2018 Volume 68 Issue 3 Pages 360-366
2018 Volume 68 Issue 3 Pages 360-366
Lilies (Lilium spp.) are one of the most important floricultural crops. As most lily cultivars have originated from interspecific hybridization, they usually have complex genome composition and occasionally fail to develop normal gametes. Further improvement of lily cultivars by sexual crossing requires evaluation of gamete development and subsequent male and female fertility. Although male fertility is easily evaluated through microscopic observation after staining or by pollen culture for germination, evaluation of female fertility is difficult, because gametes develop inside an ovule within an ovary. Lilium species have the Fritillaria type of embryo sac, which, at maturity, consists of a haploid egg apparatus, including one haploid egg cell and two haploid synergids, two polar nuclei (one haploid nucleus and one triploid nucleus) and three triploid antipodal cells. Compared to the Polygonum type of embryo sac, composition of the embryo in the Fritillaria type of embryo sac is complex. We developed an efficient microscopic observation technique for ovules using the clearing procedure, which allowed us to categorize abnormal patterns of female gametes and to elucidate the frequency of abnormal female gamete development. The relationship among normal embryo sac, pollen stainability and seed formation in lily cultivars is discussed.
The genus Lilium includes more than 100 species (McRae 1998, Nishikawa et al. 1999), which are classified into 6 sections as follows: Lilium (Liriotypus), Pseudolirium, Martagon, Sinomartagon (Asiatic hybrids), Archelirion (Oriental hybrids), and Leucolirion (Comber 1949). All cultivated lilies belong to the genus Lilium and have been improved mainly by interspecific hybridization. Lilies are cultivated worldwide as cut flower, potted or garden plants. A number of cross breeding programs have been attempted to produce novel cultivars by using various species in these sections. However, usually cross combinations between species belonging to different sections have difficulty in producing hybrids, due to incompatibility and incongruity, which act as pre-fertilization or post-fertilization sexual barriers (Comber 1949, Van Tuyl et al. 1991).
While attempting interspecific hybridization of lily, Asano and Myodo (1977) reported that pre-fertilization barriers could be overcome by using the cut-style technique. Furthermore, cut-style pollination technique combined with treatment with plant growth regulators effectively produced hybrids (Li and Niimi 1995, Van Tuyl et al. 1988). On the other hand, post-fertilization barriers in lily could be overcome by embryo rescue culture (Niimi et al. 1996, Okazaki et al. 1994), ovules-with-placental-tissue culture (Obata et al. 2000) and ovary slice culture (Arzate-Fernández et al. 1998, Fernandez et al. 1996, Niimi et al. 1995, Ohashi et al. 1999). Culture of ovaries or ovules after in vitro pollination was also used to overcome post-fertilization barriers (Van Tuyl et al. 1991).
Up to the present date, numerous lily cultivars have been produced by applying these procedures. As a result, the genome composition of cultivars derived from inter-specific or inter-sectional crosses are extremely complex. Further breeding based on these hybrids, requires scrutiny of the fertility of male and female gametes. Pollen fertility is routinely assessed by simple methods, such as aceto-carmine staining and pollen germination in vitro. On the other hand, evaluation of female gamete fertility is still difficult, because female gamete development proceeds deep inside an ovule which in turn is borne within an ovary. Observation of female gametes by the clearing procedure has been performed in Dianthus (Hoshino et al. 2000) and Alstroemeria (Hoshino et al. 2006), without sectioning. Here, we developed an effective procedure to observe female gamete development in Asiatic hybrid cultivars and L. longiflorum by applying clearing procedures. Additionally, we discuss the relationship between male and female gamete fertility and seed formation.
Asiatic hybrid cultivars ‘America’, ‘Sanciro’, ‘Mona’, ‘Las Vegas’, ‘Stella’ and ‘Sweet Kiss’ and L. longiflorum ‘Hinomoto’ were used as experimental materials. These plants were grown in a greenhouse under standard conditions. We divided flower bud development of lilies into 5 stages (Table 1, Fig. 1), as follows: stage 1; 7–6 days before anthesis, stage 2; 5–4 days before anthesis, stage 3; 3 days before anthesis, stage 4; 2 days before anthesis, stage 5; 1 day before anthesis. Table 1 lists the bud length of each cultivar used in these experiments.
| Developmental stage | Budb length (mm) | ||
|---|---|---|---|
| ‘Mona’ | ‘Las Vegas’ | ‘America’ | |
| 1 | 36.3 ± 1.08 | 44.0 ± 1.50 | 37.5 ± 1.30 |
| 2 | 50.8 ± 0.54 | 51.5 ± 1.15 | 44.0 ± 0.61 |
| 3 | 60.3 ± 1.52 | 58.3 ± 1.14 | 56.0 ± 1.12 |
| 4 | 70.3 ± 0.41 | 72.0 ± 1.17 | 68.8 ± 0.96 |
| 5 | 80.5 ± 1.82 | 83.0 ± 1.77 | 80.5 ± 1.64 |
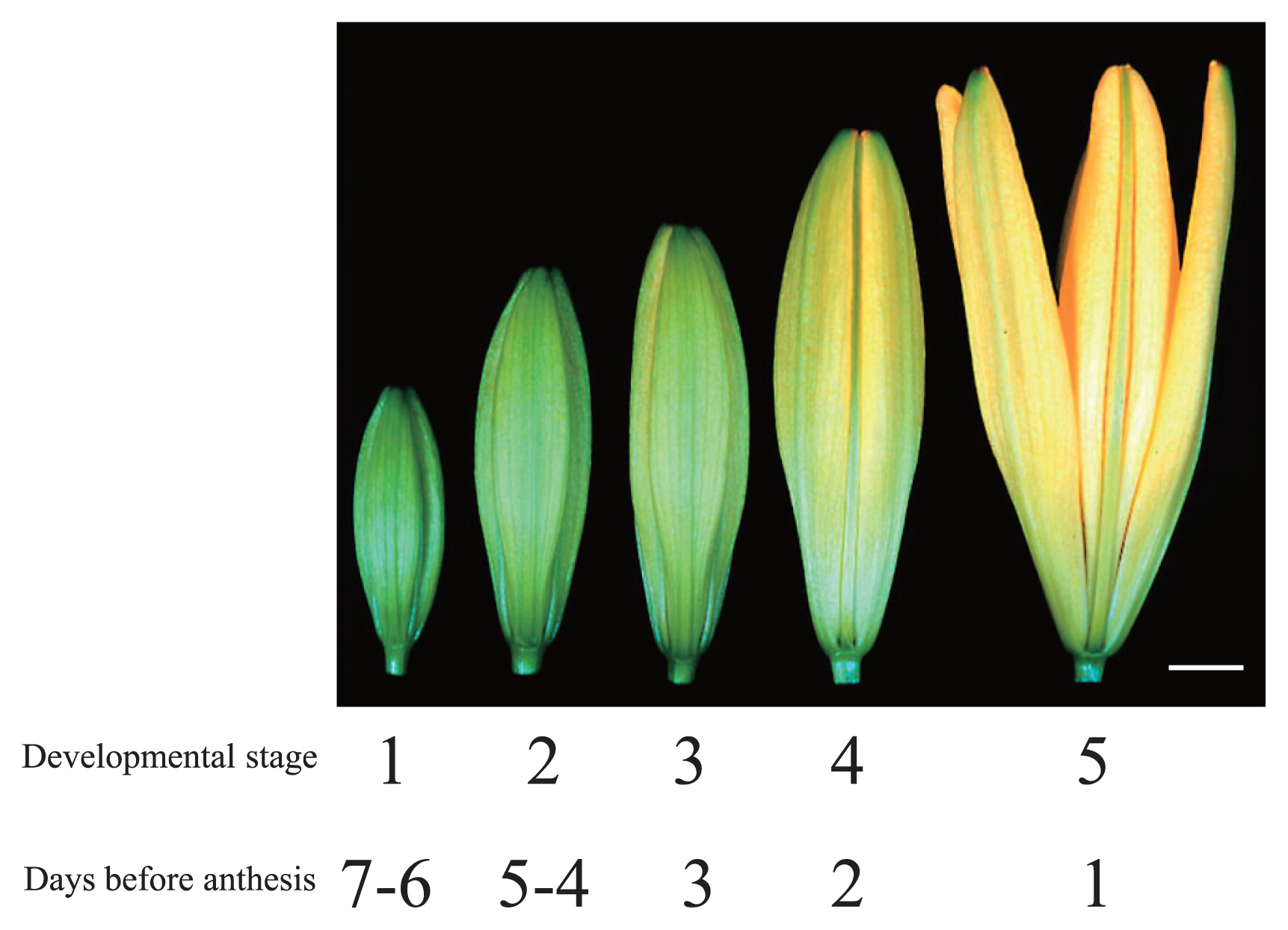
Developmental stages of flower buds in lily ‘Mona’. Bar = 1 cm.
After harvesting the flower buds, perianths were removed and ovaries were fixed with FAA solution (formalin, acetic acid, 50% ethanol, 5:5:90 by vol.; Sass 1958) or an ethanol: acetic acid (9:1, by vol.) for 1–3 days. For histological studies to observe female gamete and embryo development, ovules were subjected to one of three clearing procedures; namely, with chloral hydrate (Yadegari et al. 1994), with benzyl benzoate and dibutyl phthalate-based fluid (BB-DP-4 1/2, Herr 1992) or methyl salicylate (Stelly et al. 1984) with slight modifications as follows. Briefly, for chloral hydrate treatment, the samples were fixed in ethanol: acetic acid (9:1) for more than 24 h, which was sequentially replaced by a series of ethanol solutions (90, 70, 50 and 30%). Then, the samples were cleared with chloral hydrate: glycerol: water solution (8:1:2, w:v:v). For BB-DP-4 1/2 treatment, the samples were fixed in FAA, and dehydrated with a series of ethanol solutions (50, 70, 80, 90 and 100%). Then, the samples were cleared with a mixture of lactic acid, chloral hydrate, phenol, dibutyl phthalate, xylene, benzyl benzoate (2:2:2:2:1:1, by weight). For methyl salicylate treatment, the samples were fixed in FAA, and cleared by successive transfers to the mixtures of methyl salicylate : absolute ethanol (1:2, by vol.), methyl salicylate : absolute ethanol (2:1, by vol.), and methyl salicylate. After clearing treatments, the samples were observed using an inverted microscope (IX-70, Olympus) with Nomarski differential interference equipment.
Pollen viability and seed formationPollen viability of each cultivar was evaluated by staining with aceto-carmine. Pollen grains were observed under a microscope, IX-70 (Olympus).
For pollination tests, one day before anthesis, anthers were harvested and stored at 4°C on silica gel. All six Asiatic hybrid cultivars were used in reciprocal pollination experiments and evaluated for embryo and seed formation.
Three clearing procedures, namely, with chloral hydrate, BB-DP-4 1/2 or methyl salicylate treatment, were examined for observation of female gamete development without sectioning. Chloral hydrate did not increase the transparency of ovules. Both, BB-DP-4 1/2 and methyl salicylate were effective to allow observation of embryo sacs in whole ovules without sectioning; however, the methyl salicylate treatment made the sample fragile. As a result, BB-DP-4 1/2 was selected as the most suitable agent to observe embryo sacs in the ovules sampled.
Next, using the clearing procedure with BB-DP-4 1/2, we analyzed female gamete development in each lily cultivar. Typical Fritillaria-type embryo sac development was observed in these cultivars (Fig. 2). At maturity, the embryo sac contained haploid egg apparatus consisting of: one haploid egg cell, two haploid synergids, two polar nuclei—with one haploid and one triploid nuclei—and three triploid antipodal cells (Fig. 3). The female gamete development process is illustrated in Fig. 4.
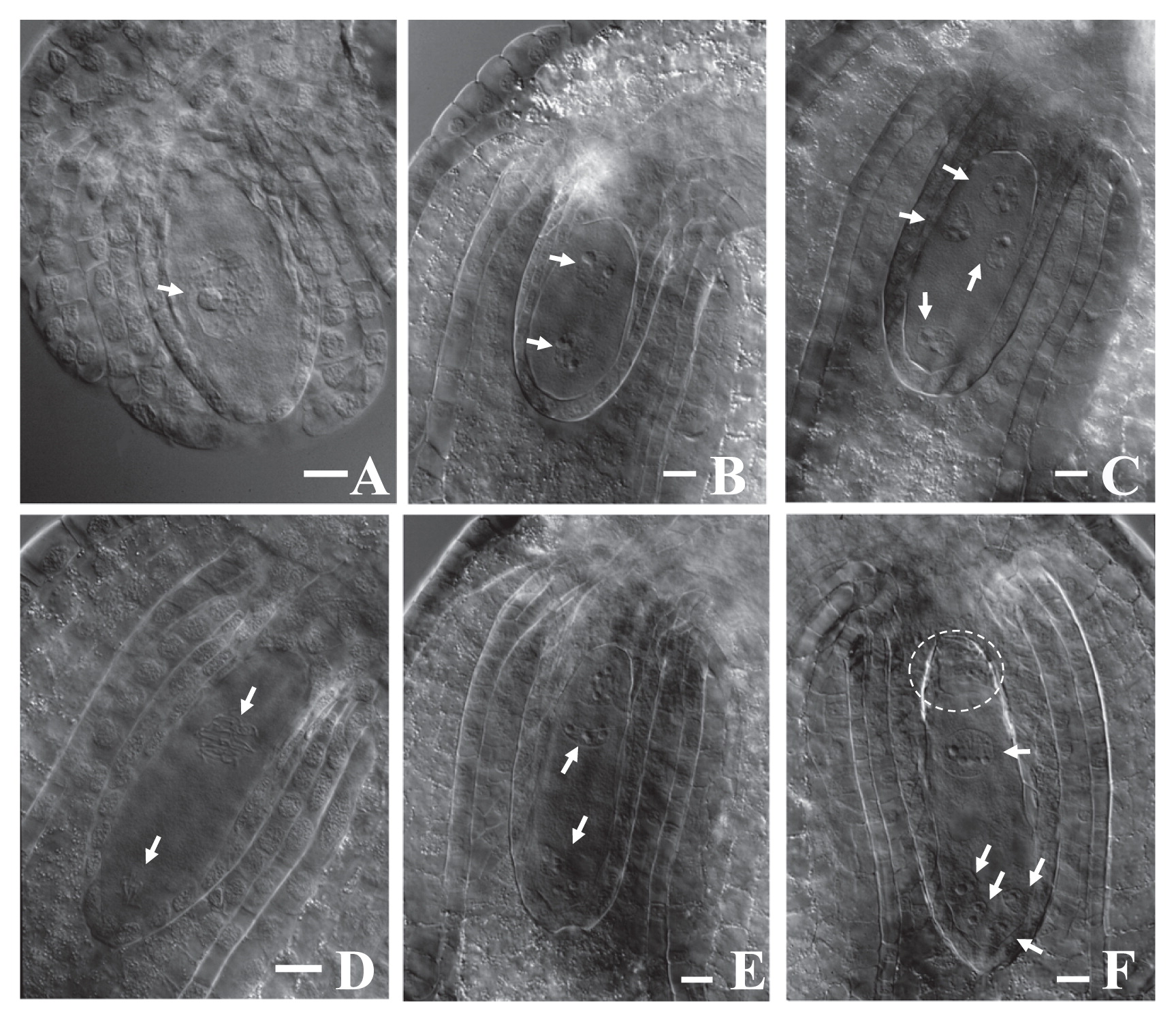
Female gamete development in lily ‘Las Vegas’. (A) megaspore mother cell (bar = 50 μm), (B) meiosis I (bar = 25 μm), (C) meiosis II (bar = 25 μm), (D) megaspore (bar = 25 μm), (E) mitosis I (bar = 25 μm), (F) mitosis II (bar = 25 μm). Arrows indicate position of nuclei in female gamete. Dotted circle shows antipodal cells.
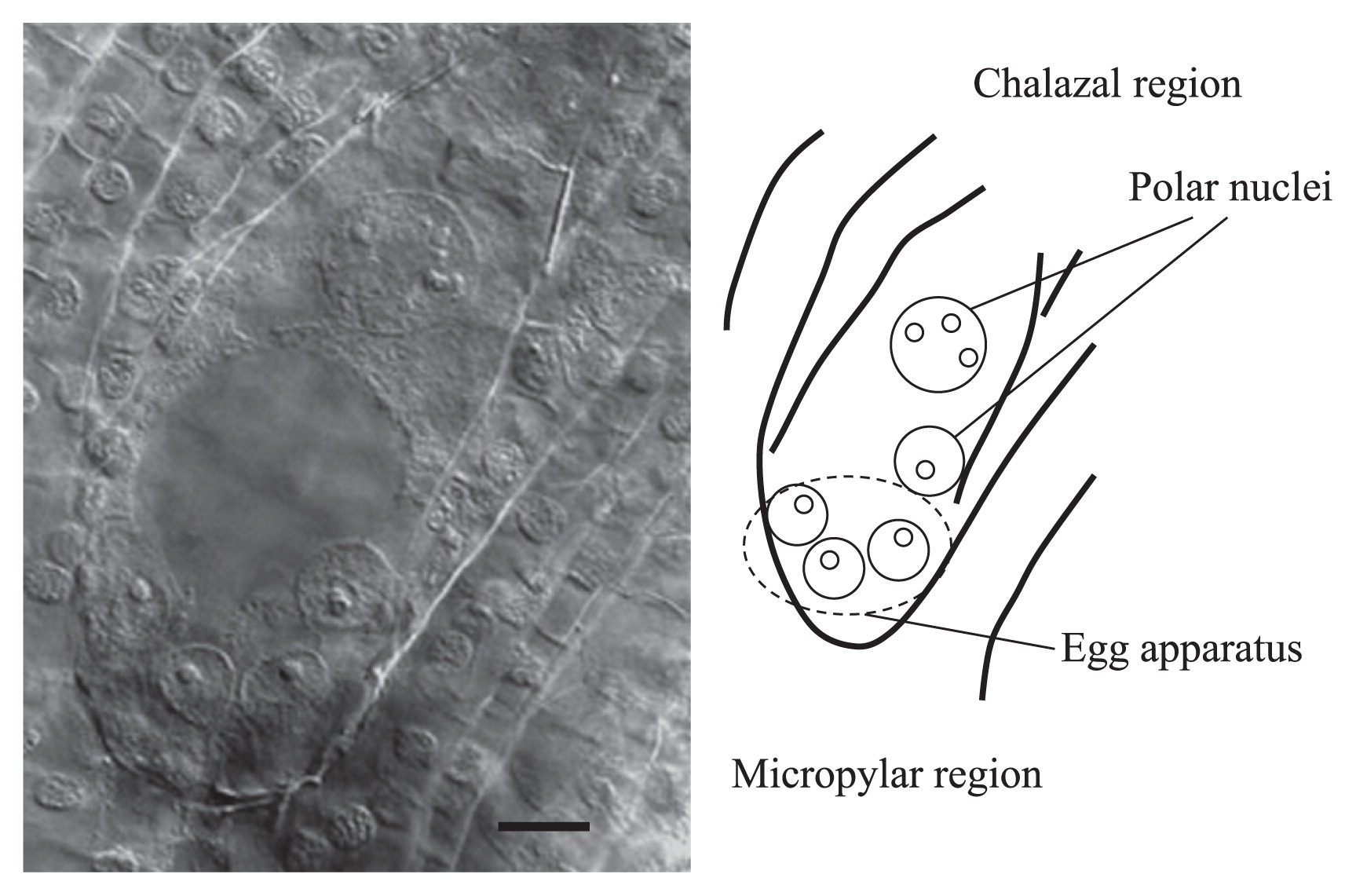
Normal embryo sac development in lily ‘Sanciro’. Bar = 25 μm.
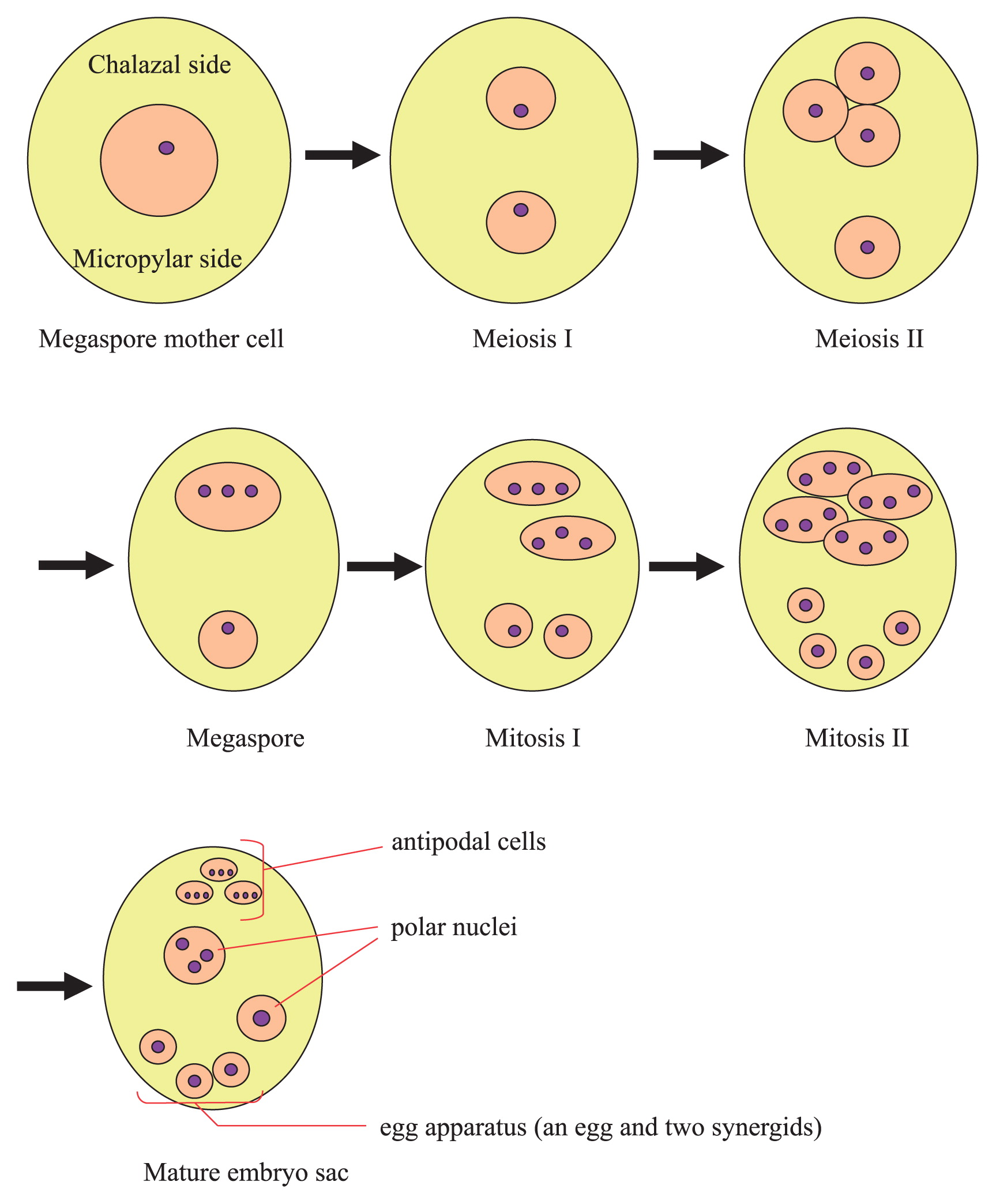
Illustration of female gamete development process in Lilium ovule in manner of Fritillaria type of embryo sac formation.
Abnormalities of embryo sacs were detected by the clearing procedure in seven cultivars on the day of anthesis (Table 2). At this stage, frequencies of normal mature embryo sacs varied among cultivars from 36.1% for ‘America’ to 92.4% for ‘Las Vegas’ and the value of L. longiflorum ‘Hinomoto’ (72.6%), which was selected from the wild population (Sakazono et al. 2009), was not so high as compared with the cultivars belonging to Asiatic hybrid group examined in the present study. Three abnormal types of embryo sac were recognized as follows: (1) lacks of both egg apparatus and one polar haploid nucleus at micropylar region, but the presence of one triploid nucleus at chalazal region (Fig. 5); (2) multicellular antipodals in chalazal region (Fig. 6), and (3) multinuclear egg apparatus in micropylar region (Fig. 7). We also observed cultivar-dependent incomplete embryo sac development, in which, embryo sac formation was arrested at Meiosis II, Megaspore stage, or Mitosis I (Table 2).
| Cultivar | Number of ovulesa examined | Normal mature embryo sac (%) | Immature embryo sac (%) arrested at | Morphologically abnormal embryo sac (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Megaspore mother cell/Meiosis I | Meiosis II | Megaspore | Mitosis I | Sum | Lacking in micropylar region | Multi-nuclear in micropylar region | Multi-nuclear in chalazal region | Sum | |||
| ‘America’ | 61 | 36.1 | 0 | 6.6 | 1.6 | 52.5 | 60.7 | 3.2 | 0 | 0 | 3.2 |
| ‘Sanciro’ | 72 | 63.9 | 0 | 0 | 0 | 5.6 | 5.6 | 30.5 | 0 | 0 | 30.5 |
| ‘Stella’ | 67 | 65.7 | 0 | 0 | 0 | 1.5 | 1.5 | 1.5 | 4.5 | 26.8 | 32.8 |
| ‘Sweet Kiss’ | 84 | 78.6 | 0 | 0 | 0 | 2.4 | 2.4 | 14.3 | 4.8 | 0 | 19.0 |
| ‘Mona’ | 203 | 90.6 | 0 | 0.5 | 6.9 | 1.0 | 8.4 | 1.0 | 0 | 0 | 1.0 |
| ‘Las Vegas’ | 210 | 92.4 | 0 | 0 | 0 | 2.8 | 2.8 | 4.3 | 0 | 0.5 | 4.8 |
| ‘Hinomoto’ | 73 | 72.6 | 0 | 1.4 | 1.4 | 24.7 | 27.4 | 0 | 0 | 0 | 0 |
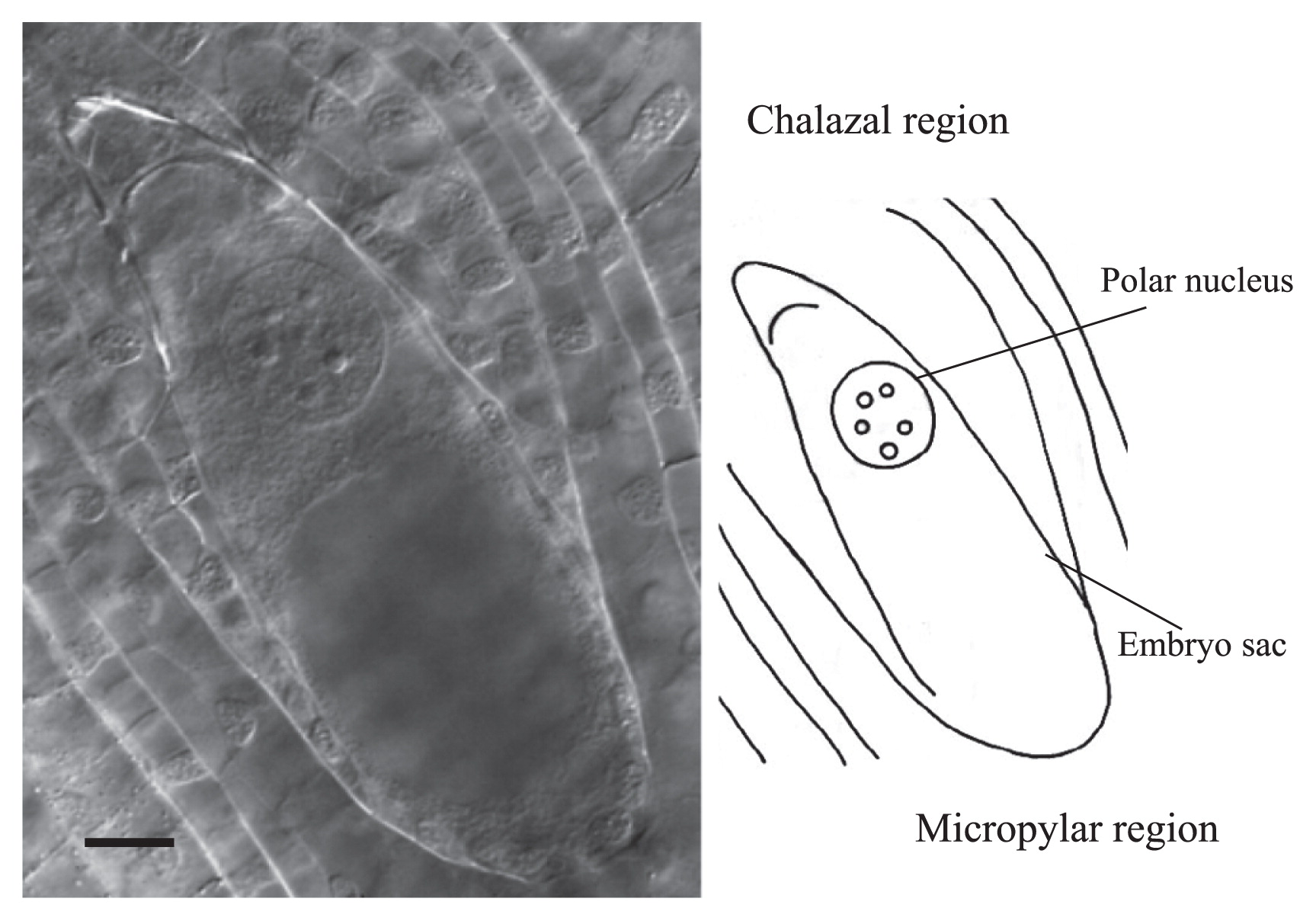
A representative ovule of lily ‘Sanciro’ showing lack of both, egg apparatus and one polar haploid nucleus at micropylar region, but presence of one triploid nucleus at chalazal region. Bar = 25 μm.
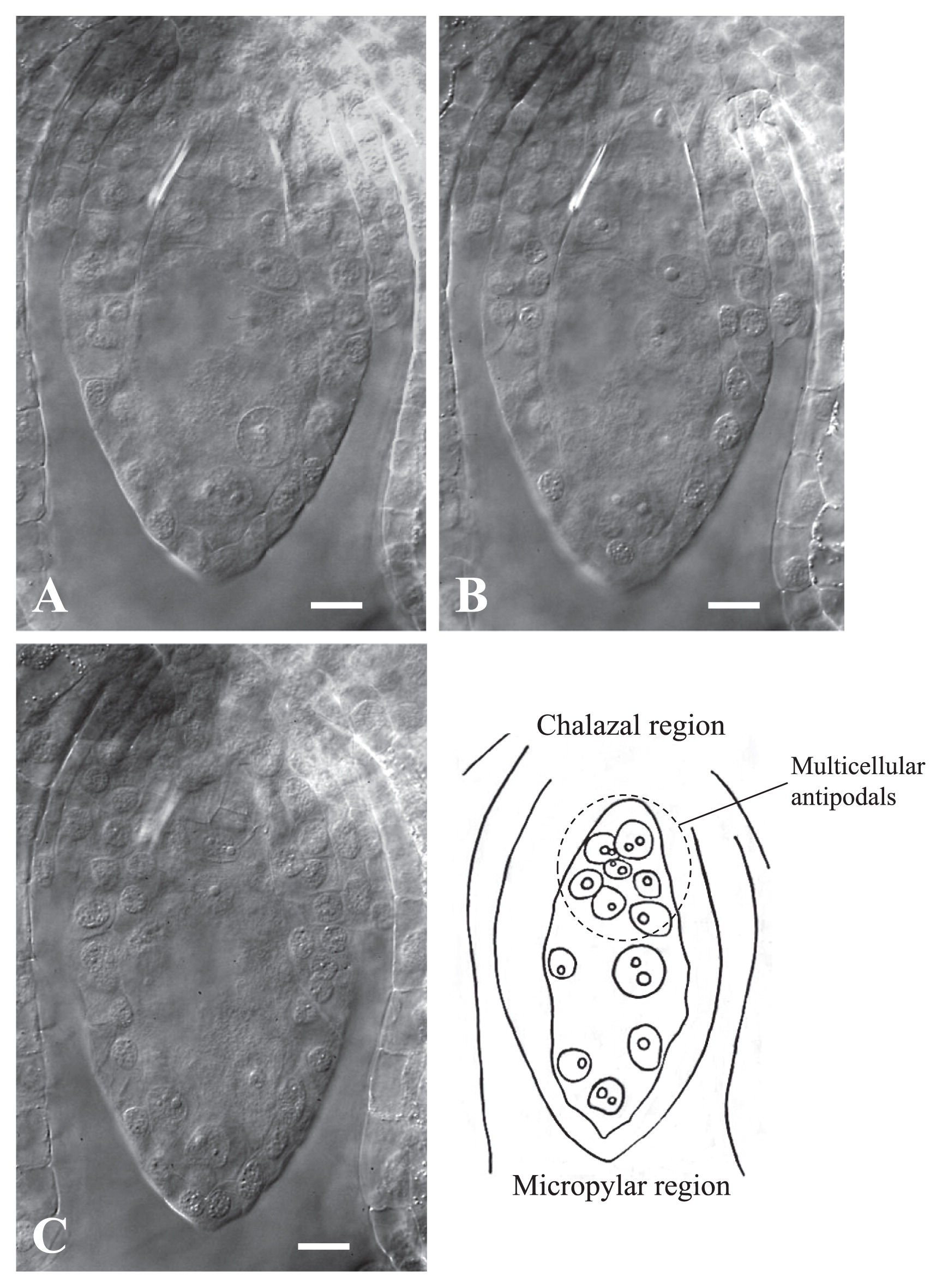
A representative ovule of lily ‘Stella’ showing multicellular antipodals in chalazal region. (A)–(C) images of the ovule sections captured with different focal planes after the clearing treatment. The illustration is composed from images of (A)–(C). Bars = 25 μm.
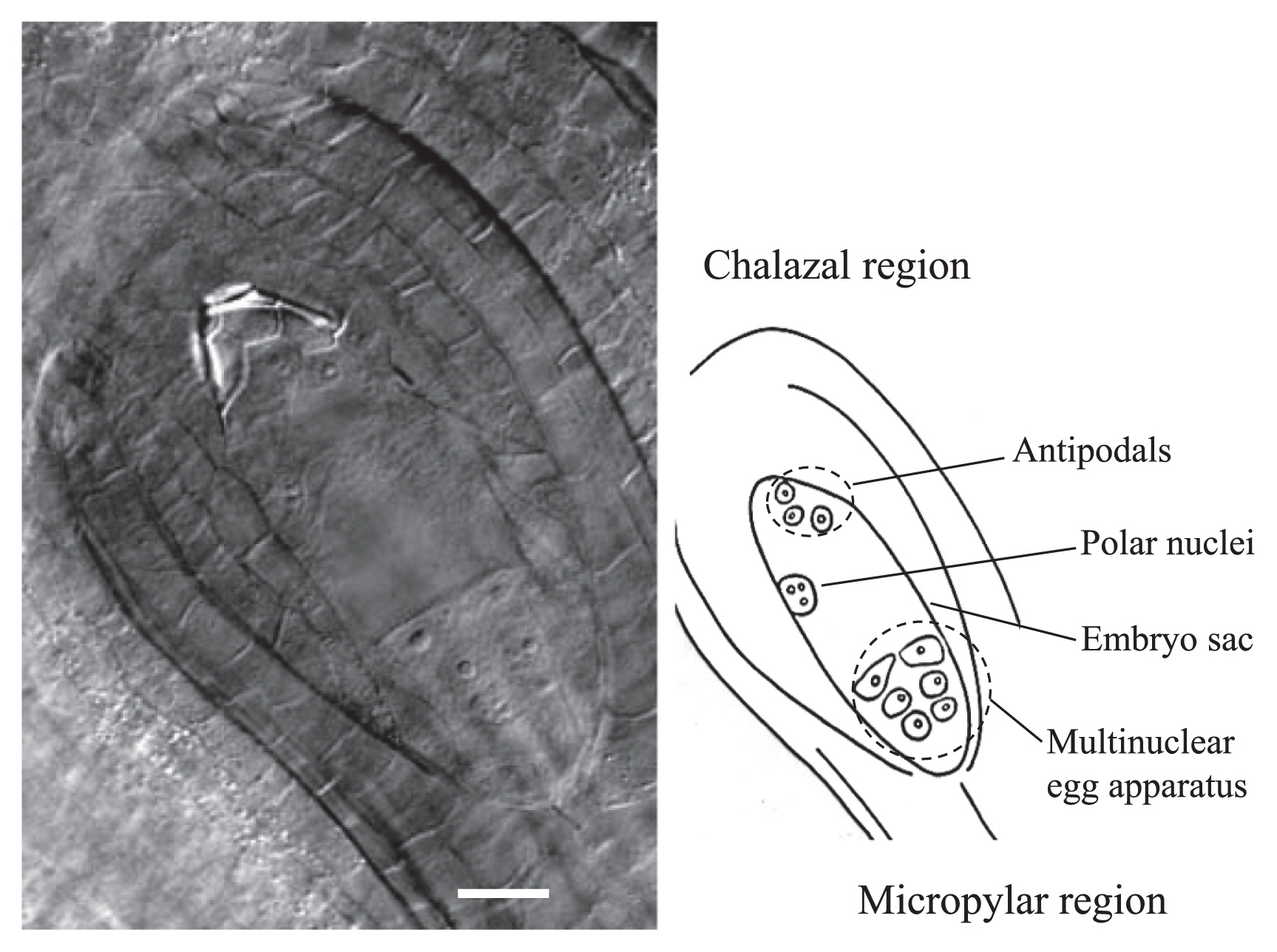
A representative ovule of lily ‘Las Vegas’ showing multinuclear egg apparatus in micropylar region. Bar = 25 μm.
Development of female gametes was examined at different developmental stages of flower buds in three cultivars, ‘Mona’, ‘America’ and ‘Las Vegas’ (Table 3). In contrast to Table 2 showing frequencies of abnormal embryo sac on the day of anthesis, 5 developmental stages of flower buds before anthesis were examined in Table 3. All three cultivars showed slightly asynchronous development of female gametes. For example, in cultivar ‘Mona’ female gametes developed as follows: megaspore mother cell was mostly found in stage 1 ovules, at a frequency of 86.3%, followed by meiosis I and meiosis II, at 11.8% and 1.9% frequency, respectively. In stage 2, approximately 85% of the observed ovules attained meiosis II (46%) or mitosis I (39%), but megaspore mother cells, remained at 10%. In stage 3, 69% of the ovules attained mitosis I, but 5% remained at the stage of megaspore mother cell. In stage 4, 72.9% of ovules underwent mitosis I, while 14.3% proceeded to mitosis II. On the other hand, megaspore mother cells were also observed in 1.6% of ovules. In stage 5, more than 88% of ovules developed into mitosis I stage, while 50.2% were observed at mitosis II stage. In ‘America’ and ‘Las Vegas’, different asynchronous development of female gametes was observed (Table 3). Female gamete development in ‘America’ seemed delayed compared to that in ‘Mona’.
| Cultivar | Stagea | Number of ovules examined | Developmental stage of female gamete (%) | Morphologically abnormal embryo sac (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Megaspore mother cell | Meiosis I | Meiosis II | Megaspore | Mitosis I | Mitosis II | Lack of nucleus in micropylar region | Multinuclei in chalazal region | Lack of megaspore mother cell | |||
| ‘Mona’ | 1 | 51 | 86.3 | 11.8 | 1.9 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 59 | 10.2 | 5.1 | 45.8 | 0 | 38.9 | 0 | 0 | 0 | 0 | |
| 3 | 42 | 4.8 | 7.1 | 19.0 | 0 | 69.1 | 0 | 0 | 0 | 0 | |
| 4 | 63 | 1.6 | 0 | 4.8 | 0 | 72.9 | 14.3 | 3.2 | 3.2 | 0 | |
| 5 | 72 | 1.4 | 1.4 | 5.6 | 0 | 37.4 | 50.2 | 4.2 | 0 | 0 | |
| ‘America’ | 1 | 52 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 69 | 95.7 | 1.4 | 0 | 0 | 0 | 0 | 0 | 0 | 2.9 | |
| 3 | 61 | 85.3 | 4.9 | 4.9 | 0 | 0 | 0 | 0 | 0 | 4.9 | |
| 4 | 67 | 9.0 | 9.0 | 82.0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 5 | 64 | 10.9 | 7.8 | 76.6 | 0 | 4.7 | 0 | 0 | 0 | 0 | |
| ‘Las Vegas’ | 1 | 77 | 13.0 | 2.5 | 61.3 | 2.5 | 20.7 | 0 | 0 | 0 | 0 |
| 2 | 59 | 0 | 6.8 | 1.7 | 0 | 91.5 | 0 | 0 | 0 | 0 | |
| 3 | 50 | 0 | 0 | 0 | 0 | 96.0 | 0 | 0 | 0 | 0 | |
| 4 | 63 | 0 | 0 | 0 | 0 | 33.3 | 66.7 | 4.0 | 0 | 0 | |
| 5 | 64 | 0 | 0 | 0 | 0 | 7.8 | 92.2 | 0 | 0 | 0 | |
Although no abnormal embryo sac was observed in stage 1, abnormalities, such as lack of nucleus in micropylar region, presence of multi-nuclei in chalazal region and abortion of megaspore mother cell, were detected in ovules during stage 2 to stage 5 in all three cultivars. These abnormal embryo sacs might be formed by aberration of mitosis in antipodal cells or by degradation of the egg apparatus and the polar nuclei.
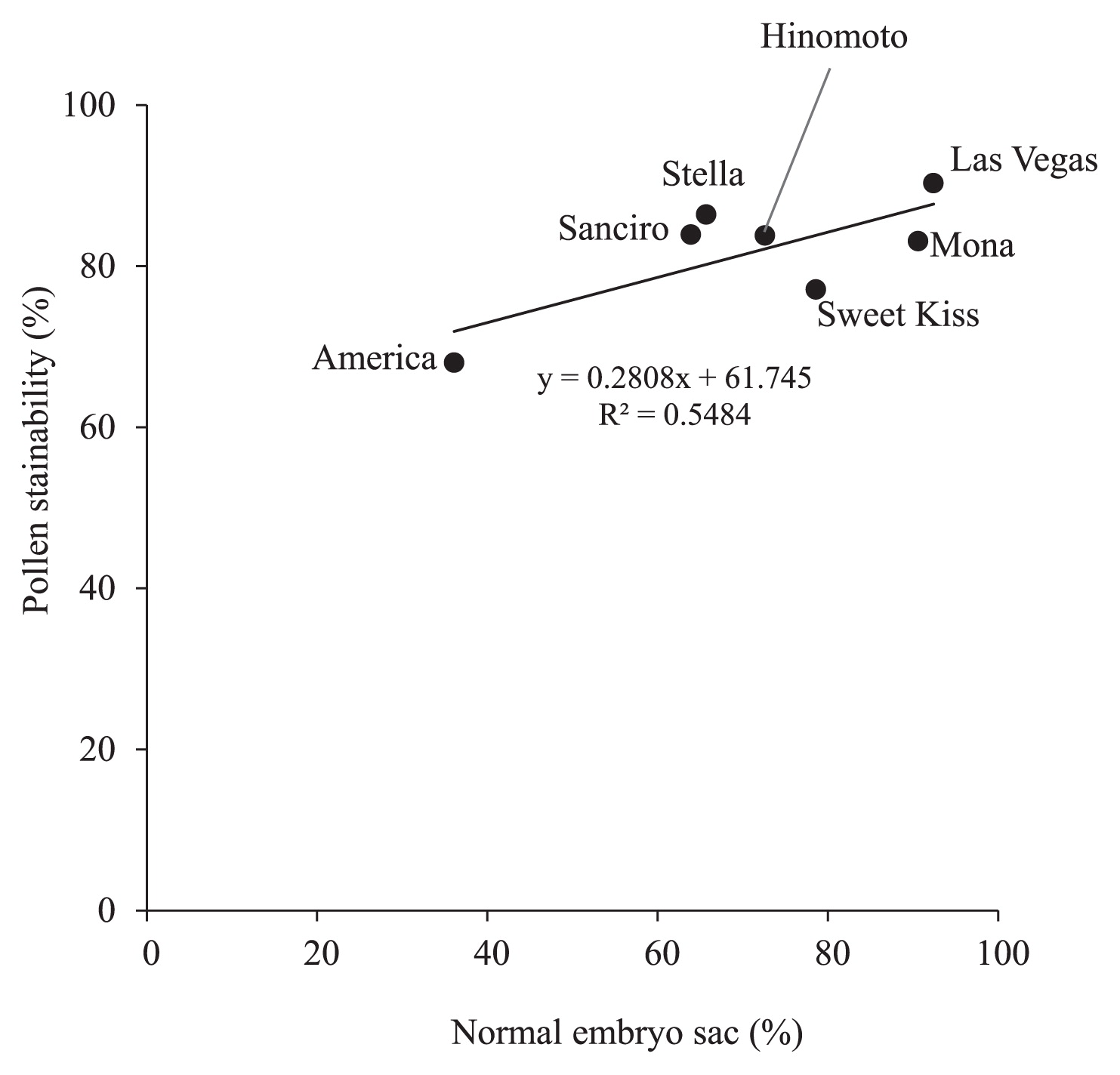
Evaluation of seed set and relationship between abnormal embryo sac formation and pollen stainabilityPollen fertility of each cultivar was evaluated by staining with aceto-carmine (Table 4). ‘America’ showed a relatively low fertility (68.1%), whereas ‘Sanciro’, ‘Sweet Kiss’, ‘Stella’ and ‘Mona’ showed higher fertility, varying from 83.1% to 90.3%. When these pollen fertility data were compared with the data of normal embryo sac frequencies, correlation was observed between them (Fig. 8). Especially, cultivar ‘America’ with high abnormal embryo sac frequency showed low pollen stainability, and cultivar ‘Las Vegas’ with high normal embryo frequency showed high pollen stainability.
| Cultivar | Number of pollen grains examined | Pollen stainability (%) | |
|---|---|---|---|
| Asiatic hybrid | ‘America’ | 506 | 68.0 |
| ‘Sanciro’ | 392 | 83.9 | |
| ‘Sweet Kiss’ | 280 | 77.1 | |
| ‘Stella’ | 561 | 86.4 | |
| ‘Mona’ | 508 | 83.1 | |
| ‘Las Vegas’ | 514 | 90.3 | |
| L. longiflorum | ‘Hinomoto’ | 361 | 83.8 |
Subsequently, cross-pollination was performed among these cultivars and the frequencies of seed formation and seed abnormalities were evaluated (Table 5). Two types of seed abnormality—embryo-less seed with normal endosperm, and completely sterile seed without embryo nor endosperm—were recorded. When seed formation frequency was compared with the frequency of normal embryo sac formation (Fig. 9), the data did not evidence any clear correlation between frequency of normal female gametes and seed formation. Cultivar ‘America’ showing low frequency of normal embryo sac—used as the female parent—produced seeds at 42.4% to 59.2%. Other cultivars showing high frequencies of normal embryo sac, ‘Las Vegas’ and ‘Mona’ gave variable seed formation (7.5% to 75.5%).
| Cross combination | Number of ovules examined | Number of seeds with normal embryo and endosperm (%) | Number of seeds without embryo but with normal endosperm (%) | Number of seeds without embryo nor endosperm (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Female (Ovule) | Male (Pollen) | |||||||
| ‘America’ | ‘Las Vegas’ | 1178 | 697 | (59.2) | 108 | (9.2) | 373 | (31.6) |
| ‘America’ | ‘Mona’ | 594 | 252 | (42.4) | 120 | (20.2) | 222 | (37.4) |
| ‘Sweet Kiss’ | ‘America’ | 245 | 0 | (0.0) | 1 | (0.4) | 244 | (99.6) |
| ‘Sweet Kiss’ | ‘Las Vegas’ | 258 | 18 | (7.0) | 20 | (7.6) | 220 | (85.3) |
| ‘Mona’ | ‘Las Vegas’ | 612 | 462 | (75.5) | 40 | (8.6) | 110 | (23.8) |
| ‘Mona’ | ‘America’ | 811 | 234 | (28.8) | 287 | (35.4) | 290 | (35.8) |
| ‘Las Vegas’ | ‘Mona’ | 680 | 414 | (60.9) | 92 | (13.5) | 174 | (25.6) |
| ‘Las Vegas’ | ‘America’ | 683 | 51 | (7.5) | 39 | (5.7) | 593 | (86.8) |
| ‘Stella’ | ‘America’ | 879 | 26 | (3.0) | 34 | (3.9) | 785 | (89.3 |
| ‘Stella’ | ‘Mona’ | 809 | 1 | (0.1) | 20 | (2.5) | 788 | (97.4) |
| ‘Stella’ | ‘Stella’ | 571 | 4 | (0.7) | 4 | (0.7) | 563 | (98.6) |
| ‘Stella’ | ‘Las Vegas’ | 558 | 0 | (0.0) | 17 | (3.0) | 541 | (97.0) |
| ‘Sanciro’ | ‘Mona’ | 815 | 29 | (3.6) | 87 | (10.7) | 699 | (85.7) |
| ‘Sanciro’ | ‘Stella’ | 799 | 232 | (29.0) | 171 | (21.4) | 396 | (49.6) |
| ‘Sanciro’ | ‘America’ | 769 | 55 | (7.2) | 81 | (10.5) | 633 | (82.3) |
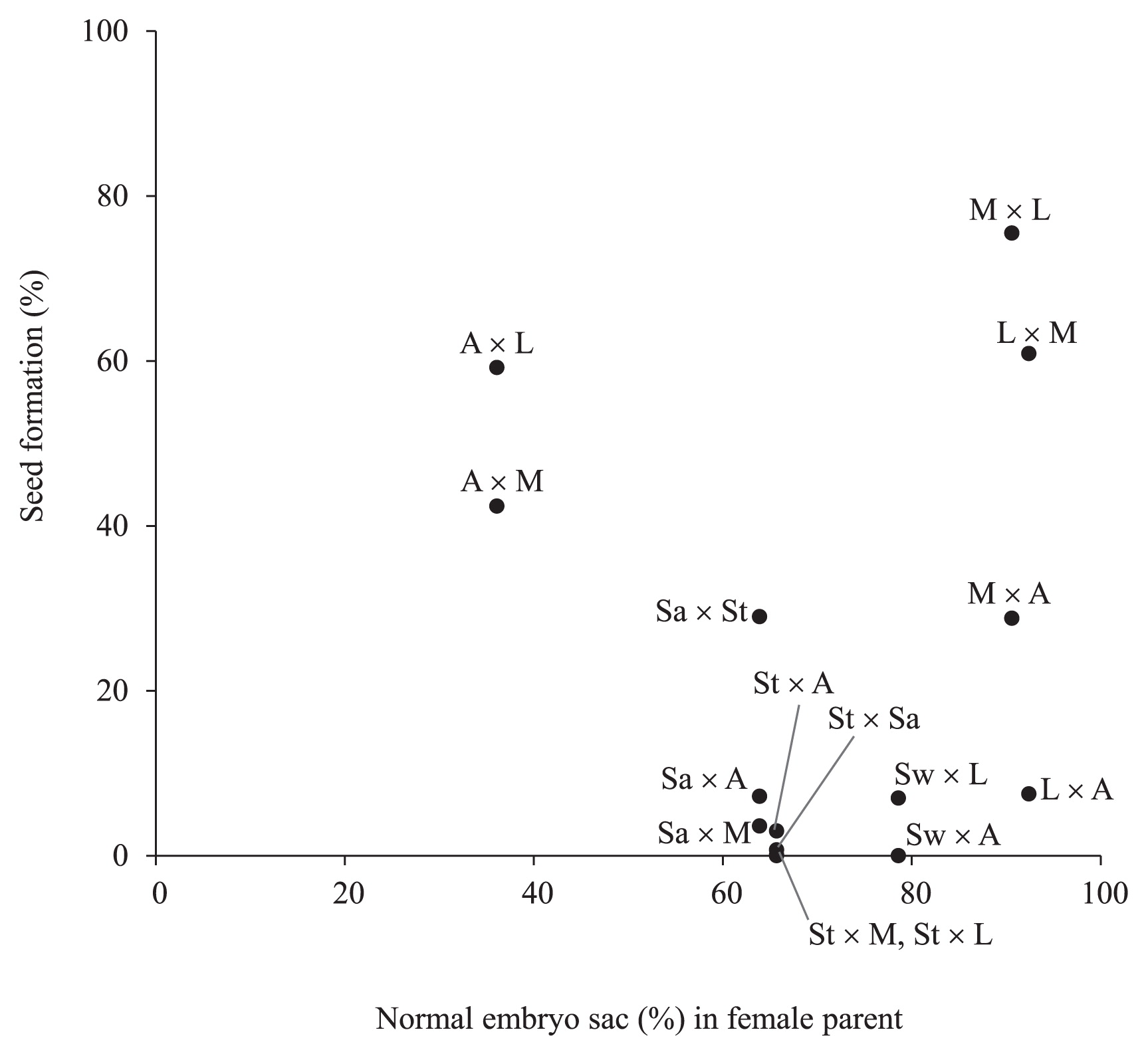
Relationship between seed formation from several cross combinations and normal embryo sac formation in Lilium cultivars. The data of normal embryo sac formation (%) and seed formation showing number of seeds with normal embryo and endosperm (%) are derived from Table 2 and Table 5, respectively. (A) ‘America’, (L) ‘Las Vegas’, (M) ‘Mona’, (Sa) ‘Sanciro’, (St) ‘Stella’, and (Sw) ‘Sweet Kiss’.
As they develop deep inside ovules, routine observation of female Lilium gametes is very difficult. Therefore, up to the present date, few studies had demonstrated abnormalities in these gametes. In the present study, we tried to observe female gamete development in Lilium cultivars by using clearing techniques which allow whole ovule observation without sectioning. As Cooper (1935), Sargant (1896) and Zhou et al. (2012) described previously, the genus Lilium has the Fritillaria type of embryo sac, which is much more complicated than the Polygonum type of embryo sac, commonly observed in most plant species. Our tests with three different clearing procedures identified BB-DP-4 1/2 as the one which enabled the clearest observation of the development of female gametes in Lilium cultivars having the Fritillaria type of embryo sac. Based on the normal Fritillaria type of embryo sac development, we were able to categorize abnormal patterns of female gametes as follows: 1) lack of both, egg apparatus and one polar haploid nucleus, but with one triploid nucleus present, 2) multicellular antipodal cells, and 3) multinuclear egg apparatus. These abnormalities might relate to the complicated genomic backgrounds of the most cultivars used in the present study, which are Asiatic hybrids originated from interspecific hybridization by using various species of the section Sinomartagon (van Tuyl and Arens 2011).
Zhou et al. (2013) described that the odd-tetraploid Lilium ‘Honesty’ showed high female fertility, despite its male-sterile nature. Our data indicated that pollen viability was related to normal embryo sac development in each cultivar (Fig. 8). Asiatic hybrid cultivar ‘Las Vegas’, an interspecific hybrid, showed higher normal embryo sac frequency and pollen stainability than those of L. longiflorum ‘Hinomoto’. Thus, it is necessary to evaluate female and male fertility independently. When they were crossed as female parents with other cultivars, no clear correlation was observed between frequency of normal female gametes and seed formation. For example, cultivar ‘America’ with low normal embryo sac formation generally exhibited relatively high seed formation rates (Fig. 9). Since the frequency of morphologically abnormal embryo sac of ‘America’ was relatively low at 3.2% just after anthesis (Table 2), it might be considered that remaining embryo sacs were still immature at this stage but developed normally soon after anthesis. Therefore, such cultivars still may have the chance to produce some seeds, depending on the choice of male cultivars by utilizing remaining normal female gametes. On the other hand, cultivars with high normal embryo sac formation also displayed different seed formation ability, depending on the kind of cultivars used for the cross. Hence, these results suggest that seed formation was mainly determined by the cross incompatibility between female and male parents, rather than by female gamete abnormality of one kind or another.
Thus, we demonstrated an easy and successful clearing procedure that made it possible to observe female gamete development. A clear correlation between female fertility and ability for seed formation was not observed in this study. However, our clearing method will prove useful, not only for evaluating the effect of various environmental factors on female gamete formation in Lilium species, but also, for evaluating the effects of female fertility and abnormality on various fertilization and seed production issues in any other species.