2022 Volume 72 Issue 2 Pages 115-123
2022 Volume 72 Issue 2 Pages 115-123
Clubroot resistance (CR) is an important trait in Chinese cabbage breeding worldwide. Although Crr1a, the gene responsible for clubroot-resistance, has been cloned and shown to encode the NLR protein, its allelic variation and molecular function remain unknown. Here, we investigated the sequence variation and function of three Crr1a alleles cloned from six CR F1 cultivars of Chinese cabbage. Gain-of-function analysis revealed that Crr1aKinami90_a isolated from the cv. ‘Kinami 90’ conferred clubroot resistance as observed for Crr1aG004. Because two susceptible alleles commonly lacked 172 amino acids in the C-terminal region, we investigated clubroot resistance in transgenic Arabidopsis harboring the chimeric Crr1a, in which 172 amino acids of the functional alleles were fused to the susceptible alleles. The fusion of the C-terminal region to the susceptible alleles restored resistance, indicating that their susceptibility was caused by the lack of the C-terminus. We developed DNA markers to detect the two functional Crr1a alleles, and demonstrated that the functional Crr1a alleles were frequently found in European fodder turnips, whereas they were rarely introduced into Japanese CR cultivars of Chinese cabbage. These results would contribute to CR breeding via marker-assisted selection and help our understanding of the molecular mechanisms underlying clubroot resistance.
Clubroot disease, caused by the soil-borne protozoan Plasmodiophora brassicae Wor., is a major concern in the production of cruciferous crops worldwide (Dixon 2009). Yield loss due to clubroot disease has been estimated to be approximately 50% in canola in Canada (Pageau et al. 2006) and 20%–30% in cruciferous crops in China (Chai et al. 2014). In Japan, this disease was first recorded in 1892 (Ikegami et al. 1981) and has become a major threat to Brassica vegetable production. Clubroot disease was observed in approximately 650 ha of Chinese cabbage fields in 2018. Because of the long-term survival potential of resting spores, it is difficult to control infection using agricultural practices such as crop rotation and liming. Therefore, breeding of clubroot-resistant (CR) cultivars is believed to be one of the most economical and environmental friendly methods for preventing clubroot infection (Diederichsen et al. 2009). Most of the F1 cultivars of Chinese cabbage released in Japan possess varying levels of clubroot resistance. However, variations in pathogenicity and virulence among the field populations of P. brassicae cause a breakdown of resistance on a part of the CR cultivars (Tanaka et al. 2006). Several differential tester sets have been reported by the studies of the pathotypes of single-spore isolates and field isolates (Buczacki et al. 1975, Hatakeyama et al. 2004, Some et al. 1996, Williams 1966). Four pathotypes (groups 1, 2, 3, and 4), from two CR F1 cultivars of Chinese cabbage as differential hosts, were identified using the classification system (Hatakeyama et al. 2004). This system has been used to assess the clubroot-infested fields in Japan.
Most of the sources of clubroot resistance were derived from European fodder turnips, and the CR genes derived from them have been introduced into Chinese cabbage (B. rapa), canola (B. napus), and B. oleracea crops by traditional breeding (Diederichsen et al. 2009). Genetic mapping of these resistance sources in B. rapa revealed the identification of more than 20 CR loci, some of which have been introduced into commercial F1 cultivars of Chinese cabbage (Chen et al. 2013, Chu et al. 2014, Hirai et al. 2004, Hirani et al. 2018, Karim et al. 2020, Kato et al. 2012, 2013, Laila et al. 2019, Matsumoto et al. 1998, 2012, Pang et al. 2018, Piao et al. 2004, Saito et al. 2006, Sakamoto et al. 2008, Suwabe et al. 2003, 2006, Yu et al. 2017). Because the clubroot resistance test for selection is laborious and time-consuming, marker-assisted selection is believed to be a powerful tool for resistance breeding. However, because of the differences among genetic resources and pathogens used for mapping, the precise location of most CR loci and their pathotype specificity remains unclear, which hinders effective breeding of CR cultivars of Chinese cabbage. Although numerous DNA markers linked to various CR loci have been reported, PCR analysis using these markers is insufficient for the detection of CR genes.
Among the CR genes identified in B. rapa chromosomes in recent times, the genes Crr1a and CRa have been cloned (Hatakeyama et al. 2013, 2017, Ueno et al. 2012). Cloning of CR genes leads to the development of gene-specific markers and is indispensable for increasing the efficiency and accuracy of marker-assisted selection of B. rapa crops. Identification of CRb (also referred to as CRbKato) revealed that this gene is the same allele as the previously cloned CRa (Hatakeyama et al. 2017). PCR analysis using the SCAR marker, designed based on the sequence of the CRa gene, revealed that this gene is widely distributed in the CR cultivars of Chinese cabbage (Aruga et al. 2013). However, the allelic variation of CR genes in F1 cultivars of Chinese cabbage has not been well studied. Although the susceptible Crr1aA9709 allele contained a retrotransposon-like insertion in exon 1, which was likely a cause of susceptibility, this insertion was not always a common feature in non-CR cultivars of Chinese cabbage (Hatakeyama et al. 2013). Moreover, information on the types and number of functional CR genes introduced in commercial cultivars of Chinese cabbage is still limited.
Cloned CR genes encode nucleotide-binding (NB) leucine-rich repeat (LRR) receptors (NLRs) carrying the Toll/interleukin-1 receptor (TIR) domain at the N terminus (Hatakeyama et al. 2013, 2017, Ueno et al. 2012). Plant NLRs induce disease resistance responses by recognizing effector proteins released by pathogens, either directly or indirectly, by monitoring the guradees or decoys (Adachi et al. 2019, Białas et al. 2018, Jones et al. 2016, Kourelis and van der Hoorn 2018). Some NLRs function as single genetic units for the recognition of pathogens and initiate immune signaling, whereas others work in pairs by sharing roles in recognition and signaling. The LRR domain of the NLR protein is involved in the direct or indirect recognition of the pathogen effectors (Adachi et al. 2019, Ma et al. 2020), whereas some NLRs that work in pairs contain an additional integrated domain (ID) required for effector recognition (Białas et al. 2018). However, functional analysis has not been performed, and the cloned CR genes and molecular mechanisms underlying clubroot resistance via CR genes remain unclear.
In this study, we investigated the function of three Crr1a alleles cloned from CR cultivars of Chinese cabbage and discovered another functional allele, which has the same pathotype specificity as Crr1aG004. Here, we showed that the C-terminal region is essential for the clubroot resistance conferred by Crr1a protein, through a domain-swapping experiment. Furthermore, we developed gene-specific markers for the functional Crr1a alleles and demonstrated the distribution of functional alleles in Japanese commercial F1 cultivars of Chinese cabbage and resistant European fodder turnips.
In this study, 48 CR and 12 non-CR commercial F1 cultivars of Chinese cabbage were used (see Supplemental Table 1). In addition, ‘Moonbeach’ (Musashino Seed Co., Ltd.) was used for cloning the Crr1a allele. The clubroot susceptible line A9709, resistant line G004, and resistant European fodder turnip varieties, ‘Siloga’, ‘Debera’, ‘Gelria R’, and ‘77b’ were used.
The Plasmodiophora brassica field isolate Ano-01 was used, and the pathotype belonged to group 4, as previously defined (Hatakeyama et al. 2004, 2013, 2017, Kato et al. 2012). The test for clubroot resistance in Arabidopsis thaliana and the evaluation of root symptoms were performed as described by Hatakeyama et al. (2013). Arabidopsis Col-0 was used as a susceptible control in each test to determine the virulence of the isolate.
DNA extraction and cloning of genomic clones of Crr1a allelesGenomic DNA was isolated using a DNeasy Plant Mini Kit (Qiagen, Germany). Amplification was carried out using Ex Taq HS polymerase (TaKaRa Bio, Japan) as follows: initial denaturation at 94°C was performed for 2 min followed by 30 cycles of amplification, including 10 s at 94°C, 30 s at 58°C, and 6 min at 72°C. The PCR product was ligated into the XL-TOPO vector (Thermo Fisher Scientific, USA) according to the manufacturer’s instructions. At least three clones were sequenced to verify the sequence.
Vector construction and transformationGenomic DNA fragments of the full-length Crr1a allele were amplified using KOD+ DNA polymerase (Toyobo, Japan), with the primers described in Supplemental Table 2, and inserted downstream of the Crr1aG004 promoter sequence (Hatakeyama et al. 2013).
The chimeric Crr1a genes used in this study are shown in Supplemental Fig. 1. Genomic DNA fragments of protein-coding regions including TIR, NB, and LRR domains were amplified from Crr1aHiroki_b and Crr1aKiko85_a, and the C-terminal regions were amplified from the functional Crr1aG004 and Crr1aKinami90_a using the primer pairs listed in Supplemental Table 2. These two fragments were fused downstream of the Crr1aG004 promoter of the cassette vector using the In-Fusion HD Cloning Kit (TaKaRa Bio). In addition, we constructed a chimeric gene in which the C-terminal region of Crr1aG004 was replaced with that of Crr1aKinami90_a. The sequence of the resultant gene constructs was confirmed by Sanger sequencing. Ligation of the gene cassette into the binary vector and transformation of A. thaliana were carried out as previously described (Hatakeyama et al. 2013).
Detection of Crr1a alleles in F1 cultivars of Chinese cabbage and CR turnipsGenomic DNA was extracted using a DNeasy Plant 96 kit (Qiagen). Bulked DNA samples were generated from eight plants of each cultivar of Chinese cabbage. Genomic DNA isolated from 16 plants of each variety was used for the analysis of CR turnips. The primers used are shown in Supplemental Table 2. Amplification, using Ex Taq HS DNA polymerase (TaKaRa Bio), was performed by initial denaturation at 94°C for 2 min followed by 30 cycles of 10 s at 94°C, 30 s at 58°C, 30 s at 72°C, and a final extension for 3 min at 72°C. PCR fragments were separated on a 2% agarose gel.
Compared with Crr1aG004, the susceptible Crr1aA9709 contained a 357-bp insertion in exon 1, leading to a lack of half of the TIR domain (Hatakeyama et al. 2013). PCR analysis using primers flanking this insertion (Crr1a_exon1_F and Crr1a_exon1_R) revealed the presence of a 347-bp PCR product in G004 and 704-bp in A9709. When this PCR analysis was applied to 48 CR cultivars of Chinese cabbage, the 347-bp PCR product for Crr1aG004 was observed in 21 CR cultivars and 7 non-CR cultivars, whereas a 416-bp PCR product was detected in two CR cultivars (‘Kinami 90’ and ‘W-1117’). To analyze whether these differences are associated with the function of Crr1a alleles, we attempted to clone the full-length genomic DNA fragment from CR cultivars using PCR and obtained seven clones from six CR cultivars (SCR Hiorki, Kiko 86, Kinami 90, W-1116, W-1117, Moonbeach). Four types of alleles were identified based on sequence differences (Fig. 1).
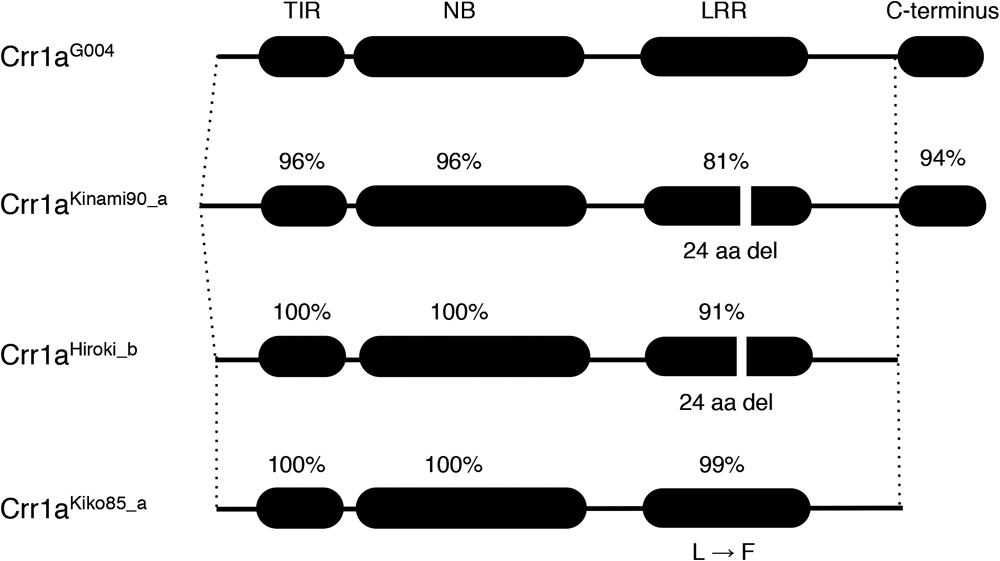
Schematic representation of the deduced protein sequences of Crr1a alleles. Amino acid sequence identities of TIR, NB, LRR domains, and C-terminal region against Crr1aG004 are indicated above each domain. The sequences of Crr1a alleles were deposited in the GenBank database (LC593254, LC593255, and LC593256).
Comparison of the predicted proteins of the four alleles showed that the TIR and NB domains were well conserved, while the LRR and C-terminal domains were variable (Supplemental Figs. 1–4). Of the two clones obtained from ‘Super CR Hiroki’, one was identical to Crr1aG004 and another clone, which was also amplified for ‘Moonbech’, contained a 72-bp deletion and 898-bp insertion in exon 4 compared with Crr1aG004. The latter clone was named Crr1aHiroki_b. The 72-bp deletion resulted in the lack of 24 amino acids in the LRR domain and the 898-bp insertion lead to the production of an immediate stop codon, resulting in the lack of 172 amino acids at the C-terminus (Supplemental Figs. 2–4). The Crr1aKiko85_a allele derived from ‘Kiko 85’ and ‘W-1116’, had a 1-bp non-synonymous substitution, resulting in Leu to Phe substitution, and an 898-bp insertion in exon 4, as found in Crr1aHiroki_b. The Crr1aKinami90_a, which was derived from ‘Kinami 90’, contained lots of base substitutions and several insertion/deletions. The 416-bp PCR product amplified from ‘Kinami 90’ and ‘W-1117’ was caused by the insertion of 69-bp in exon 1. The predicted protein had 59 amino acid substitutions, two insertions of 6 amino acids and 17 amino acids in the N-terminal region upstream of the TIR domain, and 24 amino acid deletions in the LRR domain. Among the 59 amino acid substitutions, 1, 9, and 21 were found in the TIR, NB, and LRR domains, respectively.
Functional analysis of Crr1a alleles using transgenic ArabidopsisTo determine whether the three alleles cloned in this study were functional or not, genomic DNA fragments of Crr1aG004, Crr1aHiroki_b, Crr1aKiko85_a, and Crr1aKinami90_a were transferred into the clubroot-susceptible Arabidopsis, Col-0, and T2 plants derived from independent T1 lines, which were then inoculated with the isolate Ano-01. Transgenic plants with Crr1aG004 showed complete resistance against Ano-01, whereas those harboring Crr1aHiroki_b and Crr1aKiko85_a were fully susceptible (mean disease index >2.0, Figs. 2A, 3). Transgenic plants with Crr1aKinami90_a showed high resistance (mean disease index = 0.0). Furthermore, we confirmed that Crr1aKinami90_a conferred resistance against pathotypes 2 and 4, and showed susceptibility to pathotype 3, as observed for Crr1aG004 (data not shown). These results indicated that Crr1aKinami90_a is another functional allele.
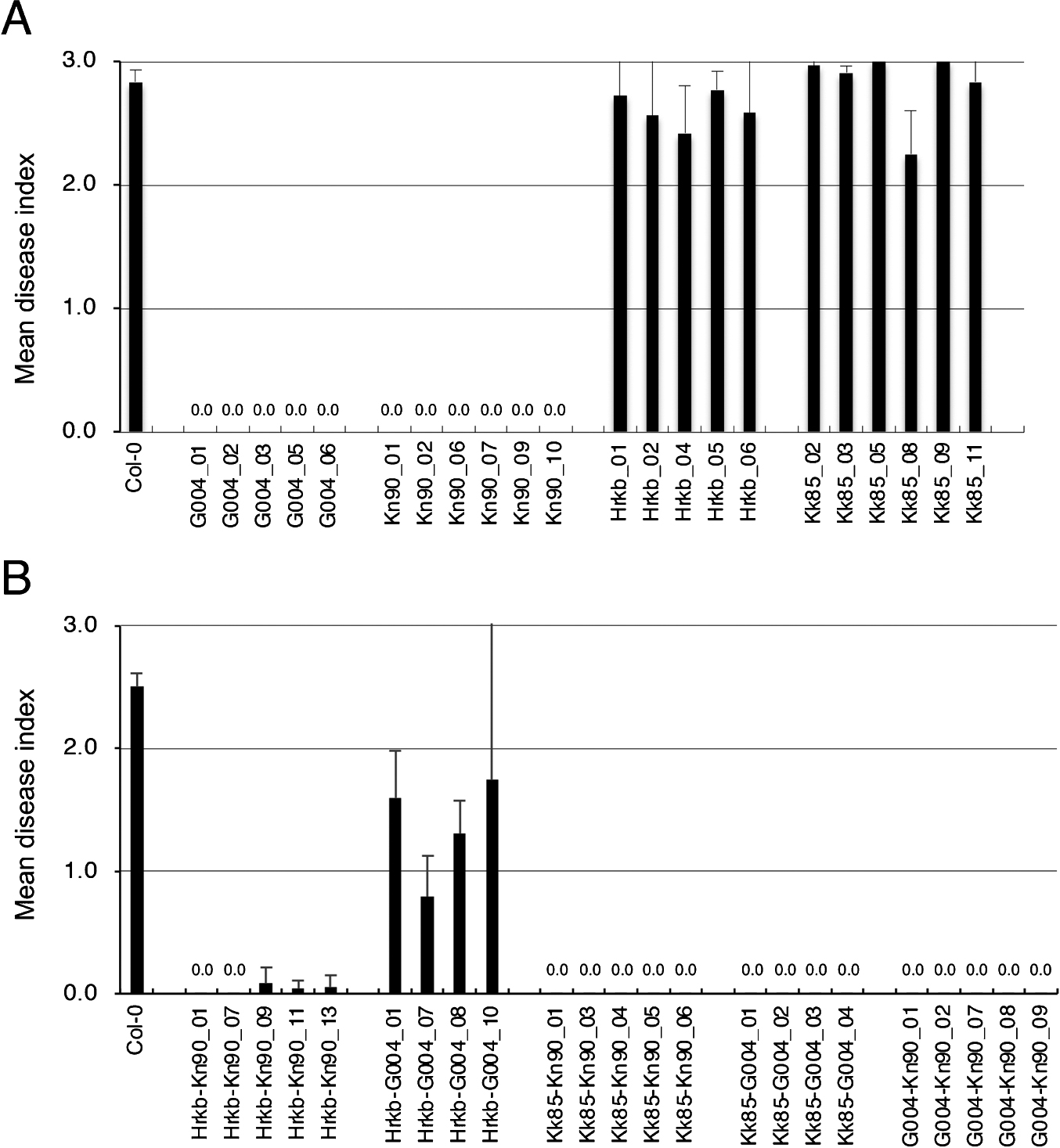
Resistance of the transgenic T1 lines carrying the genomic DNA fragments of Crr1a alleles (A) and the chimeric Crr1a genes (B) to Ano-01. A, G004, Kn90, Hrkb, and Kk85 indicate the T1 lines carrying Crr1aG004, Crr1aKinami90_a, Crr1aHiroki_b, and Crr1aKiko85_a, respectively. B, Hrkb-Kn90, Hrkb-G004, Kk85-Kn90, Kk85-G004, and G004-Kn90 indicate the T1 lines carrying Crr1aHrkb-Kn90, Crr1aHrkb-G004, Crr1aKk85-Kn90, Crr1aKk85-G004, and Crr1aG004-Kn90, respectively (Supplemental Fig. 1). Mean disease indices (±SD) of the T1 lines were calculated based on the average of two to three clubroot resistance tests (~9 T2 plants per test).
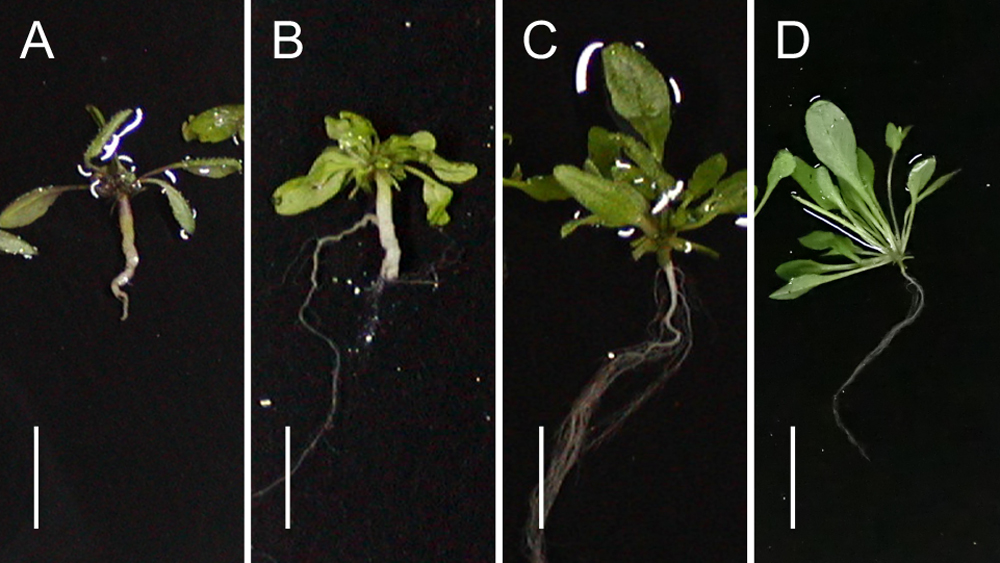
Typical root phenotype of the inoculated Arabidopsis wild type (A) and transgenic plants with Crr1aKiko85_a (B), Crr1aKinami90_a (C), and Crr1aKiko85-G004 (D). Scale bar indicates 10 mm.
The common feature of the two susceptible Crr1a alleles, Crr1aHiroki_b and Crr1aKiko85_a, is the lack of 172 amino acids at the C-terminus. To study whether the C-terminal region of the Crr1a gene is essential for conferring clubroot resistance, we constructed chimeric genes, in which the susceptible alleles were fused with the C-terminal region of the functional allele, and transferred them into Col-0 plants. We then examined the resistance of the transgenic plants with the chimeric Crr1a allele. All the transgenic plants harboring the chimeric Crr1a gene showed resistance against Ano-01, while the transgenic plants harboring the chimeric Crr1aHiroki_b gene fused with the C-terminal region of Crr1aG004 showed partial resistance (mean DI: 0.8–1.7) (Figs. 2B, 3). Furthermore, we constructed a chimeric Crr1a gene in which the C-terminal region of Crr1aG004 was replaced with that of Crr1aKinami90_a and evaluated the resistance of the transgenic plants. The resultant chimeric gene (Crr1aG004-Kn90) conferred resistance against Ano-01 (Fig. 2B). These results strongly indicate that the C-terminal region of the Crr1a gene could compensate the resistant phenotype in susceptible alleles lacking the C-terminus and therefore, is essential for clubroot resistance.
Detection of functional Crr1a alleles in CR Chinese cabbage and CR turnipsWe investigated the presence of functional Crr1a alleles in commercial CR cultivars of Chinese cabbage. Because the positions of mutations were variable among the Crr1a alleles (Supplemental Fig. 6), we considered it reasonable to develop allele-specific markers for each functional allele. We designed primer pairs to detect a 69-bp insertion in exon 1 that is found only in the Crr1aKinami90_a allele. PCR analysis using the primers Crr1a_exon1_F and Crr1a_exon1_R, could detect a 416-bp PCR product in ‘Kinami 90’ and 347-bp in G004 (Supplemental Table 2, Supplemental Fig. 6). Because there is a 357-bp insertion in exon 1 of the susceptible A9709, a 704-bp PCR product was detected in A9709. PCR analysis of 48 CR and 12 non-CR cultivars revealed that the 416-bp PCR product was detected only in ‘Kinami 90’ and ‘W-1117’, but not in any of the non-CR cultivars (Fig. 4A). Cloning of the full-length Crr1a gene from ‘W-1117’ confirmed that this cultivar possessed Crr1aKinami90_a. These results indicated that the exon 1 marker (Crr1a_exon1_F and Crr1a_exon1_R) can be useful for specifically detecting functional Crr1aKinami90_a. The 347-bp PCR product for Crr1aG004 was observed in 21 CR cultivars and 7 non-CR cultivars, and the 704-bp PCR product was detected in 26 CR cultivars. It is likely that ‘Kinami 90’ and ‘W-1117’ possessed Crr1aKinami90_a in heterozygous and homozygous forms, respectively. Furthermore, we tested this marker in the CR turnips. The 416-bp product, corresponding to Crr1aKinami90_a, was detected in more than one plant from ‘Debra’, ‘Gelria R’, and ‘77b’, but in none from ‘Siloga’ (Fig. 5A).
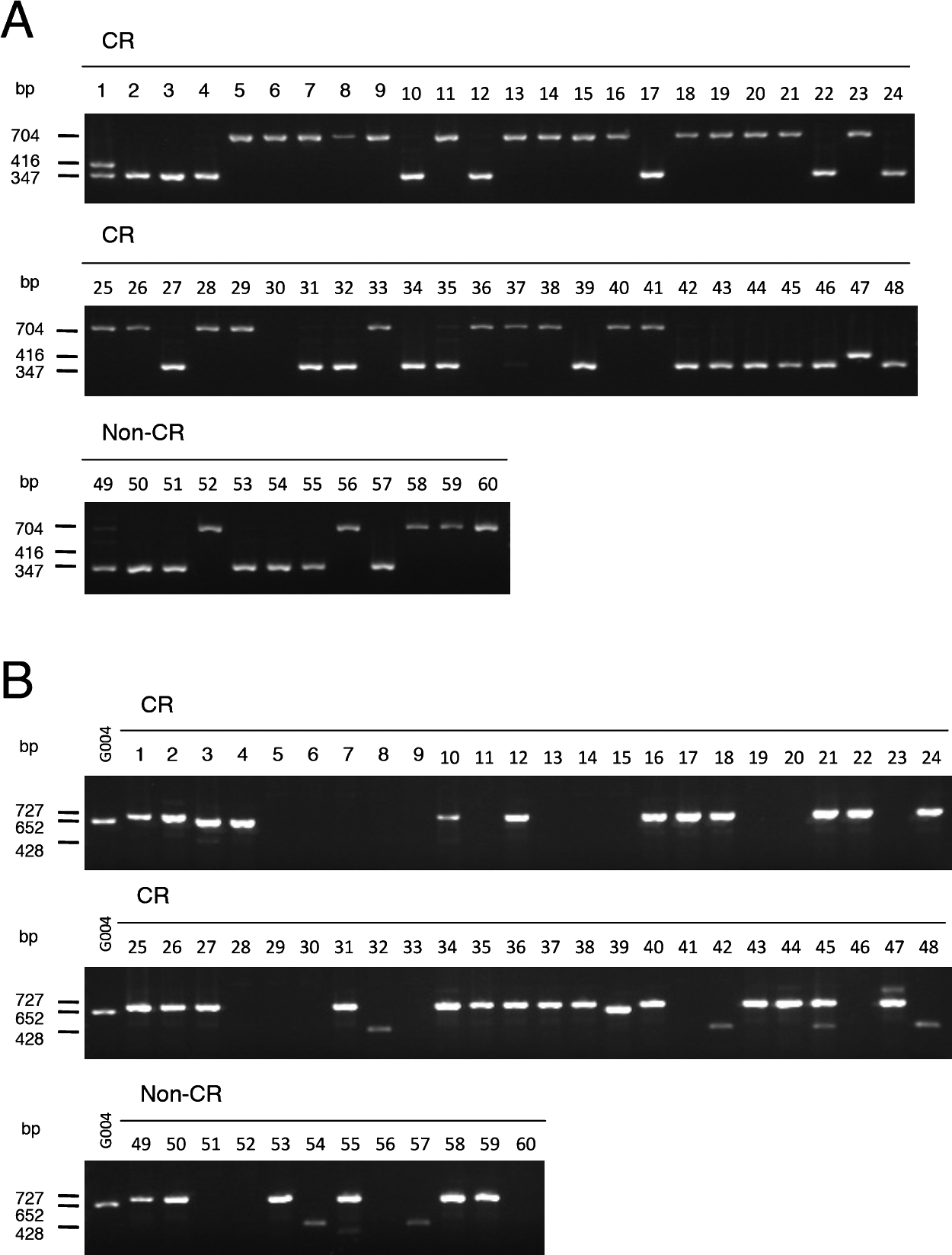
Detection of the functional alleles of Crr1a gene in 48 CR and 12 non-CR cultivars of Chinese cabbage. Details of the used cultivars used are indicated in Supplemental Table 1. A, Detection of the Crr1aKinami90_a allele using the primers Crr1a_exon1_F and Crr1a_exon1_R. B, Detection of the Crr1aG004 allele using the primers Crr1a_exon4_F, Crr1a_exon4_R1, and Crr1a_exon4_R2.
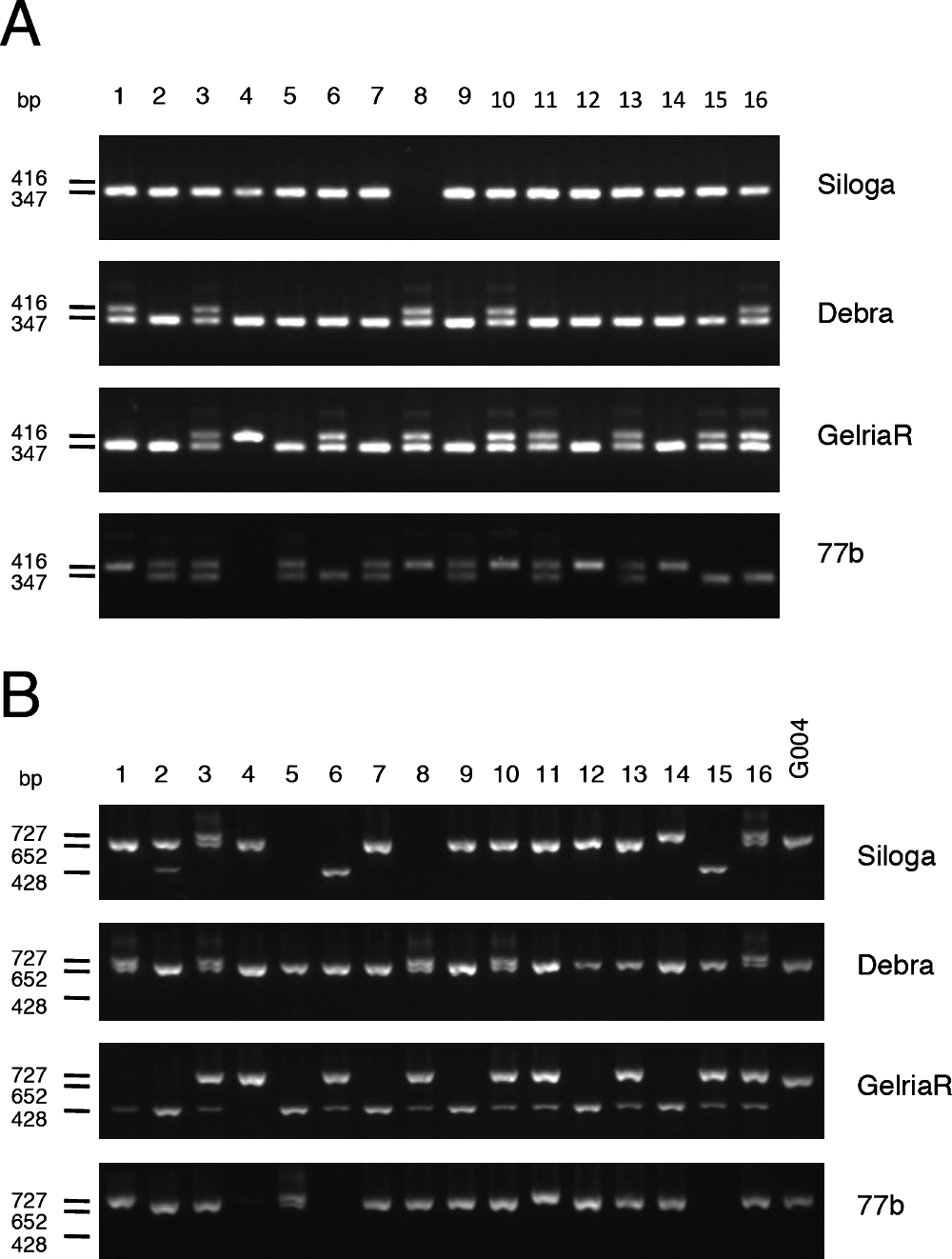
Detection of the functional alleles of Crr1a gene in 16 plants of European fodder turnips. A, Detection of the Crr1aKinami90_a allele using the primers Crr1a_exon1_F and Crr1a_exon1_R. B, Detection of the Crr1aG004 allele using the primers Crr1a_exon4_F, Crr1a_exon4_R1, and Crr1a_exon4_R2.
To develop a marker to discriminate between Crr1aG004 and Crr1aHiroki_b, we focused on sequence differences in the 3ʹ region of Crr1a alleles and designed three primers: Crr1a_exon4_F, Crr1a_exon4_R1, and Crr1a_exon4_R2 (Supplemental Table 2, Supplemental Fig. 6). Crr1a_exon4_F and Crr1a_exon4_R2 flank the 898-bp insertion found in the susceptible Crr1aHiroki_b and Crr1aKiko85_a and Crr1a_exon4_R1 is located in the reverse orientation within this insertion. A 652-bp PCR product was expected to be obtained from G004. A 428-bp PCR product flanked by Crr1a_exon4_F and Crr1a_exon4_R1 was expected to be detected in Crr1aHiroki_b and Crr1aKiko85_a, and no PCR products were expected from the susceptible A9709 because this line has more than 5-kb insertion in exon 4 (Hatakeyama et al. 2013) (Supplemental Fig. 6). A 727-bp PCR product was detected in ‘Kinami 90’, which was attributed to a 75-bp insertion located immediately downstream of the stop codon of Crr1a. The PCR analysis of 48 CR and 12 non-CR cultivars of Chinese cabbage revealed that a 652-bp product was detected in three cultivars, ‘Akimeki’, ‘SCR Hiroki’, and ‘SCR Kimi 85’ (Fig. 4B). The 428-bp PCR product was detected in 5 CR cultivars, ‘SCR Hiroki’, ‘Chiyobuki 70’, ‘Kiko 85’, ‘Kiai 65’, and ‘W-1116’. PCR analysis of CR turnips revealed that the 652-bp product corresponding to Crr1aG004 was detected in more than one plant from ‘Siloga’ and ‘77b’, and in all plants from ‘Debra’, whereas none was detected from ‘Gelria R’. Surprisingly, the 428-bp PCR product for Crr1aHiroki_b was detected in most of the plants from ‘Gelria R’ (Fig. 5B).
In this study, we investigated the sequence variation and function of three Crr1a alleles cloned from six CR F1 cultivars of Chinese cabbage. The TIR and NB domains of the predicted proteins encoded by these alleles were well conserved, but the LRR domain and C-terminus were variable (Fig. 1). We demonstrated that Crr1aKinami90_a conferred resistance to the clubroot isolate, while the other two did not (Fig. 2A). The two susceptible alleles, Crr1aHiroki_b and Crr1aKiko85_a, showed high sequence identity to the functional allele Crr1aG004 though they commonly contained a 898-bp insertion in exon 4, resulting in a lack of 172 amino acids at the C-terminus (Fig. 1, Supplemental Fig. 6). Gain-of-function analysis using the chimeric Crr1a gene revealed that fusion of the C-terminal region derived from the functional alleles could restore the ability of clubroot resistance in the two susceptible alleles (Figs. 2B, 3). This result suggests that the lack of a C-terminal region is a cause of the loss of resistance.
The significance of the C-terminal domain has been reported for several NLR proteins. Truncation and mutation of the C-terminal non-LRR region of the P2 flax rust resistance protein causes loss of function (Dodds et al. 2001). This domain is shared by other TIR-NLR disease resistance proteins such as RPS4, RPP1, and RPP5 in Arabidopsis and the N in tobacco, suggesting a possible role in specificity determination. Some NLRs possess an integrated domain at the C-terminus, which plays a role in effector recognition and NLR activation. Rice RGA5 possesses an HMA domain for Avr recognition at the C-terminus (Cesari et al. 2013). The Arabidopsis RRS1-R protein recognizes effectors via the integrated WRKY domain at the C-terminus, and its C-terminal region is also involved in the regulation of active and inactive forms of this protein (Guo et al. 2020, Sarris et al. 2015). Although we could not find any known motifs and domains in this region, these findings suggest that the C-terminal region of the Crr1a protein has a similar function. Recent findings suggest that some NLRs form complexes as singletons, or in pairs to activate immunity (Adachi et al. 2019). Therefore, we cannot exclude the possibility that the lack of C-terminus disrupts the formation of such complexes, resulting in the loss of resistance.
The resistance response of the susceptible Crr1aHiroki_b allele depended on the fused C-terminal region of the functional allele. The chimeric Crr1aHrkb-Kn90 conferred complete resistance, whereas Crr1aHrkb-G004, in which Crr1aHiroki_b was fused with the C-terminal region of Crr1aG004, resulted in partial resistance (Fig. 2B). The deduced chimera Crr1aHrkb-G004 protein was almost identical to the functional Crr1aG004 except for the 24-aa deletion in the LRR domain, which might also be involved in inducing resistance. Arabidopsis TIR-NLR receptor RPP1 confers strain-specific immunity through the recognition of Hyaloperonospora arabidopsidis effector ATR1. RPP1 was reported to be activated by direct binding of the effector to the C-terminal JID domain and LRRs, leading to the induction of an RPP1 tetrameric assembly required for host cell death (Ma et al. 2020). Although Crr1aKinami90_a, which also contained a 24-aa deletion in LRR, could confer complete resistance (Fig. 2A), this allele conferred complete resistance. It is possible that the difference in resistance between Crr1aKinami90_a and Crr1aHrkb-G004 is due to the amino acid sequence differences found in the LRR and C-terminal regions of the Crr1a protein (Fig. 1, Supplemental Figs. 1, 5). In the case of Crr1a-mediated signaling, the combination of LRR with 24-aa deletion and C-terminus of Crr1aG004 in Crr1aHrkb-G004 may affect the affinity of effector binding or cause a conformational change in the NLR immune receptor complex, resulting in incomplete resistance. Further investigation using the chimeric or mutagenized Crr1a gene would be necessary to understand the cause of partial resistance conferred by the chimeric Crr1aHrkb-G004 and provide insights into the function of Crr1a in clubroot resistance.
Because the clubroot test for selection is laborious and time-consuming, marker-assisted selection is considered the most effective way to develop cultivars with high levels of resistance (Diederichsen et al. 2009). In this study, we found another functional Crr1a allele, Crr1aKinami90_a, in addition to Crr1aG004. Based on the sequence comparison, we successfully developed PCR-based markers to identify Crr1aKinami90_a and Crr1aG004 alleles. Genotyping analysis of the 48 CR F1 cultivars revealed that five of the cultivars possessed the functional Crr1aG004 or Crr1aKinami90_a alleles (Fig. 4). These cultivars could be used as genetic resources to breed CR cultivars via marker-assisted selection. Twenty-five and six cultivars, in which the 704-bp and 428-bp were detected by exon 1 markers (Crr1a_exon1_F and Crr1a_exon1_R) and exon 4 markers (Crr1a_exon4_F, Crr1a_exon4_R1, and Crr1a_exon4_R2), respectively, were speculated to possess the susceptible allele. Kawamura et al. (2016) predicted clubroot resistance of inbred lines using DNA markers developed based on the sequence differences in exon 4 of Crr1a between G004 and A9709, and demonstrated that larger and smaller amplified fragments were associated with susceptibility and resistance, respectively. When this marker was used to detect the Crr1a allele isolated in this study, a smaller amplified fragment was obtained from the Crr1aKiko85_a allele (data not shown), suggesting the risk of selecting plants with the susceptible Crr1a allele. Information on allelic variation would help to develop gene-specific markers to prevent such a risk.
Surprisingly, the two functional Crr1a alleles were frequently found in European fodder turnips, but were rarely detected in Japanese CR cultivars (Figs. 4, 5). This result contrasts with that of the other CR genes, CRa and/or CRb (CRbKato), which were probably introduced into many CR cultivars of Chinese cabbage released in Japan (Aruga et al. 2013, Kato et al. 2013). Because CRa and/or CRb are known to function as a single dominant gene (Kato et al. 2012, Matsumoto et al. 1998), the plants heterozygous for this gene can be selected for backcross breeding. In contrast, Crr1a is an incomplete dominant gene (Hatakeyama et al. 2013). It is possible that the resistance of the heterozygous plants for Crr1a is insufficient, leading to loss of functional alleles during selection. Another possibility is that a certain CR cultivar harboring the functional Crr1a allele was used as a genetic resource in CR breeding. However, our findings suggest that Crr1a has the potential to increase the level of clubroot resistance in existing CR cultivars. The DNA markers developed in this study could facilitate the efficient and precise selection of plants possessing Crr1a.
In conclusion, we demonstrated the sequence variation and functionality of Crr1a alleles in B. rapa. Gain-of-function analysis using the chimeric genes revealed that the C-terminal region of the Crr1a gene is essential for clubroot resistance. We discovered another functional allele, developed DNA markers for the functional alleles, and showed their distribution in CR F1 cultivars of Chinese cabbage. These results would contribute to CR breeding via marker-assisted selection and help our understanding of the molecular mechanisms underlying clubroot resistance.
KH conceived the idea and designed the study. KH and SY conducted the experiments, analyzed the data, and wrote the manuscript. YT, KT, and SM provided advice on the experimental implementation and helped to draft the manuscript. All authors read and approved the manuscript.
The authors thank S. Negoro, T. Yamakawa, Y. Higashi, T. Niwa, and M. Tsukazaki for their technical assistance. We would like to thank Editage (https://www.editage.com) for English language editing. This study was supported by the Ministry of Agriculture, Forestry, and Fisheries of Japan (Genomics for Agricultural Innovation, grant number: HOR1002) and MEXT KAKENHI (Grant number: 18K05568).