as LA and OT hyborids, a majority of the tulip cultivars that exist today are still diploid. Only among Darwin Hybrids tulips (DH), triploids and tetraploid cultivars dominate on the market, but in some crosses the number of polyploids DH seedlings can be less than 1% (Van Tuyl personal communication). Some triploid tulip cultivars are of spontaneous origins resulting from 2n gamete producer (Marasek et al. 2006, Van Scheepen 1996), whereas polyploid cultivars have been synthesized through somatic chromosome doubling in Tulipa (Chauvin et al. 2005, Eikelboom et al. 2001, Podwyszynska 2012, Van Tuyl et al. 1992). In tulips, mitotic polyploidization with colchicine is difficult, because the meristems are hidden in noses inside the bulbs, and colchicine is cancerigenic to bulbous plants (Van Tuyl et al. 1992). Chauvin et al. (2005) has developed a method of in vitro chromosome doubling in tulip using oryzalin during the stem-disc regeneration process. The final yield of tetraploids was low (0–3.75 tetraploids for one treated flower stem), but was sufficient to initiate crossing experiments to obtain triploids by utilizing these tetraploids by crossing with diploid cultivars. Similarly, flower stem fragments isolated from cooled tulip bulbs were used as the initial explants for treatment with the antimitotic agents oryzalin and amiprophos methyl (APM) (Podwyszynska 2012). Both oryzalin and AMP induced tetraploidy and the efficiency of in vitro polyploidization was approximately 30%. Okazaki et al. (2005) successfully induced 2n pollen formation by the N2O-treatment of bulbs at 6 atmospheres for 24–48 h of bulbs containing flower buds in meiotic metaphase I. The subsequent use of pollen containing a relatively high proportion of giant pollen grains (2n pollen) in crosses with diploids tended to yield larger numbers of triploids in the progeny.
Interploidy crosses in tulipsAmong Darwin Hybrid tulips resulting from interspecific crosses between T. gesneriana and T. fosteriana, triploid (2n = 3x = 36) cultivars such as ‘Apeldoorn’, ‘Ad Rem’, ‘Pink Impression’ and some tetraploid (2n = 4x = 48) hybrids such as ‘Tender Beauty’ have been produced from meiotic polyploidization (Van Scheepen 1996). According to Marasek-Ciolakowska et al. (2014), crossing diploid tulips with 2n gamete producers (2x × 2x (2n) crosses) produced mostly diploids, whereas a few seedlings were triploid. Similarly, a low yield of polyploid tulips was also reported by Okazaki et al. (2005) in 2x × 2x crosses involving 2n pollen induced via N2O treatment. Many triploid tulips were also produced from interploidy crosses (4x × 2x or 2x × 4x) (Okazaki and Nishimura 2000, Straathof and Eikelboom 1997). For instance, triploid ‘World’s Favourite’ originated from the crossing of a tetraploid seedling (‘Denbola’ × ‘Lustige Witwe’) with a diploid T. fosteriana (Straathof and Eikelboom 1997), whereas ‘Lady Margot’, ‘Benny Neyman’ and ‘Sun Child’ have been obtained from reciprocal crosses (2x × 4x) (Van Scheepen 1996). In crosses between triploid tulip cultivars and diploid 2n gamete producers {3x × 2x (2n)}, aneuploids with 40–45 chromosomes were predominantly obtained, but also tetraploid (2n = 4x = 48) and pentaploids (2n = 5x = 60) were also observed (Marasek-Ciolakowska et al. 2014). Aneuploid seedlings of tulip were obtained from 2x × 3x and 3x × 2x crosses (Okazaki and Nishimura 2000). Mizuochi et al. (2009) reported that in the subsequent progeny derived from the cross of 2x × 3x crosses of tulips, diploids (2n = 24) and trisomics (2n = 25) were predominantly produced. Some near triploids (2n = 34) also appeared as a second class, but other class of aneuploidy were scarce, indicating a predominant fertilization of pollen having near euploidy. In the reciprocal cross (3x × 2x crosses), the chromosome numbers of the subsequent plants ranged from 24 to 36 in accordance with the binominal theoretical distribution. The distorted chromosome transmission rates via male gametes has been generally reported in several species (Lin et al. 1992). In crosses between diploid T. gesneriana cultivars and triploid Darwin Hybrid, most progenies were triploid; suggesting that they originated from the fusion of a haploid gamete from the diploid female with a 2n gamete from the triploid male (Marasek-Ciolakowska et al. 2014).
GISH analysis of interspecific hybrids of hybrid tulipsA GISH analysis of triploid Darwin Hybrid tulips derived from the cross of T. gesneriana × T. fosteriana, revealed that triploid ‘Yellow Dover’ resulted from FDR 2n eggs (Marasek et al. 2006), whereas triploid ‘Kouki’ originated from FDR 2n pollen (Marasek and Okazaki 2008). Similarly, Marasek-Ciolakowska et al. (2014) reported that triploid BC1 hybrids (GGF) originated from fertilization of a haploid egg (G) and the 2n male gamete (GF) resulted from FDR which occurred in PMCs of F1 hybrids without recombination. The occurrence of IMR during 2n gametes formation was confirmed in a pentaploid hybrid derived from the cross 3x (GGG) × 2x (GF), which was comprised of 52 G chromosomes (including 5 recombinant chromosomes with a T. gesneriana centromere and T. fosteriana chromosome segment, i.e., 5 G/F) and 8 F chromosomes (5 F/G) (Marasek-Ciolakowska et al. 2014). Both in Lilium (Barba-Gonzalez 2008, Khan et al. 2010, Lim et al. 2001, Zhou et al. 2008a) and Tulipa (Marasek-Ciolakowska et al. 2014) FDR is the basic mechanism of 2n pollen formation. The reason is probably that chromosomal composition of FDR gametes is more balanced and viable than those of IMR gametes.
In tulips, many recombinant chromosomes were detected in diploid BC1 plants (Fig. 7) derived from crossing T. gesneriana and the diploid Darwin Hybrid tulip ‘Purissima’, and in the tetraploid cultivar ‘Judith Leyster’ obtained by crossing between tetraploids (Marasek and Okazaki 2008). Similarly, a considerable amount of intergenomic recombination between the parental chromosomes of the two species was shown in both the BC1 and BC2 offspring of Darwin Hybrid tulips (Marasek-Ciolakowska et al. 2012a, 2012b). The number of recombinant chromosomes ranged from 3 to 8 in BC1 and from 1 to 7 in BC2 progenies, suggesting the occurrence of one or two crossovers per chromosome. According to Marasek-Ciolakowska et al. (2012a, 2012b), out of 130 recombinant chromosomes found in 32 BC2 hybrids, 42 were transmitted from BC1 and 20 resulted from the second cycle of homoeologous recombination, whereas 68 were new types of recombinant chromosomes. Thus, introgression of T. fosteriana chromatin into the T. gesneriana genome is currently applicable in tulip breeding. As such ‘Hatsuzakura’, ‘Kikomachi’ and ‘Momotaro’ have been developed from the BC1 offspring of diploid Darwin Hybrid tulips, and ‘Kikomachi’ is one of the leading cultivars in Japan (Marasek and Okazaki 2008).

Discrimination of chromosomes originating from T. gesneriana (G) and T. fosteriana (F) in the genomes of ‘Kikomachi’ (2n = 2x = 24). (A) Genomic in situ hybridization to somatic metaphase chromosome complement showing 18 chromosomes of G (green fluorescence) and 6 F chromosomes (red fluorescence). Recombinant chromosome is defined as F/G indicating a T. fosteriana centromere with T. gesneriana chromosome segment. (B) A diagrammatic representation of chromosomes in ‘Kikomachi’ (2n = 2x = 24). In this figure, the black color represents the T. fosteriana genome, while white represents T. gesneriana one.
In tulips, three main diseases have been identified; Fusarium oxysporum f. sp. tulipae, Botrytis tulipea and Tulip breaking virus (TBV). Tang et al. (2015) constructed the first genetic map for tulip based on single nucleotide polymorphism (SNP), AFLP, NBS and SSR markers aimed at the identification of loci associated with resistance to Fusarium oxysporum. Genetic maps have been made separately for parental forms T. gesneriana ‘Kees Nelis’ and T. fosteriana ‘Cantata’. Using a population of 125 individuals obtained from a cross between these cultivars in which Fusarium resistance trait is segregating, six putative quantitative trait loci (QTLs) for Fusarium resistance derived from both parents were identified.
Developing new cultivars of lilies has been extensively achieved since the end of 19th century, especially in a few recent decades. It is worthwhile to mention that lily breeding programs in the Netherlands have contributed to develop various interspecific hybrids such as AH, OH, LA, LO, OT, and OA hybrids. Though the origin of T. gesneriana is unclear, because of its relatively longer history of cultivation, at least one of the important cultivar groups, DH hybrid tulips, has been demonstrated to originate from the cross of T. gesneriana × T. fosteriana. In both lilies and tulips, interspecific hybridization plays an important role because it can combine the superior traits of two different species into one hybrid. In fact, agronomic traits such as flower colors, flower longevity, spotless, flower direction, and disease resistance traits have been incorporated into hybrid cultivars. Of additional importance, BC1 plants derived via 1n gametes and/or 2n FDR gametes of F1 interspecific hybrids are introgressed with homoeologous chromosome recombination in meiosis I, and their subsequent BC2 progenies have mixed genomes as a consequence of random parental chromosome assortment. This genome shuffling breeding enables the efficiently accumulation of desirable characteristics into one cultivar. To accomplish this, genetic analysis of agronomic traits is necessary, so we have introduced some studies that reported QTLs controlling disease resistance and the functional characterization of the genes regulating flower color in tepals of lilies. In lily and tulip breeding, molecular markers closely linked to agronomic traits should be developed extensively for the successful application of marker assisted selection (MAS) methods for utilization in new variety development.
The authors sincerely thank Dr. M. Mii at Chiba University, Japan, for critically reading our manuscript. This work was supported in part by a grant, the Science and technology research promotion program for agriculture, forestry, fisheries and food industry, from the Ministry of Agriculture, Forestry and Fisheries, Japan.