2020 Volume 70 Issue 3 Pages 387-395
2020 Volume 70 Issue 3 Pages 387-395
Powdery mildew (PM), caused by Erysiphe cruciferarum, is an epidemic of oil rapeseed (Brassica napus L.) growing worldwide, but PM resistant germplasm is rare in this species. We screened 102 accessions of B. napus and other cruciferous species and found an Ethiopian mustard (Brassica carinata) cultivar ‘White flower’ immune to PM in both the field and greenhouse. Outcrossing in the female parent ‘White flower’ was promoted by using a chemical gametocide tribenuron-methyl, to obtain hybrid seeds of distant hybridization with an elite B. napus cultivar ‘Zhongshuang11’. Three true F1 hybrids with B. carinata cytoplasm were obtained without using embryo rescue, which showed complete male sterility and light yellow petals. The hybrid plants and the progenies derived from backcrossing were validated using morphological traits, seed quality, and molecular markers. Five lines in the BC1F3 generation, named ‘W7-1’, ‘W7-4’, ‘W7-6’, ‘W8-1’, and ‘W8-3’, and one BC2F2 line ‘W3PS-1’, whose young leaf was yellow green, were identified to be resistant or moderately resistant to PM. The seed quality and some morphological traits of these lines resembled the parent ‘Zhongshuang11’, indicating that the resistance gene(s) has been preliminarily introduced into B. napus.
Distant hybridization usually refers to the interspecific hybridization of taxonomic species with more distant relationships than inter-variety crossing. This type of hybridization has great value for crop breeding and agricultural production because it enriches the gene pool for plant breeding or creates new polyploid species. In the genus Brassica, there are three diploid species (U 1935), including Brassica rapa (AA, n = 10), Brassica nigra (BB, n = 8) and Brassica oleracea (CC, n = 9), and three allotetraploids, including Brassica juncea (AABB, n = 18), Brassica napus (AACC, n = 19), and Brassica carinata (BBCC, n = 17). Among these species, distant hybridization can occur naturally or artificially. For example, B. napus is a compound species newly derived from B. rapa and B. oleracea after interspecific hybridization and spontaneous doubling of chromosomes (U 1935). To date, B. napus has been one of the most successful oilseed crops worldwide. Unfortunately, breeding of this young species encountered a narrow bottleneck in genetic diversification, and many studies have attempted to enrich the gene pool of B. napus by distant hybridization (Jiang et al. 2007, Li et al. 2007, Liu et al. 2018). Ethiopian mustard (B. carinata), an old crop adapted to semi-arid areas of east Africa, is now gaining the interest of researchers due to its higher yield and better resistance to diseases and silique shattering than any of the oilseed crops (Alemayehu and Becker 2001, Malik 1990). However, its seed quality is poor with low oil content, high erucic acid, and high glucosinolates, which does not meet the requirements for the varieties registry.
Ethiopian mustard harbours some resistance genes to fungal diseases including powdery mildew (PM) (Singh et al. 1997, Tonguc and Griffiths 2004). PM, caused by the pathogen Erysiphe cruciferarum (Opiz), is a worldwide disease of oilseed Brassica species including B. napus (Guo et al. 2013, Zhang et al. 2012). Because of the wet/dry alternations of a seasonal climate, PM is becoming more frequent and endangering all rapeseed-producing areas, such as China, England, France, Australia, and Argentina (Bradshaw et al. 1989, Gaetán and Madia 2004, Guo et al. 2013, Kaur et al. 2008, Penaud 1999, Shao 2006, Song et al. 2009, Zhang et al. 2012). It is necessary to study the pathogenic conditions and mechanism to guide rapeseed production. If rapeseed growers do not prevent and control the disease, PM will directly threaten seed production. Application of some chemical fungicide is usually used to control PM, but utilizing disease-resistant varieties is a more economical, effective and safe strategy, which has the advantages of environmental protection and long-term effects. Therefore, breeding PM resistant varieties is a strategy to cope with the outbreak of PM in rapeseed, although there are few PM resistant resources in B. napus and less attention has been paid to the resistance breeding of rapeseed.
In China, rapeseed PM outbreaks mainly occur on adult plants in winter rapeseed production areas including Shaanxi, Gansu, Sichuan, Hubei, Guizhou and Yunnan Province. The prevalent pathogen, E. cruciferarum, has been identified in Gansu, Shaanxi, and Henan Province (Guo et al. 2013, Shao 2006, Zhang et al. 2012). The aboveground parts of rapeseed plants, including stalks, leaves and siliques, are covered by E. cruciferarum, leading to premature chlorosis and senescence of leaves, deformation of siliques, and thin seeds. PM seriously affects the maturity and oil content of rapeseed, leading to seed yield declines of 15% to 50% (Shao 2006). It is now important to develop a PM resistant variety. In this experiment, distant crossing of PM resistant B. carinata × B. napus was made, and the progenies were compared for their resistance level, morphological traits, seed quality and polymorphisms revealed by SSR (Simple Sequence Repeat) molecular markers. This study aimed to develop an allocytoplasmic Brassica napus with PM resistance, as well as good seed quality, meeting the national standards of double low (low erucic acid and low glucosinolates).
In this study, 103 cultivars and breeding lines were used, which belong to B. napus, B. rapa, B. juncea, B. nigra, B. carinata or yellow mustard (Sinapis alba) from China, Czech Republic, Australia, Ethiopia, Britain, France and other countries (Supplemental Table 1).
Pre-screening of resistant germplasm in the fieldAn outdoor test of PM resistance was carried out in the experimental field of Northwest A&F University in the three growing seasons of 2015 to 2017. The materials were arranged randomly in the field, each in two or three rows with a row length of two metres with 15 plants in a row. The severity of disease and the type of reaction were recorded when PM infected the entire field. At least five randomly selected plants for each genotype were inspected repeatedly. It should be noted that the natural incidence of rapeseed PM in the field is mainly before the ripening stage of rapeseed. Thus, natural incidence, despite being average values over two or three years, were influenced by many factors such as climate, uniformity of the pathogen distribution, degree of rapeseed maturity degree, and time of leaf shedding. The field test without controlling of the experimental conditions was only used to roughly select the germplasm. The PM pathogen was stained by methyl blue and observed under a light microscope. The severity of disease was classified into five grades by the percentage of disease lesion or spots per leaf area (Shao 2006). The classification criteria of disease severity are as follows: Grade 0—no lesion; Grade 1—less than 5% of the leaf/stem area has lesions; Grade 3—the area of lesions accounts for 6% to 25% of the leaf/stem and there are obvious hyphae; Grade 5—diseased area accounts for 26% to 50% of leaf/stem with mycelium expansion; Grade 7—diseased area accounts for more than 51% to 75% of leaf/stem and a large number of spores are dispersed; Grade 9—PM cover nearly all leaf/stem. Finally, the disease index (DI) was calculated using the formula: Disease index = 100 × Σ [disease grade of a plant × number of plant in the grade]/[total number of plants × 9]. The resistance level was classified according to the DI (Liu et al. 2018): highly resistant with a DI = 0; resistant with DI < 10; moderately susceptible with DI between 10 and 20; susceptible with DI between 20 and 50; and highly susceptible with DI > 50.
Resistance tests in the greenhouseThe young seedlings of some elite rapeseed germplasms were transplanted into a greenhouse for cultivation and adult inoculation. The leaves of these plants were moistened by water, and fresh pathogen spores that were harvested from other diseased plants were dispersed onto the leaf surface. Then, the treated plants were watered every seven days to simulate wet/dry alternation treatment. The incidence and severity of the disease were investigated. Susceptible plants were also grown in the greenhouse to ensure all plants were continually exposed to inoculum. The results of artificial inoculation in greenhouse were more repeatable than under the field conditions and then the evaluation of PM resistance was conducted in the greenhouse. All the tested B. napus accessions including ‘Zhongshuang11’ (‘ZS11’) were susceptible to PM, except for a PM resistant strain ‘White flower’. The resistant ‘White flower’ and susceptive cultivar ‘ZS11’ were grown along with their interspecific hybrids and the derived plants as resistant/susceptible controls.
Transferring resistance into B. napusDistant crossing of B. carinata × B. napus without embryo rescue is very difficult when B. carinata was the female parent (Dr. Genyi Li, Manitoba University, private communication). The reciprocal crossing was much easy to do (Qi et al. 2013) but it was not in our interest because we desired new B. napus genotype with alien cytoplasm of B. carinata to add higher drought tolerance and disease resistance to B. napus from B. carinata. The anthers of the female parent ‘White flower’ were removed artificially before flowering using forceps. Then, pollen grains from the male parent were manually spread on the stigma of fresh flowers of the female parent. Alternatively, male sterility of the female parent was elicited by a foliar spray with 10 mL of 0.02 mg/L of the male gametocide tribenuron-methyl (TBM) (Lian et al. 2019). Then the TBM-treated plants became male sterile and were placed together with the male parent of ‘ZS11’ in a plastic bag with numerous micro holes. The plants in the bag were wobbled daily to help the cross pollination between ‘White flower’ and ‘ZS11’. The seeds were harvested and seed-setting rate was investigated later. The seeds of the F1 generation of B. carinata × B. napus were placed on a filter paper wet by water. When the germinated seeds developed root hairs, they were transplanted into greenhouse soil. The true hybrids were identified by morphological comparison and seed quality with high glucosinolates and erucic acid. Aceto-carmine (1%) staining was used to measure pollen viability. Individual resistant plants were isolated and backcrossed with the elite recurrent parent ‘ZS11’, which has large 1000-seed-weight (>4 g), high oil content (>46%), and very long siliques (>10 cm). Then, the derived seeds of BC1 generations were grown in the greenhouse and produced BC1F2 (including individual plants named as ‘W7’ and ‘W8’) and BC1F3 lines (‘W7-1’, ‘W8-1’ and so on) by successive self-pollination. Simultaneously, the sterile plants in BC1 were backcrossed again with ‘ZS11’ to get BC2 seeds (‘W3’). Then, the BC2 generated BC2F2 (samples ‘W3PS-1’ and ‘W3PS-3’ in this study) by self-pollination (Supplemental Fig. 1). In addition, some progenies of reverse BC2 (RBC2: ‘W4’ and ‘W5’) were produced from an emasculated ‘ZS11’ plant that was pollinated by the semi-fertile BC1 plants. The progenies of a same plant were denoted as the parental name with a number suffix.
Evaluation of the progenies of distant crossingThe seed quality, that is, content of glucosinolates, erucic acid, and crude fat, of the parents, hybrids, and offspring were analysed using a FOSS 5500 NIRsystem near-infrared spectrophotometer (Laurel, Maryland, USA). Resistant progenies were identified in the greenhouse and propagated to form different lines. The seedlings were compared to the recurrent parent ‘ZS11’ to verify whether the offspring resembled B. napus by morphological observation and analysis of the seed quality. Morphological traits included leaf shape, number of lobes, leaf colour, thickness of epidermal wax, silique length, and silique orientation (upward, horizontal, and drooping).
Detection of SSR markersDNA from the leaves of different lines was extracted (Doyle and Doyle 1990), and SSR markers derived from 18 primer pairs were used to detect the genetic differences among different progenies from the parent ‘ZS11’ and ‘White flower’. The sequences of some important SSR primers designed for C and A genomes (Kim et al. 2009, Li et al. 2011) as well as chloroplast DNA (Flannery et al. 2006) were listed in Supplemental Table 2. The genetic analysis of all polymorphic SSR markers in these accessions was performed using DPS v7.05 software (Tang and Zhang 2013). The samples were clustered according to the UPGMA (Unweighted Pair Group Method with Arithmetic mean) method based on Nei’s genetic distance. Based on analysis of the genetic diversity revealed by SSR markers, the derived lines were compared with recurrent parent ‘ZS11’ to identify lines that resembled B. napus, in addition to resistance level, morphological traits, and seed quality traits.
The spores of E. cruciferarum dispersed with airflow and their germination tubes could penetrate into the leaf surface (Fig. 1A). Many mycelia would grow from one pathogen spore if it invaded successfully. The mycelia attached to epidermal tissues and insert haustoria into the tissues to obtain nutrients (Fig. 1B). The powdery surface of a lesion was a mixture of mycelia, conidia, and pedicels. It had thin and long spore stalks, which were raised on the surface of the leaves (Fig. 1C). A mature cleistothecium contained many spores (Fig. 1D). In greenhouse conditions, E. cruciferarum can parasitize various green tissues, including cotyledons (Fig. 1E), leaves, stems or siliques, during the entire growth period. PM would often outbreak during the ripening stage of rapeseed, but if the outbreak was earlier, the seed yield would be reduced seriously because PM directly affected the functions of leaves and siliques. The disease caused premature ageing of tissues and the infected leaves were chlorotic and withered early (Fig. 1F). The infected plants produced thin pods and unfilled seeds.

Histochemical staining of E. cruciferarum and the symptoms of E. cruciferarum infection on rapeseed. A: A spore invades the leaf. B: One pathogen has many mycelia. C: The powdery substances of a lesion are mixtures of mycelia, conidial pedicels and conidia. D: A cleistothecium containing many spores. E: E. cruciferarum parasitizing the cotyledon. F: An infected leaf becomes chlorotic and withered early.
Under field conditions, all 90 cultivars of B. napus were susceptible or weakly resistant to PM. Although the incidence of disease differed, no B. napus germplasm showed high resistance (Table 1, Supplemental Table 1). All the germplasm of B. napus, S. alba, B. juncea, and B. nigra used in this study were somewhat susceptible to PM (Fig. 2A, 2B, 2D, 2E, 2G, 2H). Two B. rapa cultivars showed moderate incidence but its early maturity and early defoliation may have contributed to avoidance of PM infection. One accession named ‘White flower’ that was selected from the Ethiopian mustard landrace ‘Sao Tome’ was immune to PM (Fig. 2A, 2C, 2F) in comparison to other germplasms. In addition, ‘White flower’ retained the advantages of Ethiopian mustard, such as vigorous growth, pod shattering resistance, high mechanical strength of stem, and lodging resistance. Therefore, we used ‘White flower’ as the resistant donor to try to transfer the resistance gene(s) into B. napus. The resistance level of B. carinata strain ‘White flower’ and some selected cultivars was further investigated in the greenhouse. Several days after inoculation of the PM spores from infected plants, a dramatic change was observed for susceptible genotypes, and the differences between genotypes was pronounced. The greenhouse environment was favourable for PM development and all the tested cultivars, including ‘ZS11’, ‘ZH9’, ‘Zhongshuang6’, and ‘Zheyou267’, except ‘White flower’ were susceptible to PM, with more lesion spots than in the field conditions (data not shown).
| Resistance grade (DI) | List of plant accessions |
|---|---|
| Highly susceptible (>50%) | CH42-SI, B351, Mohican, Solida |
| Susceptible (20–50%) | Zhong508, Zheda619, S8II, Huazheyou10, Sap50, Ningyou14, Zheyou601, QJ5005, Yanyou2, Baoyou85, Fu green, YH2, Slog9, 86155, Qinyou26, SW, 169C, 2006-6707, Shengguang77, Zayou105, 86P25, Zheshuang6, Yangyou6, Shuangyou1, Y76, Huaye740, Qinyou33, G8white, H9958ys, Yangyou4, D636R, D615, D611, 147C, Shuangyou8, Yangguang2009, Yuyou2, Zhenyou1, Gaoyou605, Meiyouwang, Qin7, Suyou4, Suyou1, 2006C, 1521C, Qin6, Qinyou99, EXT66, Za2013, Q8II, RCAT0693, Veronica, Zlata |
| Moderately susceptible (10–20%) | Zhongshuang9, Zhongshuang11, Huiyou50S, H15R, Zhongshuang6, Zhongshuang7, Zhongshuang5, Zhongshuang12, Zhongyou589, Zheyou18, Zheyou267, Zheyou50, Zheyou51, Qz052, Youyan10, D4818, Ramiro, CDH, Q10, Zheyou758, Chu0708, Zheyou5002, A35, E718, D610, Qinyou3, ZY530, CQN4001, Yanyou3, Polo, Arm, Dwarf XD, Guosheng6, Guohuayou1208, Sapphire, Boomer, Dongyou1, Dingbianjie, Huyou21, Yayou1, Shuyang rapa, Huabanjie, Jinsijie |
| Resistant (0–10%) | Sao Tome |
| Highly resistant (0%) | White flower |

Comparison of PM resistance level in different species. A: Comparison of the resistance level of B. carinata ‘White flower’, B. nigra, B. napus, and S. alba. B: PM lesion on the leaf of B. napus cultivar ‘ZS11’. C: B. carinata ‘White flower’ immune to PM. D: Stem of ‘ZS11’ covered by PM. E: Stem of B. juncea covered by PM. F: The stem and silique of an adult plant of B. carinata cultivar ‘White flower’ resistant to PM. G: Infected silique of B. napus. H: Infected silique of B. nigra.
First, 120 flower buds of B. carinata ‘White flower’ that were artificially emasculated and pollinated by B. napus formed only two short siliques but contained no viable seeds. Hence, we treated the female parent ‘White flower’ with a gametocide TBM (Lian et al. 2019). This treatment allowed all treated plants of ‘White flower’ to be completely male sterile. The pollen grains from ‘ZS11’ were supplied to the male sterile plants whenever new flower opened. Eventually, we obtained 26 siliques of different sizes and 57 seed-like grains from a total of 200 pollinated flowers on a robust plant. Most of the seeds were not plump and had abnormal embryos and cracked seed coats. After promoting germination on moistened filter paper, the hybrid seedlings were transplanted into the greenhouse. Sixteen large seeds grew root hairs and produced seedlings with different vigor. Finally, only seven plants survived and the others died. Four plants were male fertile with the same white flowers and leaf shape as the parent B. carinata (Fig. 3A) and were identified as pseudo-hybrids. The remaining three plants were completely male sterile, and their petal colour was light yellow (Fig. 3B). The leaf was intermediate between B. carinata and B. napus. Only a few (approximately 90) BC1 seeds were obtained from the two sterile plants after repeated pollination with pollen grains from ‘ZS11’; most of the pollinations resulted in empty siliques, indicating that the fertilization process was still impeded or the development of immature embryos failed. The seeds of the BC1 generation were sown in the greenhouse, and the established plants showed different fertility levels, two male sterile, six semi-sterile and three fertile. The male sterile or semi-sterile plants could form short siliques similar to B. carinata, but the fertile plants formed long siliques similar to ‘ZS11’. The male sterile or semi-sterile plants showed higher PM resistance than the fertile plants. Thus, we discarded the fertile plants in BC1 and focused on the semi-sterile plants. Most plants of the BC1F2 generation from the semi-sterile plants were fertile, favouring the formation of BC1F3. It was found that most of the individual plants in different families (‘W7’ and ‘W8’) of BC1F2 showed moderate or high resistance. The leaf shape of BC1F3 plants from ‘W7’ and ‘W8’ families was similar to that of ‘ZS11’ but the leaf colour was yellow green (Fig. 3D, 3E). In addition, BC2 seeds were also produced as the male sterile plants in BC1 backcrossed with ‘ZS11’. Reverse BC (RBC2) were obtained after the pollen grains from the semi-fertile BC1 plants were collected to pollinate ‘ZS11’, which was emasculated prior to pollination. However, nearly all the offspring of BC2 (‘W3’) and RBC2 (‘W4’ and ‘W5’) were susceptible to PM, except a BC2F2 line ‘W3PS-1’.

Comparison of the flower and leaf of the F1 hybrid and several backcross offspring with the parents ‘White flower’ and ‘ZS11’.
The resistance of BC1F3 and BC2F2 generations of B. carinata × B. napus was identified in the greenhouse. The leaf colour of most plants of BC1F2 and BC1F3 was yellow green, but they became dark green with age (Fig. 4A). There were three BC1F3 lines, namely, ‘W7-1’, ‘W7-4’, and ‘W7-6’, with short siliques (Fig. 4B) that were resistant to PM (Fig. 4C). It seemed that the resistance had been stabilized in these lines. Some lines from the other BC1F3 groups (‘W8-1’ and ‘W8-3’) with long siliques (Fig. 4B) showed moderate resistance (Table 2). In partially resistant plants, the inhibition of E. cruciferarum was not durable and when the plant was aging, the remaining infection sites of the pathogen continued to grow. The others were all susceptible to PM. Nearly all the offspring of BC2 (‘W3’) and RBC2 (‘W4’ and ‘W5’) were susceptible to PM, with the exception of a BC2F2 line ‘W3PS-1’ that showed moderate resistance, whose parent was a partially sterile plant ‘W3PS’ in the ‘W3’ population. It is suggested that multiple backcrossing may result in loss of the chromosomal regions harbouring resistance genes, so it is better to make self-pollination in the BC1 generation to provide more chances to find resistant progenies, rather than repeated backcrossing.
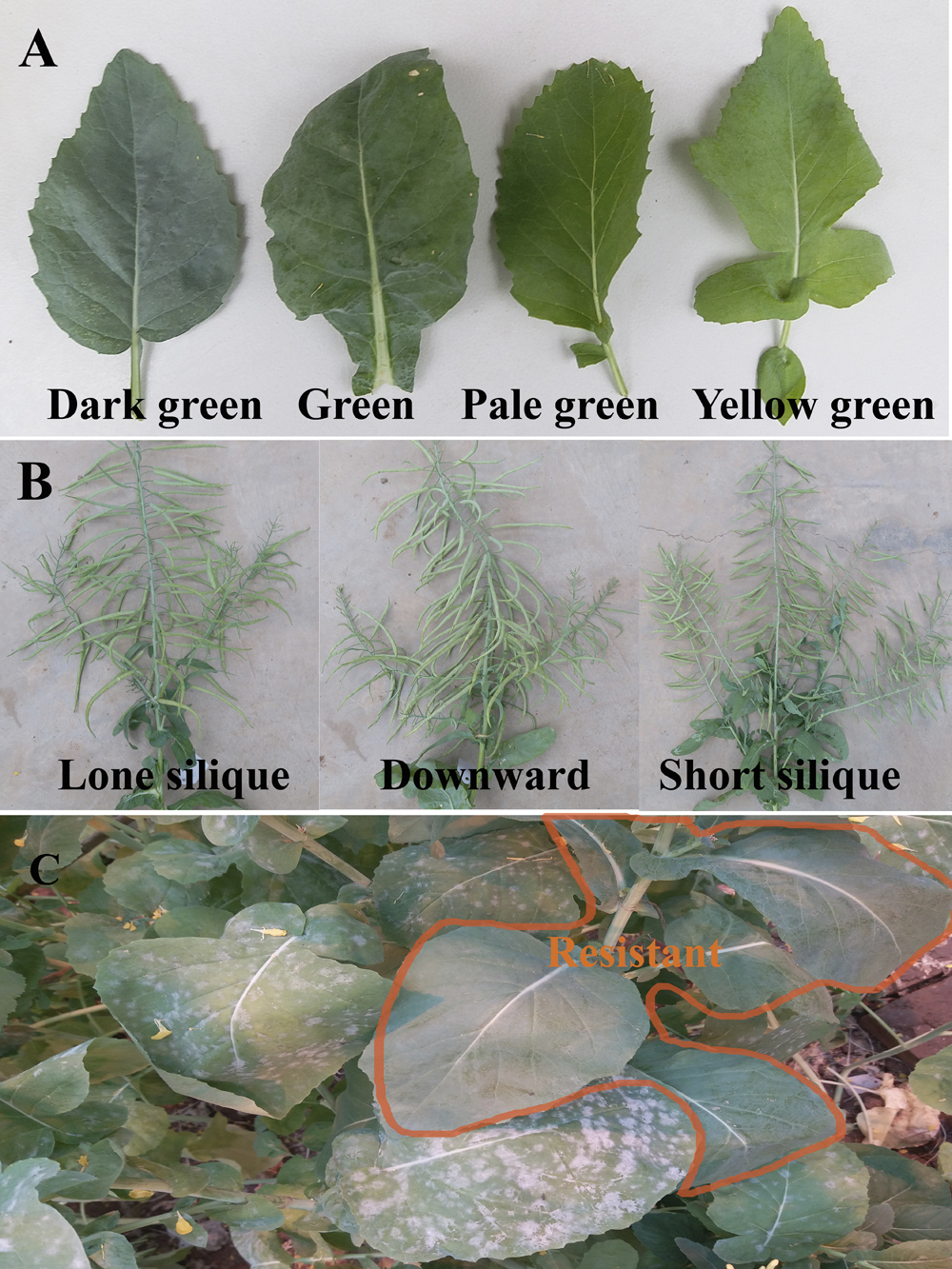
Comparison of leaf shape and colour, silique length and orientation, and resistance level in the BC1F3 offspring of distant crossing.
| Accession | Incidence of PM % | Disease index | Glucosinolates μmol/g | Erucic acid % | Oil content % |
|---|---|---|---|---|---|
| ZS11 | 80.0 | 37.2 | 21.5 | 0.3 | 46.2 |
| White flower | 0.0 | 0.0 | 108.3 | 38.4 | 34.5 |
| F1* | 0.0 | 0.0 | 72.6 | 17.1 | 28.7 |
| BC1 | 11.1 | 1.3 | 78.2 | 13.6 | 31.4 |
| W7-1 | 9.1 | 1.5 | 28.5 | 0.8 | 38.0 |
| W7-2 | 56.7 | 22.7 | 37.5 | 1.1 | 37.3 |
| W7-4 | 11.1 | 0.7 | 43.1 | 1.3 | 36.0 |
| W7-5 | 58.3 | 20.6 | 31.2 | 0.8 | 38.8 |
| W7-6 | 10.0 | 2.1 | 26.6 | 0.4 | 39.4 |
| W7-8 | 43.9 | 14.6 | 29.4 | 0.3 | 38.5 |
| W8-1 | 55.6 | 10.4 | 24.3 | 0.5 | 40.2 |
| W8-2 | 72.1 | 28.5 | 29.4 | 1.2 | 37.5 |
| W8-3 | 66.7 | 18.1 | 21.0 | 2.3 | 35.8 |
| W8-4 | 68.5 | 26.6 | 34.7 | 0.9 | 36.6 |
| W3PS-1 | 22.2 | 11.5 | 28.3 | 0.1 | 37.9 |
| W3PS-3 | 67.3 | 33.8 | 30.6 | 0.8 | 36.4 |
| W4-1 | 88.9 | 31.9 | 36.5 | 0.5 | 39.0 |
| W4-2 | 100.0 | 38.4 | 32.2 | 0.7 | 38.6 |
| W4-3 | 100.0 | 36.2 | 24.3 | 0.1 | 38.2 |
| W5-2 | 90.0 | 30.9 | 23.8 | 0.2 | 38.8 |
| W5-4 | 90.0 | 41.3 | 38.1 | 1.1 | 34.8 |
| W5-6 | 100.0 | 54.4 | 26.3 | 0.6 | 38.4 |
| W5-8 | 81.8 | 35.4 | 23.7 | 0.3 | 37.4 |
* The seed quality of F1 hybrid was estimated roughly in the near-infrared spectrophotometer by using a smaller sample cup other than the standard ring cup.
The seed quality of the two parents of the distant hybridizing and the progenies is shown in Table 2. The female parent ‘White flower’ is double high (high glucosinolates and high erucic acid) but of low oil content similar to other Ethiopian mustard, but the male parent ‘ZS11’ is an elite cultivar of double low quality with an oil content as high as 46.2%. The F1 hybrid is also of double high quality, which was estimated roughly in the near-infrared spectrophotometer by using a smaller sample cup other than the standard ring cup. The content of seed glucosinolates and oil erucic acid decreased in the BC1 seeds, and some of the BC1F3 (‘W7’ and ‘W8’ strains), BC2F2 (‘W3’), and RBC2 (‘W4’ and ‘W5’) were of double low quality with elevated oil content.
Although morphological observations suggested that there are substantial changes in leaf shape and colour, silique length and orientation, and PM resistance in the true F1 hybrid plants and their progenies, analysis of genetic diversity by using SSR molecular markers would be helpful to understand the genetic changes of the breeding materials used in this study. The electrophoresis patterns PCR products of some primer pairs were showed in Fig. 5. The PCR products from the most primers showed that most lines of BC1F3 and BC2F2 generations had similar electrophoresis patterns to ‘ZS11’. Interestingly, the electrophoretic results of primer pair BoGMS1166 showed a band (indicated by arrowhead in Fig. 5) specific to ‘White flower’, BC1F3 families ‘W7’ and ‘W8’, and BC2F2 ‘W3PS’ families. The chloroplast-specific SSR markers of primers MF-4, CCMP2, and CCMP6 showed no polymorphism between ‘ZS11’ and ‘White flower’ but the markers from primer pairs MF-2 and MF-7, which were designed for chloroplast gene rpl16 and TrnM-atpE (Flannery et al. 2006), showed a pattern (indicated by green boxes in Fig. 5) in ‘White flower’, ‘W7’, ‘W8’, and ‘W3PS’ different from ‘ZS11’ and the RBC2 ‘W4’ and ‘W5’, indicating a successful introgression of alien chloroplast components in ‘W7’, ‘W8’ and ‘W3PS’ families.

Genetic diversity of the offspring from ‘White flower × ZS11’ revealed by SSR markers. The electrophoretic band specific to ‘White flower’ is indicated by red arrowhead and green boxes. Marker is DNA ladder. WF: White flower; BCWF: F1 × White flower.
Cluster analysis (Fig. 6) using the polymorphic bands from all the SSR primers showed that these samples had considerable differences, but the genetic diversity was not very high because more than half of the rapeseed samples had genetic distances less than 0.30. The lines in ‘W4’ and ‘W5’ families were located near to ‘ZS11’ in the clustering dendrogram (Fig. 6) while moderately resistant strains ‘W8-1’, ‘W8-3’, and ‘W3PS-1’ and resistant strains ‘W7-1’, ‘W7-4’, and ‘W7-6’ had lower similarity with ‘ZS11’ and were placed far from ‘ZS11’. ‘White flower’ and the backcross BCWF located far from all the other samples in the clustering dendrogram (Fig. 6).

Dendrogram of UPGMA cluster of the offspring from ‘White flower × ZS11’ and their parents.
Infection by PM deteriorates the yield components and agronomic traits of rapeseed, which is mainly due to reduction in photosynthesis from PM lesions covering the leaves and siliques. At the same time, there is competition for nutrients for growth between the PM and the plants. According to the results of Shao (2006), there was a linear correlation between disease index, the peak of PM incidence, yield per plant, and 1000-grain weight. Several important economic traits were affected and in particular, the reduction of yield per plant and 1000-grain weight were up to 55.6% and 41.95%, respectively (Shao 2006).
The scarcity of immune/resistance resources against PM in B. napus will limit the progress of rapeseed breeding. Our data showed all 90 B. napus germplasms were susceptible or moderately resistant to PM. The resistance of the adult plants of cultivar ‘Qinyou3’, ‘Zhongshuang8’ and ‘Zhongshuang9’, which may be resistant at the seedling stage (Shao 2006), were still too low. The Ethiopian mustard ‘White flower’ has very high resistance and is seldom infected by PM. Moreover, this material has many advantages, such as vigorous growth, pod cracking resistance, high mechanical strength of the stem and lodging resistance. Interestingly, transferring resistant genes from Ethiopian mustard, when used as the male parent, may lead to cytoplasmic substitution lines with alloplasmic effects, enhancing the diversity of cytoplasmic types of B. napus (Chang et al. 2009). The introduction of new cytoplasm from tropical germplasm into rapeseed may bring some new desirable traits such as heat/drought resistance and high photosynthetic efficiency (Jiang et al. 2007, Qi et al. 2013). Thus, we need also to pay attention to the enhancement of traits, such as lodging resistance, drought tolerance and shatterproof fruits, in the progenies of ‘White flower × ZS11’.
Gametocide treatment helps to promote distant crossingFour major limiting factors of distant hybridization are incompatibility of pollination, death of immature embryos, sterility of hybrids, and separation of offspring. The genotypic influences on the success of interspecific crossing has been shown (Tonguc and Griffiths 2004). It was reported that it is possible to obtain interspecific hybrids from crosses using tetraploid Brassica species as the female parent but not from the reciprocal crosses using diploids as the female (Choudhary et al. 2000). Thus, embryo rescue was often used to produce first and second backcross generations for some distant crosses (Tonguc and Griffiths 2004). From the experiences of some colleagues, when B. napus is used as the female parent, it is easier to obtain hybrid B. napus × B. carinata (Qi et al. 2013); otherwise, when B. carinata is the female, it is very difficult (Dr. Genyi Li, Manitoba University, private communication). In this study, exposure of the female B. carinata to chemical gametocide TBM (Lian et al. 2019) allowed the plant to be male sterile and forced it to outcross with B. napus. Thus, gametocide induced male sterility combining with repeated pollination will be a useful method to overcome the problem of incomplete hybridization.
Method to identify hybrid progeniesBecause of the difficulties of distant hybridization, false hybrids will inevitably be obtained under the most circumstances. In the distant hybridization of Brassica plants, a high percentage of maternal plants often occur in the F1 population, which are regarded as pseudo-hybrids. For hybrids with different ploidy, observation of chromosome number is accurate and reliable, but cytological identification is affected by the sampling period. Chromosome banding technology is also very difficult for rapeseed studies due to the small size of the chromosome. Jiang et al. (2007) identified the offspring of distant hybridization between B. carinata and B. rapa by morphological, cytological and restriction fragment length polymorphic molecular markers. Their results showed that the hybrids identified by cytological and molecular markers were authentic, but the procedures were complex and cumbersome. Morphological identification was very easy to carry out, but some morphological traits are not accurate. These methods can be used in combination. In future, we will use these methods together to identify changes of genome composition in the hybrid generations.
Empirically, the crazy separation of offspring of distant hybridization can be avoided by backcrossing, which reduce the population of hybrid progenies. However, the backcrossed offspring resemble the recurrent parent and are likely to eliminate some unviable and male sterile plants of intermediate type of distant hybrids under high selection pressure. In our present study, some chromosomes or genes of B. carinata were preserved in the BC1F3 generations as the SSR markers and results of phylogenetic clustering showed, but most BC2 plants lost resistance. If the PM resistance gene(s) is located on the C genome of B. carinata, it will be easy to obtain a stable chromosomal substituted line in B. napus with PM resistance. However, if the gene(s) is located on the B genome, it must be translocated to the C or A genome of B. napus to maintain a stable inheritance of resistance. Thus, for our future breeding programme, it is necessary to select resistant plants with n = 19 chromosomes to evaluate genetic stability of PM resistance in the B. napus background.
PM resistance in Arabidopsis may result from different biological pathways. For example, insertion mutants in the Arabidopsis PUX2 (plant ubiquitin regulatory X domain-containing protein 2) gene significantly reduced reproduction of PM (Chandran et al. 2009). In addition, loss of function mutations of Arabidopsis MLO2, MLO6, and MLO12 (Mildew Resistance Locus O, MLO) genes conferred durable and broad-spectrum penetration resistance against PM fungi, possibly through regulating cell death and senescence-related physiology (Kuhn et al. 2017). Tissue necrosis and callose deposition are responsible for the inhibition and reduction of many fungal diseases (Coelho and Monteiro 2018). These aspects will be evaluated in future studies.
In conclusion, a strain of B. carinata ‘White flower’ that immune to PM was identified, and hybrid progenies of its crosses with B. napus were successfully obtained. Five lines with moderate or higher resistance to PM were obtained in the BC1F3 and BC2F2 generations. The morphological traits (leaf shape, petal colour, epidermal wax, and silique length) and seed quality of most resistant/moderately resistant progenies were similar to the B. napus parent ‘ZS11’, indicating that the resistant genes had been successfully introduced into the B. napus background with B. carinata cytoplasm.
CYY conceived and designed the experiments and prepared the manuscript; QG, CYD, XHZ, and XLW conducted the study; ZH, AXX, and JGD coordinated the study and provided the experimental materials. All authors drafted and approved the final manuscript.
This work was financially supported by projects of the National Key Research and Development Program of China (2018YFE0108000) and the Key Research and Development Program of Shaanxi Province (2018NY-055).