2019 年 69 巻 2 号 p. 205-214
2019 年 69 巻 2 号 p. 205-214
Generally, Pistacia species are dioecious, but monoecious strains in several populations have been found, providing excellent models for studying sex differentiation and sex determination mechanisms. Although the mechanisms of sex determination and sex evolution have been extensively studied, related research on heterozygous woody plants is limited. Here, we discuss the expressions of various sex types, which showed broad diversity and complex instability. We have also reviewed the sex determination systems in the plant kingdom and the morphological, cytological, physiological, and molecular aspects of the sex-linked markers in Pistacia trees. Moreover, hypotheses to explain the origin of monoecy are discussed, which is more likely to be the interaction between sex-related genes and environment factors in female plants. Besides, further prospects for the utilization of monoecious resources and the research directions of sex determination mechanism are proposed. This study provides important information on sex expression and provides more insights into sex differentiation and determination.
The genus Pistacia (Anacardiaceae) comprises more than 12 species or variants, including P. mexicana H.B.K., P. texana Swingle, P. lentiscus L., P. atlantica Desfontaine, P. chinensis Bunge, P. terebinthus L. (with the sub-species P. palaestina Bois), P. khinjuk Stocks (sin. P. integerrima Stewart), P. formosana Mats, (sin. P. philippinensis), P. mutica Fisher and Meyer, P. cabulica Stocks, P. vera L., and P. oleosa Lour Willd. (Avanzato and Quarta 2004, Wang 2005, Zohary 1952). It was generally believed that all Pistacia species were dioecious (Hormaza and Wünsch 2011), but a few exceptional gender types have been reported in P. atlantica (İsfendiyaroğlu 2007, Kafkas et al. 2000), P. terebinthus (Avanzato and Quarta 2004), P. chinensis (Bai et al. 2016, Hou 2009, Zhao 2011), a hybrid between P. vera and P. atlantica (Crane 1974), and a seedling of P. vera or hybrid between P. vera and P. terebinthus (Özbek and Ayfer 1958). Among these, P. vera (Pistachio) is the only species cultivated commercially, while the others are mostly used as its rootstocks (Zohary 1952). P. chinensis has become the preferred biodiesel tree species in China in current years owing to the high oil content of its fruit (Dong et al. 2016). In managed orchards, the male (non-fruit) trees are indispensable for pollination. The proportion of male to female pistachio is 1: 8–11 (Kafkas et al. 2003), while that of P. chinensis is 1:8, which greatly increases the cost, labor and field space (Bai et al. 2016). Therefore, the emergence of monoecious Pistacia species offers great prospects to break the dioecious constraints.
The sex determining mechanisms of unisexual species (monoecy and dioecy) are of biological significance for sex-specific evolution, classification, and identification. It may lead to techniques capable of altering the sex determination of species with low and unstable yields caused by unisexuality and the abortion of gametophytes, resulting in their increased economic values (Aryal and Ming 2014). However, the studies of plant sex determination have mainly focused on model herb plants (Huang et al. 2013, Kumar et al. 2012, Song et al. 2012); and little is known regarding highly heterozygous woody plants owing to their large genomes, limited genomic information, complex developmental processes (Niu et al. 2016), and polymorphic genetic backgrounds, which are typical for dioecious species (Song et al. 2013). Therefore, understanding the sex determination mechanisms in highly heterozygous woody plants is difficult because of the lack of a suitable model system. Fortunately, the various sex expressions of Pistacia species provide valuable resources for the exploration of sex determination mechanisms.
In this paper, the expression of all sex types in the genus Pistacia was described and works on sex identification and determination were summarized. In addition, hypotheses to explain the formation of the mechanism behind monoecism were presented. This review will increase the understanding of the origins of monoecy and sex determining mechanism.
Many studies have supported that separate sexes evolved from hermaphroditic ancestors (Charlesworth 2002, Pannell 2017), and the present sex expression in plants shows a broad diversity, involving hermaphroditic, dioecious (gynodioecious, androdioecious, and tridioecious) and monoecious (gynomonoecious, andromonoecious, and trimonoecious) systems (Heikrujam et al. 2015). In general, Pistacia species are known to be strictly dioecious (producing male and female flowers on separate individuals), but several exceptions have been described, and their sex expression types are listed in Table 1 (sorted by the year of discovery). The sex types were diverse at the individual level, including male, female, hermaphroditic, monoecious (male and female inflorescences on different branches, on the same branches, or male and female florets forming mixed inflorescences, mixed inflorescences see Fig. 1), gynomonoecious, and trimonoecious strains. In addition, some exceptions in a hybrid between P. vera and P. atlantica and P. chinensis trees proved to be fertile, while some individuals bore no fruit at all (Avanzato and Quarta 2004, Crane 1974, Wang et al. 2015). Also, multiple variations in typical characteristics have been found in florets, such as organ deformity or degradation, and the sexual distributions and ratios were different among individuals (Table 1).
| Finding year | Discoverer | Location | Species | Amount | Sex type | Fertility | Description | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||||
| 1958 | Özbek, Ayfer | In the vicinity of Antep, Turkey | Probably a seedling of P. vera or hybrid between P. vera and P. terebinthus | 2 | √a | –b | The plant was lost | |||
| 1974 | Crane | America | 1 P. atlantica, 2 hybrids between P. vera and P. atlantica | 3 | √ | √ | √ | Several floral abnormalities were found | ||
| 2000 | Kafkas | Manisa, Turkey | Wild population of P. atlantica | 9 | √ | √ | – | Pollen of monoecious plants could germinate | ||
| 2002 | Avanzato and Quarta | Bulgaria | P. terebinthus | 4 | √ | √ | ×c | No fruit, seem to have pollination problems | ||
| 2007 | İsfendiyaroğlu | Izmir, Turkey | P. atlantica | 1 | √ | √ | – | Flowers organs deformity or degradation phenomenon is serious | ||
| 2011 | Zhao | Lueyang County, Shanxi Province, China | P. chinensis | – | √ | – | Male inflorescences: female inflorescences: mixed inflorescences = 1:1:3, male flowers of mixed inflorescence deformities | |||
| 2008 | Forestry experts | Linzhou City, Henan Province, China | P. chinensis | 7 | √ | √ | – | Relatively concentrated distribution, 30 years old, wild trees | ||
| 2013–2014 | Wang et al. Bai et al. |
Tang County of Hebei Province, China | P. chinensis | 23 | √ | √ | √ | √ | √ | Some anther deformities and degradation, and anther number in the range of 1–6 |
| 2016 | ||||||||||
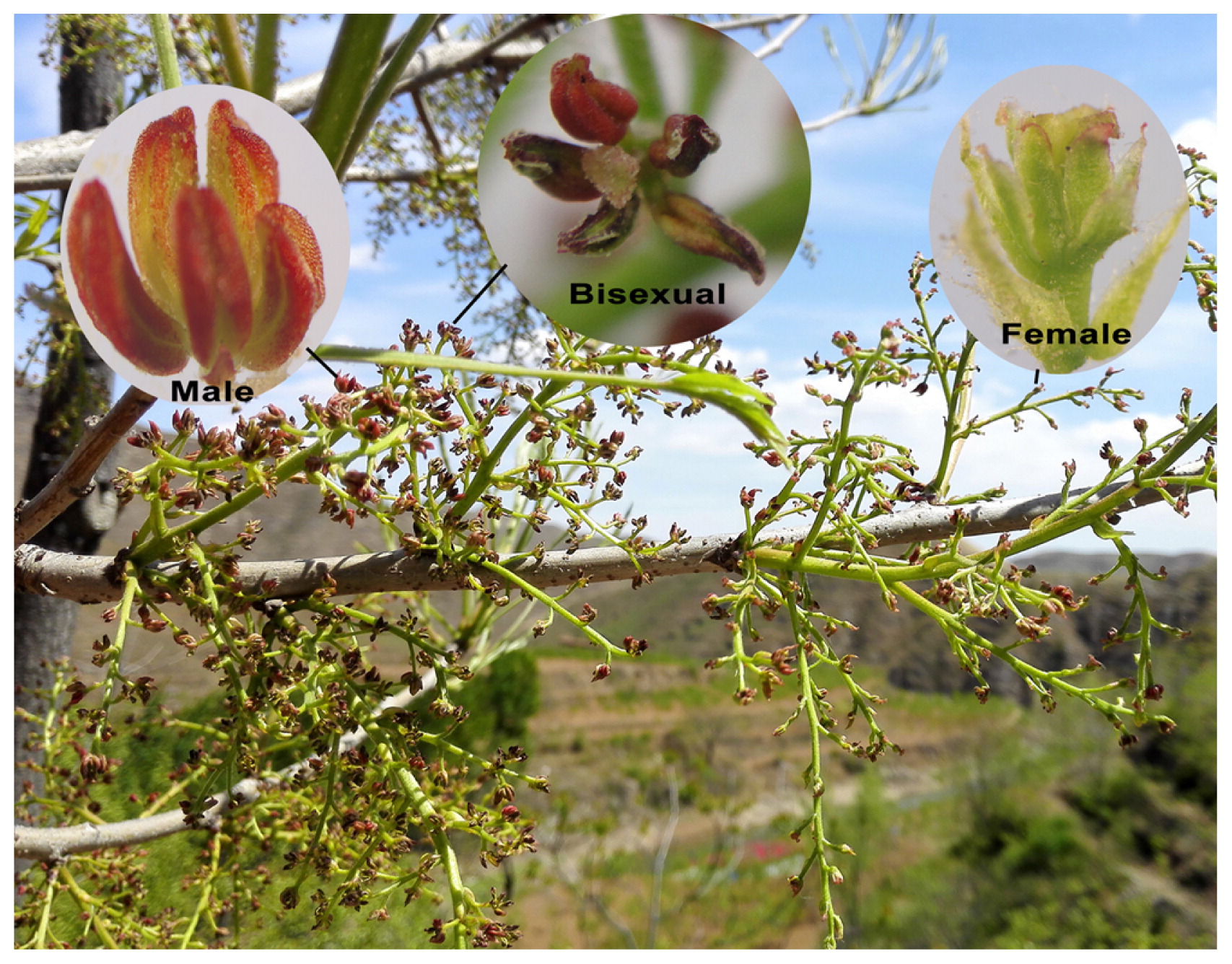
Female, male and bisexual florets on monoecious Pistacia chinensis.
More interestingly, Kafkas et al. (2000) found that the ratio of male to female inflorescences could reverse in the second year on a fully monoecious tree, and Bai et al. (2016) demonstrated that the gender type of a single shoot could change within only one year (the shoots that produced fruit last year could bear male inflorescences the next year). Therefore, Pistacia species shows various and unstable sex expressions, especially P. chinensis, which has the largest number of exceptional strains and the most varied types.
Traditionally, sex determination is considered to be the selective abortion of the gynoecium or androecium of an initially hermaphroditic floral primordia, but it should also involve the differentiation of gametophytes within the pistil or stamen (Irish and Nelson 1989). Historically, sex determination systems were often categorized as either genetic sex determination (GSD) or environmental sex determination (ESD). Sexes segregate developmentally through the meiotic segregation of alternative alleles in GSD, while from the external regulation of gene expression in ESD. Therefore, in GSD, the expression patterns are mostly invariable, while the gene contents must vary, which is reverse in ESD. However, this distinction is too strict and does not address the problem of the evolution and development of mechanisms of unisexual systems from hermaphroditic ancestors (Golenberg and West 2013).
The most influential paper on sexual evolution is that of Charlesworth and Charlesworth (1978), in which three independent and sequential mutational events were postulated (for review, see Golenberg and West 2013). This model has helped to illuminate how dioecy could evolve directly from hermaphroditism through a series of independent feminizing and masculinizing mutations that become chromosomally linked, but it is useful only when development is controlled by restrictive GSD (allelic segregation). Thus, it may not apply in monoecious and submonoecious populations, in which the development of unisexual flowers cannot be determined by allelic segregation among individual floral meristems. In monoecious species, key sex-determining genes must exist, but their expression should be regulated by the external environmental or internal physiological. In these cases, sexual developmental differentiation could be determined by internal heterogeneity within a plant (Golenberg and West 2013).
In addition, some exceptional cases of hermaphroditic, monoecious or dioecious plants have extremely labile sex systems, which may result from an inability to control sex precisely in a complex environment (Korpelainen 1998) or because lability confers an adaptive advantage (Charnov and Bull 1977). In the latter case, the environment must exert some control over sex expression (Ainsworth 2000). To date, multifarious epigenetic mechanisms have been described, involving RNA interference, DNA methylation, and histone modification (Iglesias and Cerdán 2016, Yaish 2017).
Additionally, numerous studies have proven that environmental stresses induce sexual plasticity, especially emphasizing the role of hormones in response to environmental stresses and sex determination (Aryal and Ming 2014, Heikrujam et al. 2015). Therefore, Pannell (2017) argued that the distinction between sex determination involving a genetic polymorphism and that involving responses to environmental or hormonal cues should be relaxed, because non-genetic cues might easily be converted into genetic switches.
In conclusion, the sex determination systems of plants are complicated and confusing, and their concepts, categories, and multiple cues are continuously enriched. Fig. 2 presents a combination of the existing forms of sex determination and determinants, and some factors may be associated or interact with each other. Thus, for a particular species or case, a dynamic developmental view is indispensable for discovering what determines the final sex expression.
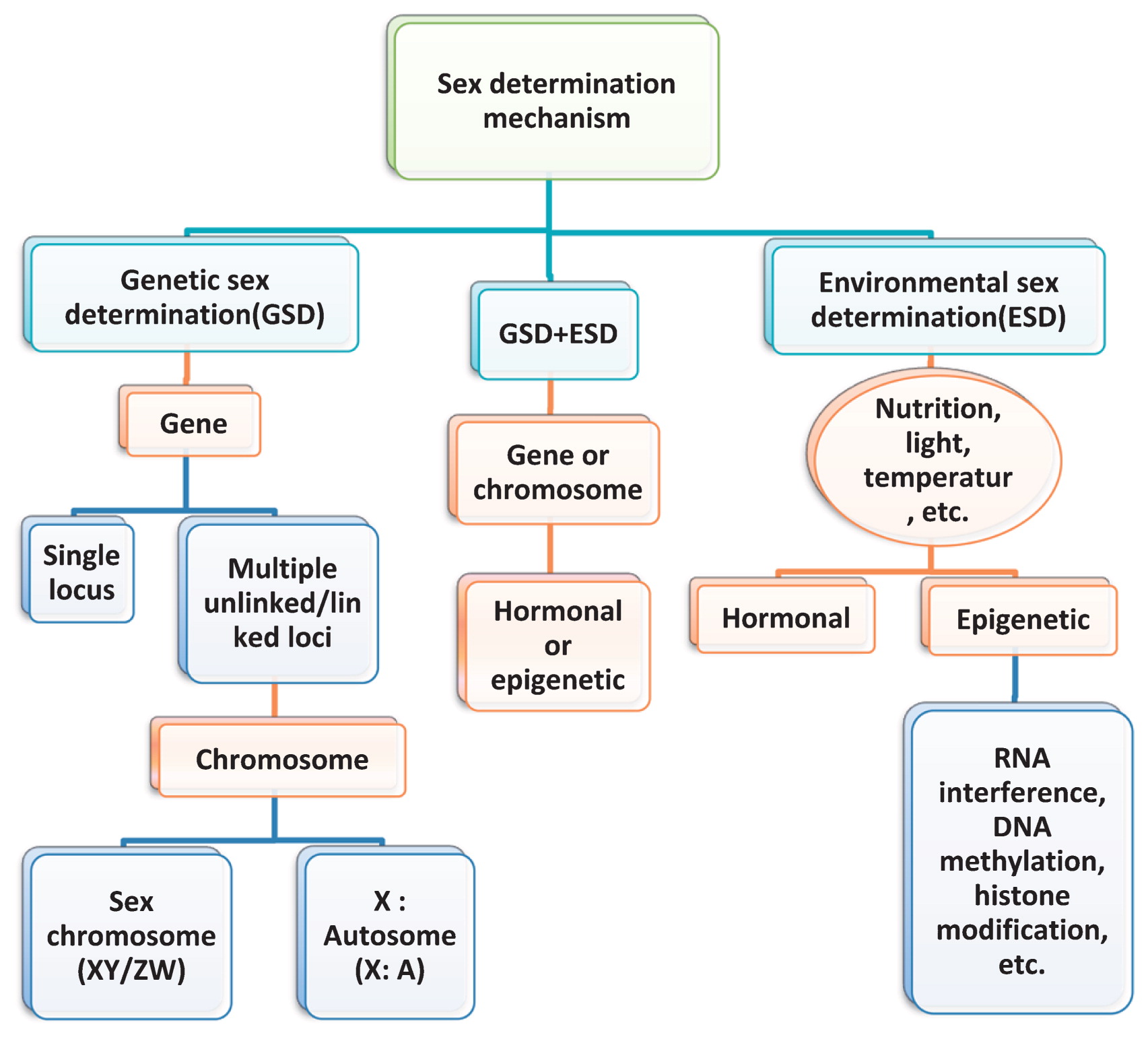
Different types of sex determination mechanisms in the plant kingdom.
Although the sex determination mechanism in Pistacia species is still far from being elucidated, a battery of studies have been performed to identify sex-linked markers at the morphological, cytological, physiological, and molecular levels (Fig. 3), providing abundant clues for understanding the potential mechanism of sex expression in this genus. Such sex specific markers are, therefore, summarized below.
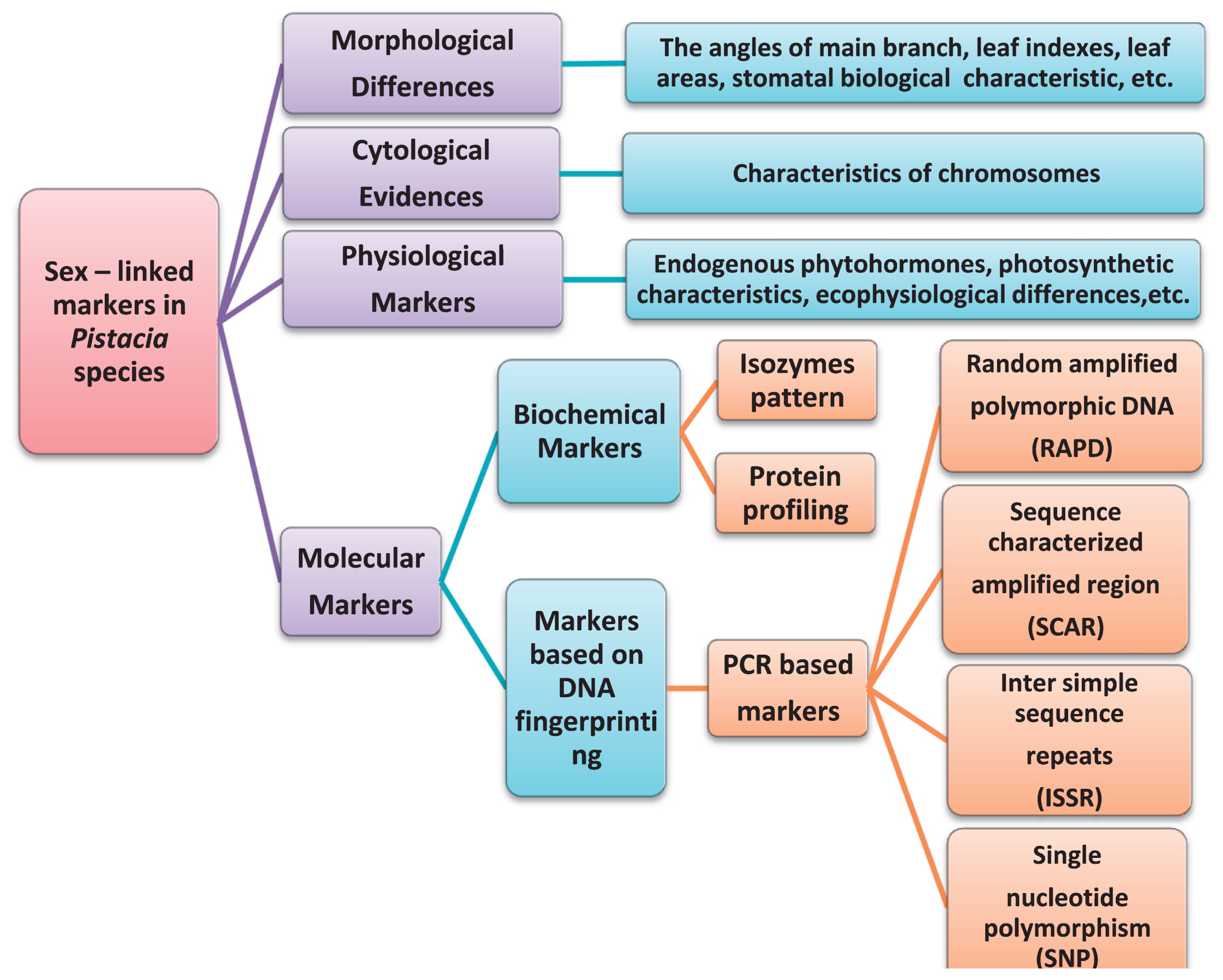
Sex-linked markers in Pistacia species.
Obviously, different sex types have significant differences at reproductive maturity, but it is difficult to identify the gender during their long juvenile periods or vegetative phases based only on morphology. Despite the many morphological differences between male and female individuals that involve the angles of main branches, leaf indices, leaf areas (Ma 2012), stomatal biological characteristic (Wang 2013), and leaf longevity (Jonasson et al. 1997), it is hard to define sex-associated markers because they are always mutable in different stages or environments. Actually, the sex of very few dioecious crops can be determined morphologically at the vegetative stage (Heikrujam et al. 2015).
Markers based on cytologyChromosome counts have been performed in some species of this genus (Table 2), and the ploidy levels of P. vera (Bochantseva 1972, Fasihi Harandi et al. 1996, Ila et al. 2003, Sola-Campoy et al. 2015, Tilkat et al. 2011, Zohary 1952), P. integerrima (Mehra 1976, Sandhu and Mann 1988), P. lentiscus (Natarajan 1977, 1978), P. atlantica (Ila et al. 2003, Vogt and Aparicio 1999, Zerey-Belaskri et al. 2018), P. terebinthus, and P. eurycarpa (Ila et al. 2003) were reported as 2n = 30, whereas those of three subspecies of P. atlantica (cabulica, kurdica, and mutica) were 2n = 28 (Ghaffari and Fasihi Harandi 2002). Additionally, the ploidy levels of P. khinjuk (Ghaffari and Fasihi Harandi 2002) and P. chinensis (Huang et al. 1989) were initially reported as 2n = 28, but Wang (2013), Wu and Yang (2014), and Yang (2013) reported that the ploidy of P. chinensis is 2n = 30. In addition, the observed species in the genus Pistacia were diploid and had basic chromosome numbers of n = 15, 12, or 14, but Al-Saghir (2010) proposed that all Pistacia species are n = 15. More importantly, P. vera, P. atlantica ssp. cabulica, P. atlantica ssp. kurdica, P. atlantica ssp. mutica, and P. chinensis were shown to have heteromorphic chromosomes (Ghaffari and Fasihi Harandi 2002, Tilkat et al. 2011, Wang 2013, Wu and Yang 2014, Yang et al. 2013). Moreover, the heteromorphic chromosomes existed in male trees of P. vera and P. chinensis (Tilkat et al. 2011, Wang 2013, Wu and Yang 2014, Yang et al. 2013), suggesting that the possible chromosomal mechanism is an XY (male) and XX (female) system in the genus Pistacia.
| Species | Ploidy | Description | References |
|---|---|---|---|
| P. vera | 2n = 30 | Male trees of P.vera L. have heteromorphic sex chromosomes (Xy). | Boczantseva 1972, Fasihi Harandi et al. 1996, Ila et al. 2003, Sola-Campoy et al. 2015, Tilkat et al. 2011, Zohary 1952 |
| P. integerrima | 2n = 30 | Mehra 1976, Sandhu and Mann 1988 | |
| P. lentiscus | 2n = 30 | Natarajan 1977, 1978 | |
| P. atlantica | 2n = 30 | Ila et al. 2003, Vogt and Aparicio 1999, Zerey-Belaskri et al. 2018 | |
| P. terebinthus | 2n = 30 | Ila et al. 2003 | |
| P. eurycarpa (syn. P. atlantica subsp. kurdica) | 2n = 30 | Ila et al. 2003 | |
| P. khinjuk | 2n = 24 | Ghaffari and Fasihi Harandi 2002 | |
| P. atlantica ssp. Cabulica | 2n = 28 | One pair of metacentric chromosomes was heterochromatic in nature (sex was unknown). | Ghaffari and Fasihi Harandi 2002 |
| P. atlantica ssp. Kurdica | 2n = 28 | ||
| P. atlantica ssp. Mutica | 2n = 28 | ||
| P. chinensis | 2n = 24 | Huang et al. 1989 | |
| 2n = 30 | A pair of the15 homologous chromosomes have not obvious morphological differences in females but are visibly different in males. | Wang 2013, Wu and Yang 2014, Yang 2013 |
Some related physiological research results seem to be conflicting. For example, Li and Yang (2012) showed that the content of water-soluble phenolics in male leaves (especially in old leaves) was higher than in female leaves for P. chinensis, whereas Wang (2013) demonstrated that the soluble phenolic content in leaves was constant in different seasons, ages, and genders.
Also, some existing conclusions are not consistent. For instance, in the studies of Ma et al. (2012 (2013), abscisic acid, indole-3-aceticacid, and spermine levels, peroxidase and catalase activity levels, and the soluble protein and soluble sugar contents in leaves of males were greater than in the leaves of females throughout the growth period, while the putrescine level was lower than that in females in each stage. For the superoxide dismutase activity, and tannin, gibberellin, and spermidine levels, their differences varied in males and females depending on the developmental period. However, the malondialdehyde and the chlorophyll contents showed no significant differences between the sexes. However, another study showed the esterase activity levels in leaves of male strains were significantly higher than in those of females, while the superoxide dismutase, peroxidase, and catalase activity levels in the leaves varied among different growth periods (Wang 2013).
Additionally, some studies showed that different physiological expression levels depended on various environmental factors. For example, for evergreen P. lentiscus trees, females exhibited higher lipid peroxidation levels in leaves than males only during the winter (when sex-related differences in reproductive effort are the highest), which was associated with reduced photoprotection (Marta et al. 2014). Additionally, in older communities, the ecological advantage of male plants is a result of a higher competition for water uptake, while in the youngest open areas, female plants have an ecological advantage resulting from their higher water use efficiency (Correia and Barradas 2000).
Therefore, most of the physiological markers in Pistacia species were controversial and unstable, and may vary in response to environmental stress or seasonal conditions.
Markers based on molecular biologySex identification using biochemical markers (isozyme patterns and protein profiling) has been performed in P. chinensis. Initially, using the leaves of male and female individuals, eight female-specific bands and two male-specific bands were found for peroxidase; nine male-specific bands and one female-specific band were found for esterase; and five male-specific bands and two female-specific bands were found for polyphenol oxidase, but only polyphenol oxidase could 100% identify the sex in further investigations (Cheng 2011). Then, Ma et al. (2013) showed that the isozyme patterns are different in leaves and petioles. One female-specific band was found in leaves, while two female-specific bands and one male-specific band were found in petioles.
In addition, 10 differentially expressed protein spots were found for vegetative organs (leaf and stem) between the two sexes, 7 of which were successfully identified by mass spectrometry and matched to 6 functional proteins. They were related to environmental stress responses that involved NB-ARC domain-containing proteins, light harvesting chlorophyll a/b-binding proteins, ascorbate peroxidase (APX), eukaryotic translation initiation factor 5A2, temperature-induced lipocalin (TIL), and phosphoglycerate kinase (PGK). The sex-related differences were displayed in a tissue-specific manner, especially in stem. For example, phosphoglycerate kinase showed a high abundance in the stem phloem of females, but was barely detected in males, while ascorbate peroxidase and temperature-induced lipocalin were highly abundant in the stems of male plants, but were much lower in female plants. Thus, ascorbate peroxidase, phosphoglycerate kinase, and temperature-induced lipocalin might be promising candidate molecular markers for sex determination in P. chinensis (Xiong et al. 2013).
In the genus Pistacia, the most popular markers for sex determination included random amplified polymorphic DNAs, sequence-characterized amplified regions, inter-simple sequence repeats, and single nucleotide polymorphisms (SNPs). Many studies tried to develop quick gender identification protocols using PCR-based molecular markers in vegetative tissues (Table 3), in which female and male genotypes could be discriminated at an early developmental stage. This would greatly facilitate breeding, selection, and management of the best seedlings.
| Marker type | Primer | Sequence | Species | References |
|---|---|---|---|---|
| RAPD | OPO-08 | CCTCCAGTGT | P. vera | Hormaza et al. 1994, Tan et al. 2003, Yakubov et al. 2005 |
| RAPD | BC1200 | GCCTGATTGC | P. vera | Esfandiyari et al. 2012, Esfandiyari et al. 2010, Esfandiyari et al. 2012 |
| RAPD | OPK-09 | CCCTACCGAC | P. terebinthus | Avanzato et al. 2004, Avanzato and Quarta 2004 |
| RAPD | OPAK09 | P. atlantica | Kafkas et al. 2001b | |
| RAPD | OPL-11 | ACGATGAGCC | P. terebinthus | Kafkas et al. 2001a |
| RAPD | BC152 | P. terebinthus | Kafkas et al. 2001a | |
| RAPD | BC156 | P. eurocarpa | Kafkas et al. 2001a | |
| RAPD | BC360 | P. eurocarpa | Kafkas et al. 2001a | |
| RAPD | FPKI106 | P. vera | Ehsanpour and Arab 2009 | |
| RAPD | FPK105 | P. vera | Ehsanpour and Arab 2009 | |
| RAPD | S218 | GATGCCAGAC | P. chinensis | Cheng 2011 |
| RAPD | S263 | GTCCGGAGTG | P. chinensis | Cheng 2011 |
| RAPD | S267 | CTGGACGTCA | P. chinensis | Cheng 2011 |
| RAPD | S1420 | AAGGCTCACC | P. chinensis | Cheng 2011 |
| RAPD | S1421 | AGCAGCGCAC | P. chinensis | Cheng 2011 |
| RAPD | S1426 | GTGGAGTCAG | P. chinensis | Cheng 2011 |
| RAPD | S1 | GTTTCGCTCC | P. chinensis | Sun et al. 2014 |
| RAPD | S281 | GTGGCATCTC | P. chinensis | Sun et al. 2014 |
| ISSR | ACACACACACACACACCG | P. vera | Ehsanpour et al. 2008 | |
| ACACACACACACACACTA | P. vera | |||
| SCAR | S1421-11CF | CTTCTCGGACCAATTAGGGAAGAC | P. chinensis | Cheng 2011 |
| S1421-11CR | CTTCTCGGACTAGCACGGCAGAG | P. chinensis | ||
| SCAR | PVF1 | GTCGTAGATGAAAACACC | P. khinjuk, P. atlantica, P. vera | Esfandiyari et al. 2012, Esfandiyari et al. 2010, Esfandiyari et al. 2012, Yakubov et al. 2005 |
| PVF2 | TAATAGAAGCCATAGA | P. khinjuk, P. atlantica, P. vera | ||
| SCAR | SCO-08-1 | CCTCCAGTGTGAATCAAGTAAAC | P. vera | Yakubov et al. 2005 |
| SCO-08-2 | CCTCCAGTGTTATGTAATACCAAAA | P. vera | ||
| SCAR | S1-1 | CGCTCCTTCTAATGTTGATGACAA | P. chinensis | Sun et al. 2014 |
| S1-2 | TCGCTCCCTCCAAATCCAATAAAC | P. chinensis | ||
| SCAR | S281-1 | CCTGGTTGCTTGTGTTGATTAG | P. chinensis | Sun et al. 2014 |
| S281-2 | GAGTGTCATCAAGCCATCTGTC | P. chinensis | ||
| SNP (SNP-PIS-112277) | F | TTACAGACACAAAACCATGACAA | P. vera | Kafkas et al. 2015 |
| R | GCATGCTGAATTTTCTTCCT | |||
| SB | GCATGCTGAATTTTCTTCCTT | |||
| SNP (SNP-PIS-127343) | F | TCACCAATATTTTACTGCAA | P. vera | Kafkas et al. 2015 |
| R | CCATTTTCACTCACCCTGTC | |||
| SB | CACCCTGTCATTACATTCTTA | |||
| SNP (SNP-PIS-133396) | F | GCAAACCGCAAAGAAGATTA | P. vera | Kafkas et al. 2015 |
| R | ACTGAAACTTGAAGATCATGGA | |||
| SB | CAAACCGCAAAGAAGATTAAAGTA/GTACTGAAACTTGAAGATCATGGATCA | |||
| SNP (SNP-PIS-135862) | F | GGTTTTGTGTCTGAATGTGGA | P. vera | Kafkas et al. 2015 |
| R | CCATGCACATTTCCCATAAT | |||
| SB | GTCTGAATGTGGATAATATATGG/TTCCCATAATCAACATGAGGC | |||
| SNP (SNP-PIS-136404) | F | GAATTCTTTTAGGGGTTGTCA | P. vera | Kafkas et al. 2015 |
| R | CCAGCTTTAGAGTTGGCAAT | |||
| SB | GAATTCTTTTAGGGGTTGTCAAA | |||
| SNP (SNP-PIS-167992) | F | CGAAAAATAACTTCATAGCGTGA | P. vera | Kafkas et al. 2015 |
| R | TGATCAATTGCAGCTTAGGG | |||
| SB | AGCTTAGGGTTGCGGTTA | |||
| SNP (SNP-PIS-174431) | F | AGTCTCTAGCCGCGTACATA | P. vera | Kafkas et al. 2015 |
| R | AACTTAACAATTTYTCCACTTTCAC | |||
| SB | CCGCGTACATATAAAAATTAACTACTCA | |||
| SNP (SNP-PIS-112277b) | F | GGACCACTTTGCAGCTATCA | P. vera | Khodaeiaminjan et al. 2017 |
| R | GGACTGGCATGCTGAATTTT | |||
| SB | GTCTGTAAGAGTTTTAAAATCACCCTCTAG | |||
| SNP (SNP-PIS-130684a) | F | AATTTTTAGGAATGCAGGGTTT | P. vera | Khodaeiaminjan et al. 2017 |
| R | ATCCCAAGGAGGAGCAAAGT | |||
| SB | CTAGCACAAGTGGACAGCAATC | |||
| SNP (SNP-PIS-130684b) | F | CGGTAAATTTGAATTCTGCTGT | P. vera | Khodaeiaminjan et al. 2017 |
| R | AGCACAAGTGGACAGCAATC | |||
| SB | GGTAAATTTGAATTCTGCTGTCA | |||
| SNP (SNP-PIS-130935) | F | GACCAAAGGGGTGAATCTTG | P. vera | Khodaeiaminjan et al. 2017 |
| R | TTACCTGTCTTCCCATTCTGA | |||
| SB | AAATTACCTGTCTTCCCATTCTGATA | |||
| SNP (SNP-PIS-133197) | F | CAAACTGGCAAGGATGTGAA | P. vera | Khodaeiaminjan et al. 2017 |
| R | TGCTGCTGCAACTTTAGGAA | |||
| SB | GGTGGGCAAGGATGATGTAGTA | |||
| SNP (SNP-PIS-136228) | F | ACGTGTCCCACCAAAATTGA | P. vera | Khodaeiaminjan et al. 2017 |
| R | CCGCACCCAATAAACTCGTA | |||
| SB | CCACCAAAATTGACTTAATAGAAAAGAC | |||
| SNP (SNP-PIS-165792) | F | GAATTCCAATAGTCCAAGTCAGA | P. vera | Khodaeiaminjan et al. 2017 |
| R | TCATGGCCTAATTATCTCCAA | |||
| SB | AGATGAGATCAATCTCATATTGTCATA | |||
| SNP (SNP-PIS-175177) | F | GCAATTAATTTATTCAGCCAAAA | P. vera | Khodaeiaminjan et al. 2017 |
| R | TCCAATCTTGGATCCAGCTAA | |||
| SB | TCTCTTAGGACTTATAGCCTCATTCTG | |||
| SNP (SNP-PIS-179354) | F | CGCAAACCAGAATCAGATCA | P. vera | Khodaeiaminjan et al. 2017 |
| R | TGTTTTATTTGATCATTTGTGGAC | |||
| SB | GCACAATTTTAGATTCCATGAATA |
F: forward, R: reverse, SB: single-base extension.
Compared with other markers, DNA-based molecular markers are stable in all tissues and are independent of environmental factors and developmental stages (Hormaza and Wünsch 2011). Unfortunately, few specific marker-based bands can accurately distinguish among different sex types. For instance, Hormaza et al. (1994) found just one sex-associated marker by screening of 1,000 primers in P. vera. However, Kamiab et al. (2014) proved that a 15% error rate in the sex identification of P. vera is possible using this marker, and it was also ineffective for sex determination in other Pistacia species (Hormaza et al. 1994, Kafkas et al. 2015). Moreover, two monoecious P. vera strains were tested using the sequence-characterized amplified region primers PVF1 and PVF2, and one was identified as a male, while the other was identified as a female (Yakubov et al. 2005). A molecular analysis showed high variability level among all of the tested accessions, including female, male, and monoecious P. terebinthus (Avanzato and Quarta 2004). Thus, sex-related loci appear to be small or few (Esfandiyari et al. 2010).
Subsequently, restriction-site associated DNA sequencing in male and female progeny (Siirt × Bağyolu F1 population) was performed, and 17 sex-linked SNP markers were found. However, they were unable to separate the sexes in wild species, like P. atlantica, P. terebinthus, P. eurycarpa, P. integerrima, and P. lentiscus (Kafkas et al. 2015, Khodaeiaminjan et al. 2017). Nevertheless, all of these SNP markers were heterozygous in the female individuals and homozygous in the male individuals, suggesting a ZW/ZZ sex determination system in P. vera (Kafkas et al. 2015, Khodaeiaminjan et al. 2017). This hypothesis could have been supported by a molecular cytogenetic characterization study that revealed only two types (type-I/II heterozygotes and type-I homozygotes) of pistachio individuals had HC1 chromosomes with differing PIVE-40 hybridization signals (Sola-Campoy et al. 2015). However, the gender of the seeds carrying heteromorphic HC1 pairs was not determined; therefore, the HC1 chromosomes cannot be unambiguously used for sex determination.
Possible origins of monoecious Pistacia speciesEven though the mechanism behind the formation of monoecism still remains unclear, several hypotheses exist (Avanzato and Quarta 2004, Bai et al. 2016, Kafkas et al. 2001c). The monoecious traits in a population may have arisen as a somatic mutation in one tree, followed by its descendants. According to the description of Kafkas et al. (2001c), a fully monoecious 500-year-old tree (PA2) was in the middle of an approximate circle of younger monoecious P. atlantica trees (of which the oldest was at most 100 years old) located within an area of approximately 3,000–4,000 m2. In this case, the fully monoecious trees were descendents of PA2, but this did not explain why the younger trees had scattered male branches/inflorescences. Moreover, Damiano and Quarta (2004) showed that the monoecious form was found within a small subpopulation of P. terebinthus that had a low probability of genetic crossover from the existing genetic population, leading to the supposition that the monoecious trait likely appeared in the progeny of the dioecious female plants.
In addition, Kafkas et al. (2001c) proposed two more explanations: 1) all of the chimeric phenotypes arose as multiple independent somatic mutations. While trees are genetically related and not chimeric, the monoecious trait may be somatically unstable or confer a non-uniform phenotype, which may be expressed only in some tree parts; or 2) the chimeric phenotypes arose as a result of a transposable element, resulting in an interaction with biotic/abiotic environmental factors.
Our studies have shown that, based on the descriptions of local people, more than one dioecious female P. chinensis became monoecious (Bai et al. 2016), suggesting that monoecious trees are more likely to originate from female trees. In association with the results of sex-linked markers (Kafkas et al. 2015, Khodaeiaminjan et al. 2017), this case supports the ZW/ZZ sex-determination system, because the female trees seem to be heterozygotes that could have a genetic foundation for male organs. If their sex differentiation is controlled by sex determination gene(s) or sex chromosomes, why do they possess labile systems instead of strictly GSD to determine a fixed sex expression? The non-fruiting male trees, which were considered to be useless, were almost all used to make wooden furniture. After that, some female trees gradually transforming into monoecious trees within two decades (Bai et al. 2016), and the monoecious trees were more than 40 years old, indicating that almost all of them arose as multiple independent somatic mutations caused by environmental stress, involving the absence of males or other biotic/abiotic factors. In addition, the unstable sex expression in successive years also indicated the influence of environmental factors.
Furthermore, when and how could the ESD (hormonal or epigenetic) play a role in the sex expression based on the restrictive GSD? Flower bud developmental processes in Pistacia species have shown that rudimentary female organs are present in mature male flowers of most Pistacia species, including P. atlantica, P. lentiscus, P. terebinthus, P. weinmannifolia, P. palaestina, and P. khinjuk (Zohary 1952). The mature florets of P. vera and P. chinensis are unisexual; however, Hormaza et al. (1994) reported that vestigial organs of the opposite sex appeared during flower bud development in P. vera, and our study showed that the male and female organs also coexisted in the early sex differentiation phase in P. chinensis (not published). These phenomena indicate that sex determination can take place during different developmental stages and that the critical period of sex determination is early in P. chinensis. More importantly, the emergence of monoecism, especially bisexual florets, has been identified (Bai et al. 2016), which supports the hypothesis that all Pistacia species evolved from hermaphroditic ancestors, and they might have the potential to become monoecious, or even hermaphroditic, if the abortion of opposite organ can be stopped. Moreover, our recent study showed that the sex expressions of grafted trees was not consistent with those of scions, indicating that monoecism probably did not originate from a stable bud mutation and that sex determination may occur during floral development instead of during an early vegetative period (Bai et al. 2019). Therefore, combined with the sexual determination and developmental mechanisms, environmental pressures might influence sexual differentiation by activating or modifying one or several “agent” genes (ancient silent genes, may also be restructured or mutated forms of existing genes), which are silent in the female trees, and would likely play roles downstream of the sex-determining gene in the process of floral development, resulting in male organs. This strategy might explain the emergence of monoecious P. chinensis.
In conclusion, the sex expression of Pistacia species showed broad diversity and complex instability. Although large studies on sex identification using morphological, cytological, physiological, and molecular markers have been performed, many questions still remain. For example, the cytological analysis suggested the XX/XY system, whereas the molecular analysis supported the ZW/ZZ system. Also, the ploidy of P. atlantica was proven to be 2n = 30 (Ila et al. 2003, Vogt and Aparicio 1999, Zerey-Belaskri et al. 2018), while its subspecies (P. atlantica ssp. cabulica, kurdica, and mutica) were reported to be 2n = 28 (Ghaffari and Fasihi Harandi 2002), suggesting that more subsequent studies should be performed based on the existing results. Therefore, the mechanism of the sex determination and the origin of monoecious Pistacia species remains an intriguing and elusive question. Future work should address multiple aspects that are summarized below.
First, monoecious strains should be preserved and utilized. The in situ, ex situ, and in vitro conservation are necessary to obtain more resources for further scientific research. Monoecious P. chinensis would have direct applications in selective breeding in determining the pathway of gender regulation, with the aim to develop monoecious plants with high and stable yields. For P. vera, despite the current lack of a monoecious strain, a long-term breeding project has been launched, which aims to transfer monoecious genetic traits to pistachio by grafting (İsfendiyaroğlu and Özeker 2009) and hybridization (Avanzato and Quarta 2004, Buffa et al. 2009, Kafkas 2002, Kafkas et al. 2005). If monoecious pistachios can be obtained by these breeding methods, planting of non-fruit-bearing male trees can be reduced and thus the yield may increase by ~10% (Gercheva et al. 2008). Additionally, more materials and observations of monoecious species are needed for studies of sex determination and evolutionary mechanisms.
Second, studies related to sex determination have mainly focused on dioecious trees, but taking the monoecious individuals into account could provide new information. From the group perspective, the genetic diversity and relationships between monoecious and dioecious strains could be analyzed by markers, such as simple sequence repeats. From individual perspective, tissues with different sex expressions should be isolated and observed, which would increase our understanding of gender differences.
Third, despite numerous studies concerning sex-linked genes that used DNA fingerprinting, comparative researches on the different developmental stages and organs at the RNA or cDNA level (for sequencing or molecular markers) are necessary. For Pistacia trees, to eliminate the noise generated by the genetic backgrounds of different individuals, RNA transcriptomic analyses and small RNA sequencing are effective ways to investigate large datasets from different sex types. Additionally, these techniques could be applied to determine candidate genes that influence or determine the sex type.
Furthermore, regardless of whether the emergence of monoecism is an atavistic or evolutionary event, it is clear that environmental factors have played an important role in the final sex expression. The instability of gender expression also hinted at its adjustability. The ability to obtain more fertile bisexual flowers would represent enormous progress in the development of economically important forest species. However, the interactions between genes and the environment may indicate a much more complicated mechanism. Subsequent researches on sex-specific evolution and sex determination should have a global view, including integrating multi-omics such as whole genome sequencing, RNA sequencing and proteomics (Yagi 2018, Yano 2004), and combining multiple levels of morphology, cytology, physiology and molecular biology. Additionally, further research and observations are indispensable to explaining the origin of monoecious Pistacia species.
This study was supported by the Fundamental Research Funds for the Central Universities (2015ZCQ-LX-02), the Special Project of International Cooperation Ministry of Science and Technology (2014DFA31140) and the Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges under Beijing Municipality (IDHT20150503). We thank International Science Editing for language editing.