2018 Volume 68 Issue 3 Pages 381-384
2018 Volume 68 Issue 3 Pages 381-384
To obtain a clear intact section of a ripened rice grain, which is suitable for biochemical and histological analysis, the Kawamoto method using a specific adhesive film was applied using a cryomicrotome. The longitudinal and sagittal sections were easily obtained together with the cross-section, and cell characteristics were clearly discerned in the ripened grain. It was demonstrated that the Kawamoto method is readily applicable for intact sectioning of hard tissue, including ripened grain. Intact section sampling may be useful for enzymatic analysis and transcriptomic analysis of plant tissue.
Rice (Oryza sativa L.) is very important as a staple food of the world, especially in Southeast Asia. Rice grains sometimes show some defective phenotypes, resulting in reduced eating quality: notched grain caused by unbalanced development of the caryopsis compared to glume size (Lin et al. 2017, Takeda and Takahashi 1970), reduction of grain size caused by high night temperatures (Morita et al. 2005), and chalkiness induced by some environmental factors including high temperature, high humidity, or cultural practices at the ripening stage (Zhou et al. 2015). These grain defects have been known to be controlled by genetic factors (Kobayashi et al. 2007, Takeda and Takahashi 1970). To improve these grain qualities, the mechanisms generating these defects need to be analyzed. For this, a sectioning sample of the grain is very useful, as Morita et al. (2005) examined the endosperm cell size and number in a cross-section of a rice grain matured under high night temperatures. They also determined the endosperm cell size and number using digitalized hand-tracing images of 20-μm thick cross-sections. For this analysis, many thin cross-section samples are needed for confirming the influence of some stresses. In this regard, it is important to rapidly produce many thin cross-section samples.
Kawamoto (2003) reported the method for preparing thin frozen-sections using an adhesive film and producing almost intact sections, which were very thin and little damaged, from many organisms such as animals, plants and insects.
In this report, we tried to obtain the cross and longitudinal sections of a whole matured rice grain using cryomicrotome by the Kawamoto method using the specific adhesive film (Kawamoto and Kawamoto 2014). We were able to obtain almost perfect sections of the whole grain with high-quality cell morphology and orientation.
The rice grain used in the study was japonica variety, Koshihikari. The grains were dipped in water at room temperature overnight, then subjected to sectioning by cryomicrotome according to the Kawamoto method (Kawamoto and Kawamoto 2014) as follows:
A cross section of the Koshihikari grain was shown in Fig. 1A. It was observed that some parts of outermost exosperm, including the pericarp and seed coat of the grain, were deleted. The darkly stained outer part is the aleurone layers. A single layer was found from the ventral side to the dorsal side of the arrows, and multiple layers were developed around the dorsal peripheral side from arrows (Fig. 1B). Very clear endosperm cell shapes were observed. Especially, cell shapes at the upper side in Fig. 1A were different from those at the lower side. There were many polygonal cells at the lower side (Fig. 1C), whereas there were many relatively lengthwise cells at the upper side. The reason for the difference in cell shapes between the upper and lower sides was unknown. At the central area from ventral to the dorsal side through the central point, slender elongated cells were observed (Fig. 1D). A longitudinal section of the grain was shown in Fig. 2A. It was observed that a few aleurone layers were developed at the bottom and dorsal peripheral side (Fig. 2B). In general, lengthwise cells stretched toward the central part were found at ventral and dorsal sides of the endosperm (Fig. 2B). The embryo facing endosperm cells seemed to be collapsed (Fig. 2C). Since all sections from 16 grains showed collapse of the embryo facing endosperm cells, these collapse phenomena were considered no to be artificial during section production. The embryo facing endosperm cells slightly collapsed with cell death due to ROS generation during seed development (Nagasawa et al. 2013). Furthermore, it was reported that water absorption led to endosperm cell collapse from embryos facing the endosperm region (Hoshikawa 1993). Thus, the collapsed of embryo facing the endosperm region may increase due to water absorption. In addition, the central portion was found to consist of a population of small cells (Fig. 2D). Fig. 3A showed the saggital section of the grain.
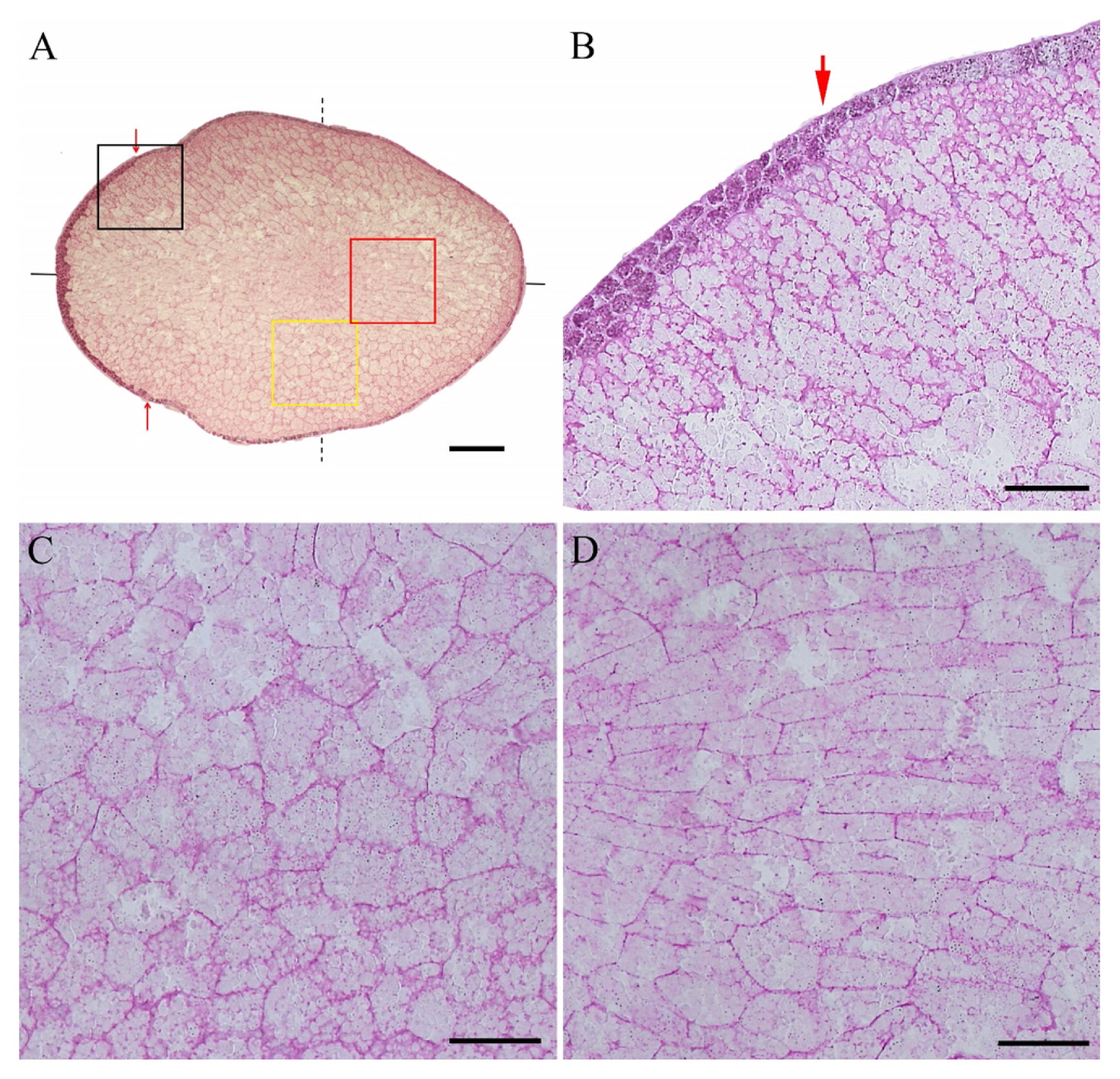
Thin cross section of Koshihikari grain (4 μm thick). A; Whole view of the grain section. Red arrow shows a change of the aleurone layer number. Black and dotted lines indicate the longitudinal section and saggital section, respectively. Bar = 400 μm. B; Magnification of aleurone layer indicated by arrow. Bar = 100 μm. C; Magnification of yellow rectangle at side portion. Bar = 100 μm. D; Magnification of red rectangle at dorsiventral portion. Bar = 100 μm.
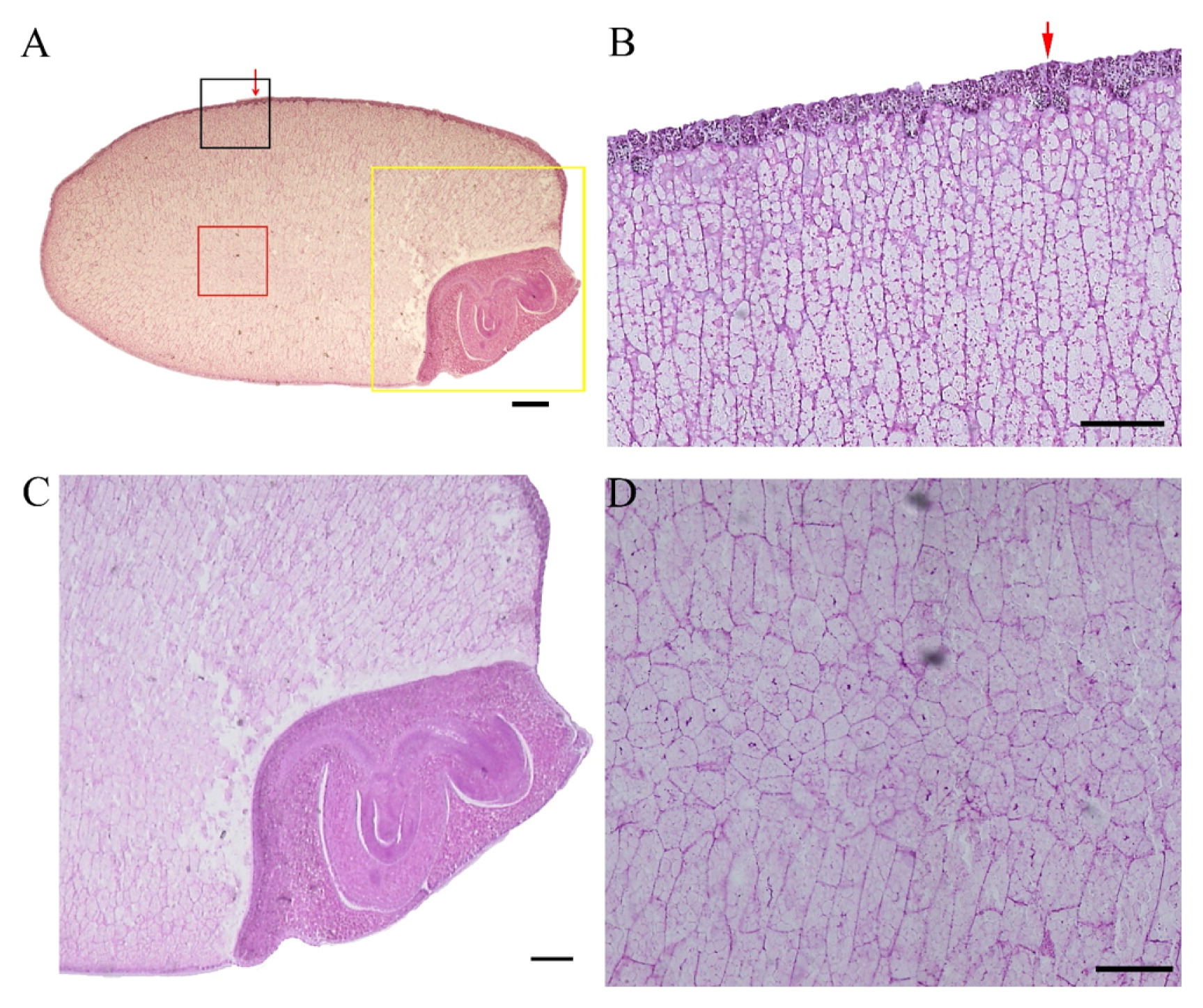
Thin longitudinal section of Koshihikari grain (4 μm thick). A; Whole view of the section. Red arrow shows a change of the aleurone layer number. Bar = 400 μm. B; Magnification of black rectangle included the red arrow–showing part in A. Bar = 100 μm. C; Magnified embryo part in yellow rectangle. Bar = 200 μm. D; Magnified central part of the endosperm in red rectangle. Bar = 100 μm.

Thin saggital section of Koshihikari grain (4 μm thick). A; whole view of the section. Bar = 400 μm. B; Top part of the grain magnified in black rectangle. Bar = 200 μm. C; Epithelium cells and vascular bundle of the scutellum magnified in yellow rectangle in A. Bar = 100 μm
Cell shapes of the endosperm were similar to those in the longitudinal section. There were polygonal cells at the top part of the grain, and multiple layers of aleurone were observed at the top of the grain (Fig. 3B). Furthermore, epithelium cells and cell shape around vascular bundle of the scutellum were clearly observed as shown in Fig. 3C. Endosperm cells accumulate starch and proteins as the grain ripens. Since the starchy endosperm is hydrophilic and becomes sticky, it is difficult to obtain an intact thin section of the ripened grain. As shown in Figs. 1A, 2A and 3A, the intact cross section and longitudinal thin sections of the ripened grain were successfully and easily obtained using the Kawamoto method (Kawamoto and Kawamoto 2014).
Intact sectioning is an essential method to observe the structures of interesting organs or tissues of an organism, and the high-quality intact sections should be easily obtained. For producing thin sections of plant tissue, the paraffin section method is usually performed. This method produces uniform thin section. Ogawa et al. (2001) made paraffin sections of whole rice grain using special adhesive tape for 3D visualization of the grain. Although the section of the whole grain was observed, the quality of the section sample was poor because cell shapes of the grain were not clear. The paraffin section method has important merits for obtaining thin section samples. However, the fixation process with some fixative and several steps for paraffin infiltration in tissue are needed before sectioning. During this process, tissue components including protein, enzymes and RNA may be removed or degenerate, and some artifacts may be produced. On the other hand, fresh frozen-sections of tissue are advantageous to retain the subcellular components including protein, DNA and RNA. However, it has been difficult to readily transfer thin sections from freshly frozen samples to slide glasses. For this purpose, Kawamoto (2003) developed a frozen sectioning system using the specific adhesive film to easily obtain a very thin section without any damages and distortions because the sections are supported with an adhesive film till being mounted. The method was also applicable for obtaining the cross section of the whole leaf of rice (Maekawa and Kawamoto 2002).
Since high-quality thin sections of all tissues, including hard tissues, are easily produced by the Kawamoto method, the section can be useful in many analytical fields, e.g., histology, enzyme histochemistry, immune histochemistry, in situ hybridization and mass imaging. For example, immunohistological analysis using frozen section of mouse brain by Kawamoto method clearly demonstrated that some pathogenic cells accumulated at specific vessels under stress conditions, resulting in brain micro-inflammation (Arima et al. 2017). Recently, the freeze-dried section was found to be highly suitable for Laser Microdissection (LMD) for gene analysis (Kawamoto and Kawamoto 2014). Actually, fresh frozen section of mouse spine produced by Kawamoto method reveal the part at which the pathogenic cells were accumulated and was effectively utilized for LMD for concerned gene expression (Arima et al. 2012). Although any applications of LMD for gene expression using frozen-sections of plants by Kawamoto method have not been reported so far, it is expected that pinpoint gene analysis of plant tissue, including the seed or grain, could be promoted by the Kawamoto method.
We thank Ms. Midori Kawamoto (Radioisotope Research Institute, School of Dental Medicine, Tsurumi University, Japan) for her technical support. This work was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan [No. 16K07561] and the Joint Research Program implemented at the Institute of Plant Science and Resources, Okayama University in Japan (No. 2942 to K.T.), and the NIBB Cooperative Research Program (17-331 to M.M.).