2019 Volume 69 Issue 4 Pages 601-610
2019 Volume 69 Issue 4 Pages 601-610
This study examined contents of nine plant hormones in developing seeds of field-grown wheat varieties (Triticum aestivum L.) with different seed dormancy using liquid chromatography—mass spectrometry. The varieties showed marked diversity in germination indices at 15°C and 20°C. Contents of the respective hormones in seeds showed a characteristic pattern during seed maturation from 30-day post anthesis to 60-day post anthesis. Principal component analysis and hierarchical clustering analysis revealed that plant hormone profiles were not correlated with dormancy levels, indicating that hormone contents were not associated with preharvest sprouting (PHS) susceptibility. Indole acetic acid (IAA) contents of mature seeds showed positive correlation with the germination index, but no other hormone. Response of embryo-half seeds to exogenous abscisic acid (ABA) indicates that ABA sensitivity is correlated with whole-seed germinability, which can be explained in part by genotypes of MOTHER OF FT AND TFL (MFT) allele modulating ABA signaling of wheat seeds. These results demonstrate that variation in wheat seed dormancy is attributable to ABA sensitivity of mature seeds, but not to ABA contents in developing seeds.
Preharvest sprouting (PHS) is precocious germination of seeds in the spike before harvest. In wheat, PHS causes starch hydrolysis by induction of amylase activities; it eventually engenders a marked decline of commercial value of flour produced from affected wheat (Nagao 1996). Plant hormones (also known as plant growth regulators) are thought to be involved in plant dormancy and germination.
Abscisic acid (ABA) is a widely accepted major determinant of seed dormancy (Fincher 1989, Kermode 1990, King 1976). Numerous studies have examined the significance of ABA in PHS susceptibility. Reportedly, ABA contents in common wheat seeds increase along with the increase of seed dry weight and decrease along with seed dehydration (Kawakami et al. 1997, King 1976, McWha 1975, Quarrie et al. 1988, Radley 1976, Walker-Simmons 1987). The ABA contents of common wheat and barley seeds are also affected by natural climate variation. Maxima of ABA content of barley and wheat shift earlier by high atmospheric temperature during seed maturation (Goldbach and Michael 1976, Radley 1976, Tuttle et al. 2015, Walker-Simmons and Sesing 1990). Moreover, ABA contents in wheat seeds differ drastically according to high and low atmospheric humidity (King 1993, Mares 1989). These findings have spurred speculation that ABA contents govern the PHS susceptibility of cereals.
The PHS susceptibility of common wheat differs among cultivars (Reddy et al. 1985). Some earlier studies have been conducted to explain these differences based on diverse ABA contents among cultivars. Goldbach and Michael (1976) reported that ABA contents of barley grains were lower in a less-dormant cultivar almost throughout seed development and the ripening process. Suzuki et al. (2000) claimed that a PHS-sensitive cultivar has a premature germination window during which ABA was lost in a particular period, thereby rendering the seed more germinable, although it could be an artifact derived from experimental procedures using half-seed embryos. Chono et al. (2013) reported that catabolic activity of ABA in wheat seeds affects germinability. By contrast, King (1976), and Walker-Simmons and Sesing (1990) demonstrated that no substantial difference can be found in ABA contents between PHS-sensitive and PHS-resistant cultivars.
Gibberellins (GAs) are thought not to be involved in seed dormancy, but they are rather important for germination after loss of dormancy (Bewley 1997, Jacobsen et al. 2002). Indole acetic acid (IAA) has been well documented as having an inhibitory effect on seed germination (Brady et al. 2003, Liu et al. 2007, 2013, Morris et al. 1988, Ramaih et al. 2003). Cytokinins stimulate germination evoked by GAs in pear, legume, and Arabidopsis (Khan 1971, Nikolić et al. 2006, Wang et al. 2011). Jasmonic acid (JA) reportedly enhances apple seed and pear seed germination (Ranjan and Lewak 1992, Yildiz et al. 2008). However, the temporal pattern of these plant hormones during seed ripening remains poorly understood. To gain insight into the roles of plant hormones in PHS susceptibility of wheat seeds, simultaneous profiling of multiple plant hormones in seeds in field grown wheat is expected to be helpful.
This study examined nine plant hormones of seven common wheat varieties with diverse dormancy levels. Based on the results, we tested the hypothesis that diverse seed dormancy among these wheat varieties is attributable to differences in plant hormone contents of the seeds.
At a rainfed field at the Institute of Plant Science and Resources, Okayama University, Kurashiki, Japan (34°59′34.1″N, 133°76′09.3″E) in 2008, 2009, 2010, 2011, and 2012 seasons, seven varieties/cultivars of common wheat (Triticum aestivum L.) were cultivated: ‘Zenkoujikomugi’ (ZK); ‘OW104’ (OW); ‘Kitakei-1354’ (KITA); ‘Tamaizumi’, formerly Kanto 123 (TAMA); ‘Halberd’ (HAL); ‘AUS1408’ (AUS); and ‘Chinese Spring’ (CS). Hokkaido Research Organization Kitami Agricultural Experiment Station provided OW and AUS. TAMA was gifted from Dr. Masaya Fujita, The Institute of Crop Science, National Agriculture and Food Research Organization (NARO). Kyushu Okinawa Agricultural Research Center, NARO provided ZK and HAL. Also, CS and KITA were from the collection of Dr. Kazuhiko Noda originally derived from The Kihara Institute for Biological Research, Yokohama City University.
Whether they were winter wheat or spring wheat, all varieties were seeded together in November as described hereinafter. We transplanted nursery plants to the field to distinguish experimental plants from contaminating plants sprouted from seeds left from the prior year. Seeds were imbibed for three days on a sheet of filter paper (No. 2; Toyo Roshi Kaisha Ltd., Tokyo, Japan) moistened with 3–5 mL of distilled water in a Petri dish (9 cm diameter). Seedlings were transferred in a nursery tray filled with gardening compost (Hanatoyasaino Baiyodo; Setogahara Kaen, Gunma, Japan) on 19 November 2007, 17 November 2008, 17 November 2009, 22 November 2010, and 21 November 2011. They were transferred to the field respectively on 17 December 2007, 18 December 2008, 18 December 2009, 28 December 2010, and 21 December 2011.
Spikes were tagged at anthesis and were harvested at 5-day intervals from 30 to 60 days post-anthesis (DPA). Seeds were collected from the primary and secondary florets of the central spikelets of spikes. Water contents and dry weights of seeds were examined at each collection as described by Himi et al. (2002). Germinability and plant hormone contents were ascertained when the water content was below 20%, where we considered the seeds as fully ripened (Supplemental Table 1 presents harvest dates), unless otherwise indicated. For plant hormone quantification, seeds were frozen immediately in liquid nitrogen after collection in the field, without dissection. The hormone status was kept at the field condition to the greatest degree possible.
Red grain varieties were CS, KITA, OW, and ZK. White grain varieties were AUS, HAL, and TAMA. Grain color is controlled by R-1 genes on chromosomes 3A, 3B, and 3D. R-1 genotypes of lines in this study were investigated as described by Himi et al. (2011). Genotypes of Vp-1B, TaSdr-B1, MKK3, and MFT-3A, which were reported to affect PHS susceptibility, were examined according to earlier reports (Chono et al. 2015, Shorinola et al. 2017, Yang et al. 2007, Zhang et al. 2014).
Germinability of seeds and effects of exogenous ABAIn a Petri dish with two layers of filter paper (No. 2; Toyo Roshi Kaisha Ltd.), 20 seeds that had been moistened with 6 mL of distilled water or aqueous solution of a given concentration of ABA were incubated at either 20°C or 15°C in darkness for 14 days. The germination index (GI) was determined according to a formula described elsewhere (Walker-Simmons and Sesing 1990), except the extended examination period (14 days instead of 9 days). Three independent experiments were conducted. To infer sensitivity to ABA, seeds were cut in half with a scalpel to break dormancy artificially (Himi et al. 2002). Germinability of the embryo-half seed was examined in the dark at 20°C and 15°C.
Quantification of plant hormones in whole seedsWe extracted ABA, IAA, JA, salicylic acid (SA), gibberellin A1 (GA1), gibberellin A4 (GA4), trans-zeatin (tZ), dihydrozeatin (DHZ), and isopentenyladenine (iP), and purified them by solid-phase extraction. Then the contents of these hormones were quantified using liquid chromatography-electrospray tandem mass spectrometry (LC-ESI-MS/MS). For simultaneous determination of these plant hormones, four seeds (approximately 100 mg) were ground in a mortar containing 4 mL of extraction solvent (1% [v/v] acetic acid in acetonitrile/water [4:1]) supplemented with the internal standards. Then they were incubated at 4°C for 1 hr. Stable isotope-labeled compounds were used as internal standards: D2-IAA (CDN Isotopes Inc., Quebec, Canada); D6-ABA, D2-GA1, D2-GA4, D4-SA, D5-tZ, D3-DHZ, and D6-iP (Olchemim s.r.o., Czech); and D2-JA (Tokyo Chemical Industry Co. Ltd., Tokyo). Supernatant was collected after centrifugation at 3,000 × g for 10 min at 4°C. The pellet was rinsed with 4 mL of the extraction solvent without internal standards. After centrifugation, supernatants were combined, evaporated using a centrifugal evaporator to give plant hormones remained in 1% acetic acid aqueous solution, and loaded to a pre-equilibrated Oasis HLB 1 cc cartridge (30 mg; Waters Corp., Milford, MA, USA). After washing the cartridge with 1 mL water containing 1% acetic acid, plant hormones were eluted with 2 mL of acetonitrile/water (4:1) containing 1% acetic acid. Acetonitrile in the eluate was evaporated to water containing 1% acetic acid. Extracts were then applied to a pre-equilibrated Oasis MCX 1 cc cartridge (30 mg; Waters Corp.). After washing with 1 mL water containing 1% acetic acid, acidic fraction containing GA1, GA4, IAA, ABA, JA, and SA was eluted with 2 mL of 1% acetic acid in acetonitrile/water (4:1). From this fraction, 200 μL was collected, evaporated to dryness, reconstituted in 50 μL of 1% acetic acid in water, and used for SA quantification by LC-ESI-MS/MS. The MCX cartridge was washed further with 5% (v/v) ammonium water. Then the basic fraction containing tZ, DHZ, and iP was eluted with 2 mL of 5% ammonia in acetonitrile/water (2:3). The basic fraction was evaporated to dryness and was reconstituted in 50 μL of 1% acetic acid in water to quantity tZ, DHZ and iP by LC-ESI-MS/MS. Acetonitrile in the acidic fraction was removed by centrifugal evaporation. The remaining acidic aqueous portion was then applied to a pre-equilibrated Oasis WAX 1 cc cartridge (30 mg; Waters Corp.). After washing with 1 mL of water containing 1% acetic acid and 2 mL of acetonitrile/water (4:1) without acetic acid, the acidic hormones were eluted with 2 mL of 1% acetic acid in acetonitrile/ water (4:1). This fraction was dried and reconstituted with 1% of acetic acid in water. Contents of analytes were analyzed quantitatively using LC-ESI-MS/MS (triple quadrupole mass spectrometer with 1260 high-performance LC, G6410B; Agilent Technologies Inc.) equipped with a column (Zorbax Eclipse XDB-C18; Agilent Technologies Inc.). Mass-to-charge ratio (m/z) transitions of analytes were described in an earlier report (Tsukahara et al. 2015). The retention time and solvents used for LC are presented in Supplemental Table 2. Contents of plant hormones were normalized to the fresh weight (FW) of seeds.
Quantification of plant hormones in dissected tissues of seedsLyophilized wheat seeds were dissected carefully into two parts using a scalpel without moistening: embryo/scutellum and endosperm/outer layers. Extraction, purification, and quantification of plant hormones were conducted as described above. Plant hormone contents were normalized to dry weight (DW) of each dissected tissue.
Principal component analysis and hierarchic clustering analysisData of six plant hormones (ABA, IAA, JA, SA, tZ, and iP) of seven wheat cultivars obtained from 30 to 60 DPA with five-day intervals, if available, were log-transformed (base 10), mean-centered, and divided by the standard deviation. The generated data matrix comprising 6 hormones × 49 data (7 cultivars and 7 DPAs) was investigated using principal component analysis with software (SPSS statistics ver. 21; IBM Corp.).
Hierarchical clustering analysis was used to compare profiles among six plant hormones or among seven cultivars in whole seeds during seed maturation. The data were log-transformed (base 10), mean-centered, and divided by the standard deviation. A data matrix comprising 6 hormones × an array of 49 data (cultivars and DPA) and of 7 cultivars × 42 data (hormones and DPA) was investigated using the hclust package of R software with ward.D method (ver. 3.2.4, R Core Team 2016).
Statistical test for difference of meansDifferences among means were examined using analysis of variance with Tukey’s multiple comparisons test or Wilcoxon rank sum test. Correlation between variables was assessed using Spearman’s rank correlation test.
Seed dormancy, a major determinant of PHS susceptibility (King 1989), is affected by ambient temperature (Mares 1989). Here, to gain insight into the variation in seed dormancy levels among these cultivars, we characterized the germinability of ripened seeds (see Materials and Methods) of seven cultivars of common wheat at two temperatures: 20°C and 15°C.
At 20°C, GIs of ripened seeds of HAL, CS, AUS, and KITA were 0.19–0.83 in 2008, 2009, and 2010 seasons (Table 1, Supplemental Fig. 1). The degree of GI sometimes showed variation during experiments (e.g., GIs of AUS and KITA in 2008 and 2010 were, respectively, 0.24 and 0.86, and 0.19 and 0.83). These variations between years might be attributed partly to precipitation events and air temperatures before and during the harvest (Supplemental Fig. 2). Precipitation events (25–27 June, 2010) overlapped with the harvest dates of AUS and KITA (27 and 26 June, respectively). Concurrently, the average temperature of the days (25 and 26 June, 2010) was reduced sharply to 19.2°C, and 21.7°C, respectively. Regarding TAMA, ZK, and OW, considerably lower GIs were observed consistently (GI < 0.04) with the exception that TAMA showed high GI in the 2009 season (GI = 0.22 ± 0.02) (Table 1). Harvest of TAMA seeds in 2009 was not accompanied with precipitation or low-temperature events. We wondered if the extremely low GIs of TAMA, ZK, and OW at 20°C are attributable to impotence of germination instead of being attributable to their strong seed dormancy. To address this question, we examined the germinability of seeds after artificial dormancy breaking. Embryo-half seeds of TAMA, ZK, and OW germinated at comparable rates to those of other cultivars (Supplemental Fig. 3), indicating that they were capable of germination and that the extremely low GIs of TAMA, ZK, and OW are attributable to their seed dormancy but not embryo dormancy. Therefore, we infer that TAMA, ZK, and OW are PHS-tolerant varieties under our cultivation conditions.
| Variety | GI at 20°C | GI at 15°C | ||||||
|---|---|---|---|---|---|---|---|---|
| 2008 | 2009 | 2010 | Mean | 2010 | 2011 | 2012 | Mean | |
| Halberd | – | – | 0.83 ± 0.14a | 0.83 ± 0.14 | 0.87 ± 0.01a | 0.97 ± 0.02a | 0.91 ± 0.03a | 0.92 ± 0.04 |
| AUS1408 | 0.24 ± 0.09b | 0.36 ± 0.11a | 0.58 ± 0.15b | 0.39 ± 0.21 | 0.86 ± 0.04a | 0.75 ± 0.01b | 0.82 ± 0.03ab | 0.81 ± 0.05 |
| Chinese Spring | 0.61 ± 0.09a | – | – | 0.61 ± 0.09 | – | 0.74 ± 0.04b | 0.82 ± 0.07ab | 0.78 ± 0.08 |
| Kitakei 1354 | 0.19 ± 0.11b | 0.25 ± 0.14b | 0.67 ± 0.03ab | 0.37 ± 0.18 | 0.83 ± 0.02a | 0.60 ± 0.10bc | 0.65 ± 0.16bc | 0.69 ± 0.19 |
| Tamaizumi | – | 0.22 ± 0.04b | 0.02 ± 0.05c | 0.12 ± 0.06 | 0.49 ± 0.17b | 0.63 ± 0.15bc | 0.55 ± 0.19c | 0.56 ± 0.30 |
| Zenkoujikomugi | 0.04 ± 0.01c | 0.04 ± 0.03c | 0.04 ± 0.04c | 0.04 ± 0.05 | 0.30 ± 0.05b | 0.47 ± 0.18c | 0.21 ± 0.16d | 0.33 ± 0.25 |
| OW104 | 0 ± 0c | 0.03 ± 0.03c | 0.01 ± 0.02c | 0.01 ± 0.04 | 0.30 ± 0.18b | 0.04 ± 0.01d | 0.15 ± 0.12d | 0.16 ± 0.22 |
Data are shown as mean ± standard deviation (n = 3).
At 15°C, higher GIs were observed consistently in all varieties than those observed at 20°C, as reported from an earlier study (Kashiwakura et al. 2016). The GIs of HAL, CS, AUS, and KITA were 0.58–0.97 in 2010, 2011, and 2012 (Table 1), which generally agreed with results presented in an earlier report (Noda et al. 1994). A particular increase of GI was observed in TAMA (from 0.02–0.22 at 20°C to 0.49–0.63 at 15°C). GIs of ZK and OW remained considerably low (0.21–0.47 and 0.04–0.30, respectively), even at 15°C. Higher germinability of ZK in 2011 for comparison with OW (Supplemental Fig. 1E) might be attributable to the occurrence of a precipitation event before the harvest (Supplemental Fig. 2). These observations suggest that low temperature promotes a dormancy break. Variation in dormancy and temperature-dependence of dormancy are apparent among the wheat varieties tested in this study.
Temporal patterns of dormancy break and plant hormone contentsA plant hormone profile in a seed might alter successively during seed development process in a field and coincide with the reduction of seed dormancy through time. We questioned whether the variation in dormancy breaking rate among cultivars is ascribable to the difference in temporal patterns of plant hormone contents along with seed development process in field. To test this hypothesis, dynamics of germinability and plant hormone profile were examined serially from 30 to 60 DPA, where the germinability of wheat seeds increases gradually (Supplemental Fig. 1G). The developmental stage of seeds at 30 to 60 DPA can be regarded as the late seed development to the fully mature stage because the gain in dry weight almost stopped and seeds were dehydrating gradually in the examined period (Supplemental Fig. 4). Germinability was examined at 15°C, so the modest difference in PHS tolerant varieties was assessed (Supplemental Figs. 1D–1F).
GIs of CS, AUS, and HAL increased sharply during the last 10–15 days toward full ripening and eventually reached greater than 0.8 (Supplemental Fig. 1G), which were concordant with their low dormancy at ripening (Table 1). The GIs of KITA and TAMA increased gradually and reached as high as 0.6 (Supplemental Fig. 1G). GIs of ZK were still below 0.2 at full ripening and continued to increase afterwards with some fluctuation (Supplemental Fig. 1G), which might be influenced by weather (Supplemental Fig. 2). Germinability of OW remained very low during measurements (<0.2).
Contents of nine plant hormones, GA1, GA4, IAA, ABA, JA, SA, tZ, iP, and DHZ, in whole seeds including endosperm were quantified simultaneously from 30 DPA to 60 DPA with a five-day interval (Fig. 1). Also, GA1, GA4, and DHZ were detected as too low to analyze quantitatively. The IAA contents of all seven cultivars decreased rapidly and similarly from 1–4 μg g−1 to <0.2 μg g−1 during 30 DPA to 40 DPA and subsequently stabilized at the low level (Fig. 1A). The ABA contents decreased rapidly from 0.2–0.7 μg g−1 to 20–30 ng g−1 during 30 DPA and 40 DPA and subsequently stabilized in all cultivars as the IAA contents did, although the slopes of the decrement in HAL, AUS, TAMA, and OW were less steep than that of CS, KITA, or ZK (Fig. 1B). This decreasing pattern generally matched earlier reports of results from gas-chromatography analyses conducted at the late developmental stage of wheat seeds (King 1976, McWha 1975), but not with findings reported from enzyme-linked immunoassay (Suzuki et al. 2000). Temporal pattern of JA contents showed no apparent concordance among cultivars (Fig. 1C). The JA contents in seeds decreased consecutively along maturation in AUS, CS, and OW. The pattern of those in HAL, KITA, and TAMA showed a broad peak at 40 DPA. ZK demonstrated a sharp transient increase at 45 DPA. The temporal pattern of SA contents generally showed a decreasing trend but its contents among varieties were divergent. Moreover, those of some varieties fluctuated over time (Fig. 1D). Also, tZ contents decreased gradually along with the ripening process from 30 DPA to 60 DPA in all seven varieties with a little fluctuation, although absolute concentrations of tZ were diverse. A marked exception was found after ripening: a sharp rise in ZK (60 DPA, Fig. 1E). Contents of iP appeared very low and decreased gradually (Fig. 1F).

Plant hormone dynamics in wheat seeds during late seed maturation. Plant hormone contents in seven cultivars were examined in 2011. HAL, Halberd; CS, Chinese Spring; AUS, AUS1408; KITA, Kitakei1354; TAMA, Tamaizumi; ZK, Zenkoujikomugi; OW, OW104. Dynamics of indole acetic acid (IAA) contents (a), abscisic acid (ABA) contents (b), jasmonic acid (JA) contents (c), salicylic acid (SA) contents (d), trans-zeatin (tZ) contents (e), isopentenyladenine contents (f), and dihydrozeatin of whole seed harvested in the field as indicated in Materials and Methods. DPA represents the days post-anthesis. Error bars represent standard error of the mean (n = 4).
To investigate the relation between differences in germinability and the plant hormone profile during seed maturation, a principal component analysis (Fig. 2A, 2B) and a hierarchical clustering analysis (Fig. 2C) were conducted. Plots of the principal component analysis showed a diagonal distribution (Fig. 2A). Eigenvector analysis revealed that primary component 1 (PC1) was explained chiefly by the contents of IAA and ABA (Fig. 2B). Results show that PC1 decreased along DPA following the decreasing patterns of IAA and ABA. PC2 was explained by variations of JA, SA, and cytokinins. Data points of PHS-sensitive cultivars (HAL, CS, AUS, and KITA) largely overlapped with those of PHS-tolerant cultivars (TAMA, ZK, and OW). This result indicates that the plant hormone profile is not correlated with dormancy levels among these seven varieties. Hierarchical clustering analysis revealed that PHS-sensitive varieties and tolerant varieties can be grouped in the same cluster (Fig. 2C). Given that dynamics of plant hormones during seed development are associated with germinability, varieties with similar germinability would be grouped in a close cluster. However, PHS-susceptible cultivars AUS and CS were distributed in different clusters. The PHS-tolerant varieties OW and ZK were grouped in distal clusters. Collectively, these results do not support that the difference in germinability of these wheat varieties is associated with different plant hormone profiles.

Profile of contents of six plant hormones in whole seeds among seven wheat varieties and relation among plant hormones dynamics during seed maturation. Data were collected from grain harvested in 2011, which was used in Fig. 1. (A) Principal component analysis plot of first two principal components (PC) of hormone profiling of wheat cultivars. HAL, Halberd; CS, Chinese Spring; AUS, AUS1408; KITA, Kitakei1354; TAMA, Tamaizumi; ZK, Zenkoujikomugi; OW, OW104 from 30–60 DPA. The darkest color symbols represent 30 DPA and progressively become a lighter color along DPA proceeds. (B) Eigenvectors of each hormone corresponding to the principal component analysis shown in panel A. (C) Heat map of seven plant hormones and dendrogram of hierarchic clustering analysis. DPA denotes days post anthesis. HAL, Halberd; CS, Chinese Spring; AUS, AUS1408; KITA, Kitakei1354; TAMA, Tamaizumi; ZK, Zenkoujikomugi; OW, OW104 from 30–60 DPA. White represents missing data. Data of hormone contents were log-transformed (base 10) and then normalized prior hierarchic clustering analysis. (D) Heat map of six plant hormones and dendrogram of hierarchic clustering analysis. DPA denotes days post anthesis. IAA, ABA, JA, SA, tZ, and iP respectively stand for indole acetic acid, abscisic acid, jasmonic acid, salicylic acid, trans-zeatin, and isopentenyladenine. White represents missing data. Data of hormone contents were log-transformed (base 10) and were then normalized with prior principal component analysis and hierarchic clustering analysis.
Hierarchical clustering analysis among hormone species was conducted to investigate the similarity of temporal patterns among plant hormones (Fig. 2D). The profiles of IAA and ABA were similar, but distinct from those of other hormones. Two cytokinins showed similar patterns: tZ and iP. Apparently, JA and SA were unrelated to other hormones in terms of their temporal patterns during the development process in wheat seeds. King (1993), based on original reports by McWha (1975) and Wheeler (1972), reported that temporal profiles of IAA and ABA during seed development were similar when assayed using bioassay and gas chromatography. Our result examined using LC-MS shows fundamentally equivalent results for these two hormones.
Hypothetically, uneven redistribution of plant hormones within seeds might produce varied germinability, even when the total contents in whole seeds are similar. Therefore, plant hormone contents in embryo/scutellum and other parts (endosperm and outer layers) were analyzed separately following dissection of the lyophilized seeds. Results obtained for the high-germinability cultivar CS and a low-germinability variety OW were compared (Supplemental Fig. 5). Significant differences in the ratio of hormone contents in embryo/scutellum and endosperm/outer layers between two varieties were found at 30, 35, and 50 DPA in IAA, at 35 DPA in JA, at 35 DPA in tZ and at 50 DPA in iP. Differences in the ratios of ABA contents were not observed, suggesting that the redistribution of ABA within seeds is not involved in differences in seed dormancy levels among wheat varieties.
Plant hormone contents in ripened seedsTo elucidate the relation between germinability and hormone contents further, correlation of GIs and contents of each hormone in ripened seeds (Table 1, Supplemental Fig. 6) were investigated using Spearman’s rank correlation test using data from 2011. The IAA contents were found to be highest in CS and lowest in OW, with a 6.9-fold difference between these varieties (Supplemental Fig. 6A). Also, ABA contents were found to be highest in CS and lowest in OW but with much less variation (2.5-fold) (Supplemental Fig. 6B). JA contents and SA contents were significantly high only in single-cultivar ZK and TAMA, respectively (Supplemental Fig. 6C, 6D). Contents of tZ and iP in wheat seeds varied among the varieties (Supplemental Fig. 6E, 6F). Significant and strong positive correlation between GIs and hormone contents were found only in IAA (rs = 0.786, P = 0.036). The P values of other hormones were higher than 0.05: ABA, 0.535; JA, 0.879; SA, 0.702; tZ, 0.383; iP, 0.535. These results indicate that differences in contents of ABA, JA, SA, tZ, and iP in seeds are not associated with differences in germinability of the seven varieties, except IAA, which positively correlated with germinability. Whereas hormone data were acquired only in 2011, this result would be extended reasonably to other years because the rank of GI in each year was almost consistent.
Differences in ABA response among wheat varietiesFinally, we investigated the response of ripened seeds to exogenously applied ABA to look into variation of germinability in the presence of ABA among the cultivars. GIs of seven varieties were examined in the absence and presence of ABA after artificial dormancy breaking, at 20°C and 15°C (Table 2, Fig. 3). GIs decreased by the application of ABA in a concentration-dependent manner (0, 1, 10, and 100 μM). Furthermore, unambiguous variation in germinability was found in the presence of ABA among varieties. Table 2 shows GIs examined in the presence of exogenous 10 μM ABA, where the differences in GI among varieties were rather apparent at 15°C than 20°C. No significant difference was found in GIs of HAL, CS, AUS, and KITA in 2008, 2009, 2010, or 2011, with the exception of a significant difference found between AUS and KITA in 2010. Differences in GIs among TAMA, ZK, and OW were not found to be significant in any experiment. This result suggests that these seven cultivars are classifiable into two groups based on germinability in the presence of 10 μM ABA (Table 2). This grouping resembles that of the results found for seed dormancy tests (Table 1). To infer the relation of seed dormancy and ABA response of seeds, correlation between GIs of whole seeds and those of half seeds in the presence of 10 μM ABA was examined. The values of rs for germination assays at 20°C (dataset of Tables 1, 2) and 15°C were 0.85 (p = 0.020) and 0.62 (p = 0.025), respectively, suggesting that significant correlation exists between GIs of whole seeds in water and that of embryo-half seeds in the presence of ABA. Although GI of embryo-half seeds in the presence of ABA does not necessarily imply ABA sensitivity of seeds, the difference in germinability of wheat seeds can be explained partly by ABA sensitivity to ABA.
| Variety | GI at 20°C | GI at 15°C | ||||
|---|---|---|---|---|---|---|
| 2008 | 2009 | Mean | 2010 | 2011 | Mean | |
| Halberd | – | – | – | 0.74 ± 0.07ab | 0.46 ± 0.11ab | 0.60 ± 0.13 |
| AUS1408 | 0.27 ± 0.06a | 0.48 ± 0.23ab | 0.37 ± 0.24 | 0.57 ± 0.02b | 0.51 ± 0.11ab | 0.54 ± 0.11 |
| Chinese Spring | 0.31 ± 0.12a | – | 0.31 ± 0.12 | – | 0.41 ± 0.09ab | 0.41 ± 0.09 |
| Kitakei 1354 | 0.24 ± 0.12ab | 0.58 ± 0.18a | 0.41 ± 0.22 | 0.99 ± 0.03a | 0.64 ± 0.11a | 0.76 ± 0.11 |
| Tamaizumi | – | 0.31 ± 0.28ab | 0.31 ± 0.28 | 0.12 ± 0.09c | 0.11 ± 0.06c | 0.11 ± 0.11 |
| Zenkoujikomugi | 0 ± 0b | 0.02 ± 0.03b | 0.01 ± 0.03 | 0.29 ± 0.04c | 0.13 ± 0.09c | 0.21 ± 0.10 |
| OW104 | 0.05 ± 0.05b | 0 ± 0b | 0.02 ± 0.05 | 0.19 ± 0.09c | 0.30 ± 0.06bc | 0.25 ± 0.11 |
Different lowercase letters represent a significant difference according to Tukey HSD test. Data are shown as mean ± standard deviation (n = 3).
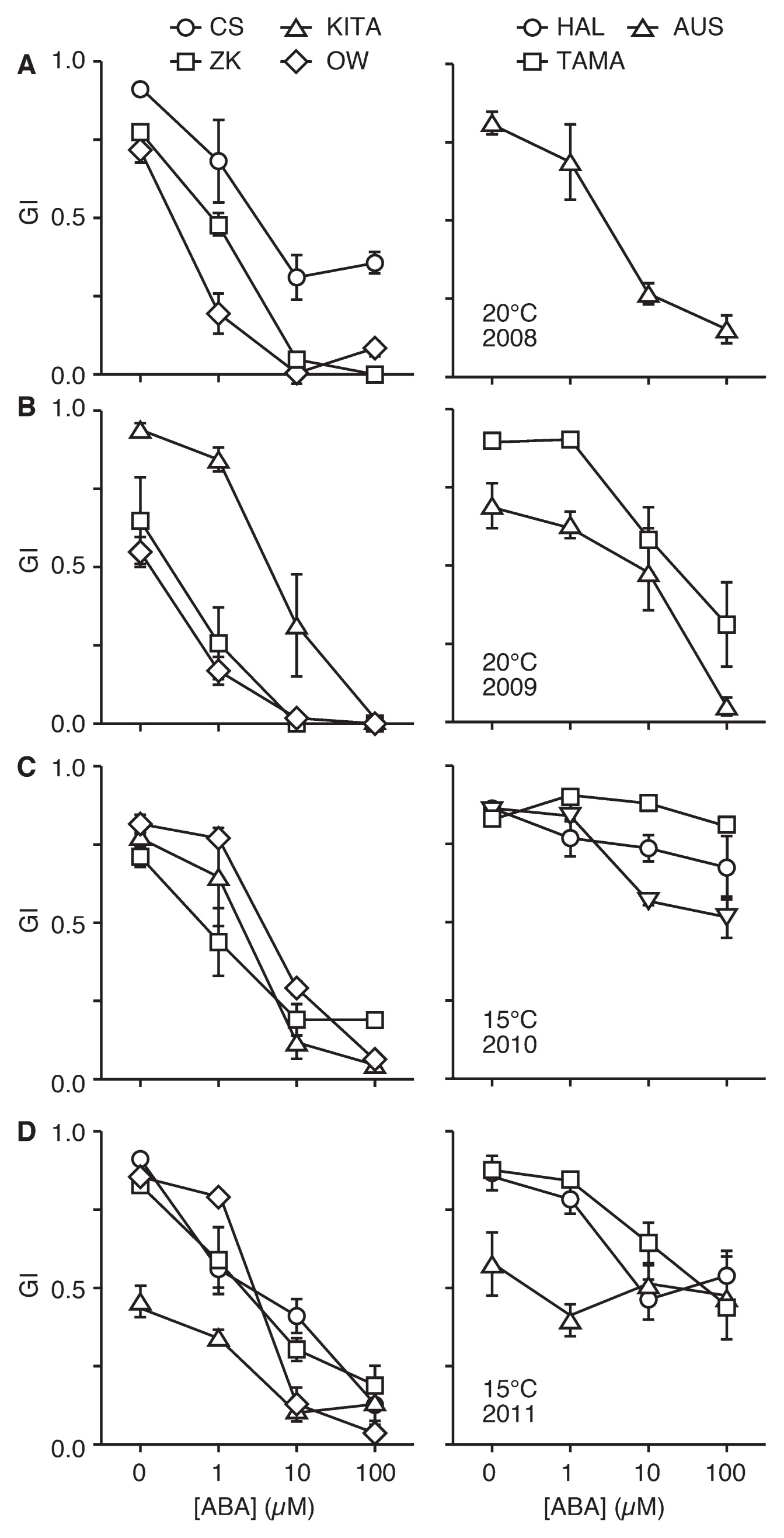
Inhibition of germination of half seed of seven wheat cultivars by ABA. GI of seven cultivars, HAL, Halberd; CS, Chinese Spring; AUS, AUS1408; KITA, Kitakei1354; TAMA, Tamaizumi; ZK, Zenkoujikomugi; OW, OW104 at full ripe (water content becomes ≤20% as presented in Materials and Methods). Error bars represent the standard error of the mean (n = 3).
Several polymorphisms related to PHS resistance have been documented. We examined genotypes in seven genes involved in PHS resistance and grain colors. The R-1 gene is responsible for seed color; it is also linked genetically to seed dormancy (Himi et al. 2002, 2011). Significant difference in ABA contents was not found between red-grain varieties possessing one or two R-1 dominant gene(s) in B and /or/ D genomes (CS, KITA, OW, ZK) and white-grain varieties possessing only recessive alleles in all subgenomes (AUS, HAL, TAMA) (Supplemental Table 3, Fig. 3B, Wilcoxon rank-sum test, P = 0.72). Nakamura et al. (2011) demonstrated that single nucleotide polymorphism (SNP) types of TaMFT-3A in the seven varieties showed good agreement with the order of the dormancy phenotypes: HAL, CS, AUS, and KITA have non-Zen type (non-dormant) and TAMA, ZK, and OW have Zen type (dormant) (Chono et al. 2015 and Supplemental Table 3). These SNP types in MFT-3A can well explain the ABA sensitivity difference among these seven cultivars. Alleles of Vp-1B and TaSdr-B1 did not explain the difference in seed dormancy among the seven varieties examined for this study (Supplemental Table 3). The genotype of MKK3 was found to be the PHS-resistant allele in all seven varieties (Supplemental Table 3).
In this study, we examined the contents of nine plant hormones of seven varieties of field-grown common wheat, as well as germinability at 15°C and 20°C to test the working hypothesis that differences in seed dormancy levels among wheat cultivars is attributed to the difference in plant hormone profile during seed maturation.
King (1993) pointed out that the contents of IAA and ABA in wheat seeds change in a parallel manner based on data reported by McWha (1975) and Wheeler (1972). McWha (1975) examined ABA contents in cultivars Aotea and Arawa using gas chromatography. Wheeler (1972) examined the amounts of auxin in cultivar Kloka by bioassay. A peak of ABA contents and a peak of auxin-like activity were observed concomitantly with the cessation of dry weight increase. In this study, we examined the contents of IAA and ABA simultaneously from the same seed specimen by LC-ESI-MS/MS after stopping of dry weight gain and the onset of seed dehydration. The temporal change in IAA and ABA contents in seeds showed similar patterns: a rapid decrement (Fig. 2) that strongly supports the conclusions of King (1993), although we examined only the late stage of seed development.
Seeds with higher contents of IAA showed higher GI, indicating a positive relation between germinability and auxin (Table 1, Supplemental Fig. 5). Earlier reports suggest that exogenous IAA inhibits wheat seed germination (Morris et al. 1988, Ramaih et al. 2003). IAA contents were lower in high-amylase-inducing inbred lines of wheat, which demonstrate high PHS susceptibility (Barrero et al. 2013). A mutant study in Arabidopsis suggests that endogenous IAA enhances seed dormancy through the regulation of ABA signaling component (Liu et al. 2013). These reports are apparently opposite to our field experiment in wheat. Our hormone analysis results might indicate that IAA content was increased in cultivars exhibiting high GI at the ripened stage by initiation of germination process/cell division in the spike at the time of harvest in the field, rather than having an inhibitory function of IAA on seed germination.
Wheeler (1972) reported that wheat seeds include cytokinins of two distinct kinds, which are separable by paper chromatography: one is similar to zeatin; another has a different Rf value. Our result indicates that wheat seeds contain both tZ and iP. Also, DHZ is detectable but only at a very low level for comparison with tZ and iP. It is most likely that cytokinin activities, which Wheeler (1972) detected, are tZ and iP.
Reportedly, application of exogenous jasmonic acid to apple embryos and pear seeds promotes germination (Ranjan and Lewak 1992, Yildiz et al. 2008). The results of endogenous JA content analysis in wheat seeds suggest that endogenous JA contents are apparently not associated with germinability. Differences in JA contents cannot explain the differences in germinability of wheat cultivars, although this result does not necessarily contradict the involvement of JA in promotion of germination. Jacobsen et al. (2013) documented that JA reverses light-inhibition of wheat seed germination. In this study, wheat germination assays were conducted in the dark. Endogenous JA contents were not associated with germinability in this study. That fact might be attributed to the lack of light inhibition effects on the seed germination. However, JA contents varied among the varieties and timing of harvest. This variation in JA contents might be explained by abiotic and biotic stresses occurring in the field, rather than developmental processes. Similar variation in contents was also found in SA, which can also be brought by abiotic and biotic stresses.
We detected only trace amounts of GA1 and GA4 in whole seeds (<0.05 ng g−1). For that reason, the results were not of sufficient quantitative precision. Barrero et al. (2013) found high contents of the precursor of active GAs GA29 and GA44 in the PHS-susceptible high-amylase-inducing inbred lines by LC-ESI-MS/MS. Contents of GAs might be attributed to the degree of seed dormancy or to the initiation of germination processes in spikes.
Some studies have examined whether the diverse PHS susceptibility of wheat, barley, and rice cultivars is attributed to ABA contents in seeds (Chono et al. 2013, Goldbach and Michael 1976, King 1976, Lee et al. 2018, Suzuki et al. 2000, Walker-Simmons and Sesing 1990). One reason for the controversy might be the numbers of varieties/cultivars examined in the studies. Many of those studies compared two varieties/cultivars of wheat with different dormancy levels. The present study examined seven varieties during a late stage of seed maturation. Results showed divergence in ABA contents among varieties, but the divergence was not correlated significantly with seed dormancy. Our observations support the notions presented by King (1976) and by Walker-Simmons and Sesing (1990). This does not necessarily contradict the effectiveness of breeding strategies in increasing ABA contents aimed at mitigating PHS susceptibility.
This study found no significant difference in ABA contents among the seven varieties. Reportedly, the identified quantitative trait loci of ABA sensitivity in chromosomes 5A, 4D, and 6D of common wheat were not involved in ABA contents (Iehisa et al. 2014a, 2014b). The ABA contents were not changed either in the high seed dormancy wheat mutant Zak ERA8 (Martinez et al. 2016). Genotypes of TaMFT-3A, which are involved in differences of wheat seed dormancy (Nakamura et al. 2011), apparently agreed with the germinability propensity (Supplemental Table 3). At low temperatures, variation in germinability among the Zen genotype varieties was apparent (Supplemental Fig. 1, Supplemental Table 3), which suggests that other loci aside from TaMFT-3A are involved in ABA sensitivity. Three other genotyped genes, Vp-1B, TaSdr-B1, and MKK3 showed no an apparent relation to the order of germinability (Supplemental Table 3). We found no apparent relation between genotypes related to ABA sensitivity and ABA contents in seeds, except for TaMFT-3A.
Accumulated results from many studies have indicated the association of variations in dormancy break timing and PHS susceptibilities among wheat varieties with the temporal change and difference in ABA sensitivities of seeds (King, 1976, 1993, Tuttle et al. 2015, Walker-Simmons and Sesing 1990). Our results obtained from comparison of seven wheat varieties support this notion. Nevertheless, we do not deny that diverse dormancy levels among varieties are based partly on differences in the sensitivity of seeds to other plant hormones.
T.M., I.C.M., and E.H. performed experiments. I.C.M. analyzed data. I.C.M. and T.H. conceived the study. T.M. and I.C.M. wrote the manuscript. All authors read the manuscript and agreed to its submission.
This research was supported by the Japan Advanced Plant Science Network and The Ohara Foundation for Agricultural Research.