2024 Volume 25 Issue 3 Pages 59-64
2024 Volume 25 Issue 3 Pages 59-64
In this study, we developed composite membranes of biorenewable and biodegradable polyhydroxyalkanoate (PHA) and cellulose for depth filtration. The membranes were prepared by coating PHA solutions in N,N-dimethylformamide onto cellulose lint cloth, followed by the phase separation method. Approximately half of the PHA porous layer was integrated into the cellulose fiber layer of the lint cloth. The average permeation flux was five-fold higher when yeast cell suspensions were filtered from the cloth side of the membrane than from the PHA side. The permeation behavior of filtration from the PHA side followed the cake filtration model, resulting in a dense cake layer. However, when the yeast suspension was filtered from the cloth side, yeast cells were captured in the cellulose fiber network, indicating a less increase in filtration resistance. These composite membranes are expected to facilitate the development of sustainable and efficient filter media in food and biochemical processes.
生物由来の生分解性プラスチックであるポリヒドロキシアルカノエート(PHA)とセルロース繊維との複合膜をデプス濾過のために開発した.複合膜はセルロース繊維製のリント布上にPHAのジメチルホルムアミド溶液をコーティングした後に,相分離を行うことによって作製した.PHAの多孔質層の約半分がリント布のセルロース繊維層と複合化していた.酵母懸濁液の濾過を行ったところ,リント布側から濾過を行うとPHA側から濾過した場合の5倍の平均速度で濾過を行うことができた.PHA側から濾過した場合の濾過の挙動はケーク濾過モデルに従い,濾過後には密な濾過ケーク層が複合膜のPHA側に形成していた.一方,リント布側から濾過を行った場合,酵母はセルロース繊維の網目構造に捕捉されており,濾過抵抗の増加が低減されたことが示唆された.この複合膜は食品・バイオプロセスにおける,持続可能な社会の実現のための効率的な濾材として役立つことが期待される.
Filtration membranes are often used in various food and bioproduction processes to separate solid particles from liquids [1,2]. Filtration membranes composed of glass fibers [3] or conventional synthetic polymers such as polysulfone [1] pose disposal problems. Biopolyesters, including polyhydroxyalkanoate (PHA) and poly(l-lactic acid) (PLLA), have garnered attention in recent years as key materials for sustainable technologies because they are derived from biomass and degrade readily during composting and in the natural environment [4-8]. Biopolyester filtration membranes reduce waste in the food and biochemical industries by degrading via composting.
PHA filtration membranes have been developed by a few methods although the number of reports on PLLA filtration membranes [9–16] are much more than that on PHA ones. Mas et al. used a nonsolvent-induced phase separation (NIPS) method to prepare poly (3-hydroxybutyrate) (PHB) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) membranes from polymer solutions in chloroform or mixed chloroform solvents containing tetrahydrofuran and dimethylformamide (DMF) in a coagulation bath of mixed nonsolvents of ethanol and water [17,18]. Tomietto et al. fabricated PHA membranes using the NIPS method with polymer solutions in N-methyl-2-pyrrolidone (NMP) containing ethylene glycol [19] and the evaporation-induced phase separation method for chloroform containing poly(ethylene glycol) 8000 [20]. Tabata et al. reported on PHA membranes prepared via NIPS using DMF as the solvent and water as the nonsolvent. The PHA membrane remained stable at 25 °C even under wet conditions and degraded at 60 °C [21].
Filtration properties are critical for membrane use. There are two primary types of filtration membranes: screen and depth. Screen filtration membranes capture particles on the membrane surface, whereas depth filtration membranes capture particles in the membrane. Particle layers (filter cakes) formed on the surfaces of screen membranes are known for their high filtration resistance. Depth filter membranes, however, capture particles to prevent filter cake formation [3,22-25]. The filtration characteristics of previously reported PHA membranes are similar to those of screen filter membranes. In this study, we developed composite membranes composed of PHA and cellulose fibers, which are sustainable materials derived from biomass. The particles were partially captured in the network of the cellulose fiber layer. A PHA layer of the composite membrane was formed on the cellulose layer to enhance particle retention.
PHA was purchased from GoodFellow (PH326300, Huntingdon, England; extrusion grade, melt flow rate at 170 °C and 2,160 g = 3.0 g/10 min, ASTM D-1238). Analytical-grade DMF and dry yeast were purchased from Fujifilm Wako Pure Chemical Industries (Osaka, Japan). The lint cloth, which is made of cotton cellulose fiber, used in this study was purchased from Matsumotokiyoshi Co., Ltd. (Matsudo, Japan).
2.2 Membrane preparationPHA was dissolved in DMF in a sealed 100 cm3 flask. All solutions were prepared on a mass percentage basis. The majority of mixtures were first stirred with a polytetrafluoroethylene (PTFE) stirring bar and warmed on stirrer/hot plates at 85 °C for 9 h. Polymer solutions were cast onto a lint cloth (100 mm × 100 mm) that adhered to a glass plate (100 mm × 100 mm) using double adhesive tape. The cloth had an 80 mm × 80 mm PTFE frame on it. The frame also adhered to the lint cloth using double adhesive tape. The thickness of the frame was 0.5 mm. The flasks containing the polymer solutions were immersed in a water bath at 50 °C. The glass plates with lint cloths were preheated to the same temperature. Following the removal of excess polymer solution, the glass plate was immersed in a room-temperature (25 ± 2 °C) coagulation bath for 2 h. The membrane was then removed from the glass plate, washed thoroughly, and stored in water until further use.
2.3 Filtration experimentsDead-end filtration experiments were performed in an unstirred filtration cell (Amicon Model 8010, 4.1 cm2, Millipore, Bedford, MA) and a support screen (19301203, ADVANTEC TOYO, Tokyo, Japan). Water was used to measure the permeation resistance of the membranes. Filtration was mainly performed at a transmembrane pressure of 10 kPa and 25 ± 2 °C. The membrane resist-ance (Rm) was calculated using Eqs. (1)-(3).
 | (1) |
 | (2) |
 | (3) |
where J, J0, R, t, v, ΔP, and μ represent the permeation flux, permeation flux for water, filtration resistance, filtration time, permeation volume per unit filtration area, transmembrane pressure, and viscosity of the permeate, respectively. The viscosity of water at 25 °C is 0.89 mPa·s [26]. Suspensions of 0.1% dry yeast in purified water were used to measure particle retention. The absorbance of the initial 30 min permeate at 660 nm (or the initial 10 cm3 permeate if filtration was completed within 30 min) was used to monitor particle leakage, and the retention (= 100×(A0 - A1)/A0) was calculated using the absorb-ance of the leakage (A1) and that of the suspension before filtration (A0).
2.4 Scanning electron microscopyEach membrane was immersed in liquid nitrogen and then fractured. It was mounted vertically and horizontally on a sample holder. The surface of the sample was coated with gold-palladium using a sputter coater (MPS-1S, Vacuum Device, Mito, Japan). The membrane cross sections and surfaces were examined using scanning electron microscopy (SEM, TM-1000, Hitachi, Tokyo, Japan) at an acceler-ating voltage of 15 kV.
2.5 Infrared spectroscopyInfrared spectra were obtained using Fourier-transform infrared (FTIR) spectroscopy (IR Affinity-1S, Shimadzu, Kyoto, Japan) with a single-pass attenuated total reflection (ATR) sampling accessory (Quest ATR Diamond Accessory, Specac, Orpington, England). PHA spectra were measured using a 3 mm × 3 mm × 1 mm pellet.
2.6 Tensile testingTensile testing was performed at 25 ± 2 °C under wet conditions using a desk-top tensile testing machine (EZ-S-500N, Shimadzu). The gauge length and crosshead speed were 30 mm and 1.0 mm/min, respectively.
Fig. 1 depicts cross sections of the lint cloth and PHA-lint cloth composite membranes. The lint cloth was 1 mm thick and composed of entangled 10 µm cellulose fibers (Fig. 1(a)). The composite membrane prepared with the 25% PHA solution in DMF had a 0.7 mm-thick PHA layer, with approximately half of the PHA layer infiltrating the lint cloth (Fig. 1(b)). The top layer was smooth (Fig. 2(a)), while the bottom layer was composed of cellulose fibers (Fig. 2(b)). The top layer (PHA) and bottom layer (cellulose) components were confirmed using IR spectroscopy (Fig. 3). The breaking strain and breaking force of a 0.8 mm-thick PHA membrane prepared without lint cloth were 0.0144 and 0.93 N, respectively. The breaking strains of the composite membrane were 0.1256 and 0.5163 in the lateral and longitudinal directions, with breaking forces of 51.45 and 54.83 N in the two directions, respectively. Compositing with lint cloth increased the breaking strains and forces of the PHA membrane in both directions.

SEM images of the cross sections of lint cloth and PHA-lint cloth composite membrane prepared from a 25% PHA solution in DMF. (a) Lint cloth; (b) composite membrane.
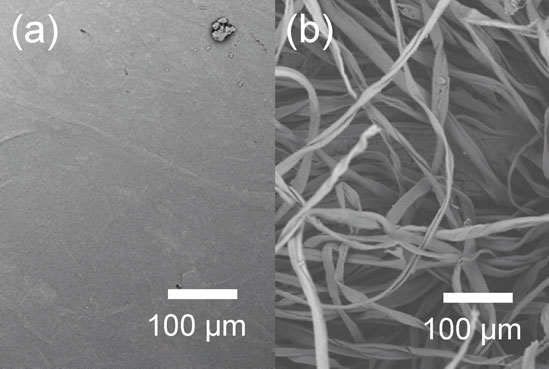
SEM images of PHA-lint cloth composite membrane prepared from PHA solutions in DMF. (a) PHA side; (b) cloth side.

ATR-FTIR spectra of PHA and cloth sides of a PHA-lint cloth composite membrane. Those of a PHA pellet and lint cloth are also shown for comparison. One unit of scale on the y-axis corresponds to 20%.
Fig. 4 depicts how polymer concentration affects membrane resistance (Rm) and yeast cell retention. Here, a 0.1% dry yeast cell suspension (approximately 3-5 µm) was used as a model suspension for food processing. The wet/dry ratio (3.7 g g-1) was used to determine the concentration of hydrated wet yeast cells, which was found to be 0.37%. The ratio was calculated using the mass of wet yeast cells recovered after suspending 1.1 g of dry yeast in 10 mL of purified water for 1 h and centrifuging at 800×g for 5 min. Filtration was performed on the PHA side of the membrane. The lint cloth leaked 40% of the yeast cells. The retention of yeast cells and membrane resistance were increased by compositing the lint cloth with PHA. The retention was higher than 98% when 18-25% PHA polymer solutions were used. The resistances at the PHA concentration of 18-25% were comparable to that of the polysulfone depth filter membrane (2×1010 m-1) [22] and lower than PLLA depth filtration membranes (6×1010-2×1011 m-1) [13,25]. In the following experiment, PHA-lint cloth composite membranes prepared using a 25% PHA solution were investigated to reduce the risk of cell leakage when the yeast cell suspension was filtered from the cloth side in depth filtration.

Effect of PHA concentration on membrane resistance and retention of yeast cells by PHA-lint cloth composite membranes prepared from PHA solutions in DMF (n = 4). Filtration was performed using PHA side of the membranes.
Fig. 5 depicts yeast cell filtration via the PHA and cloth sides of the PHA-lint cloth composite membranes. The filtration rate (slope of filtration volume vs. filtration time) decreased rapidly during PHA side filtration, whereas the rate decreased significantly slowly during cloth side one. On both sides, yeast cells were retained at rates > 99%. Fig. 6 depicts the reciprocal of the permeation flux (1/J) vs. filtration volume per unit area (v), plotted to analyze filtration behavior. The nearly straight line for filtration from the PHA side represents typical cake filtration behavior. In the cake filtration model for constant-pressure filtration, the filtration resistance (R) increases linearly with v, as shown in Eq. (4) when the particle concentration (C) is low.
 | (4) |
where α represents the specific resistance of the filter cake [2,27]. Eq. (5) was derived from Eqs. (2) and (4).
 | (5) |
Therefore, the curve for 1/J vs. v is straight in the cake filtration model. The intercept of the curve on y-axis, 2.0×103 m-1, was nearly equal to the constant in Eq. (5) (µRm/ΔP), 2.6×103 m-1, which was calculated at 10 kPa, and the value was much smaller than those of 1/J except during the very initial stage of the filtration. The curve for filtration from the cloth side is also nearly straight. However, the slope was significantly lower than that from the PHA side. The higher permeation flux resulted from the slow increase in resistance. Some depth filtrations also produced almost linear curves in the 1/J vs. v graphs [13,23-25].

Filtration of yeast cell suspensions by PHA-lint cloth composite membranes from PHA and cloth sides.

Reciprocal of permeation flux vs. filtration volume per unit area in filtration of yeast cell suspension by PHA-lint cloth composite membranes.
The filtered membranes were observed using SEM (Figs. 7 and 8). The yeast cells (3-5 µm) were captured on the top surface of the membrane, forming a dense filter cake when the yeast suspension was filtered from the PHA side (Figs. 7(a) and 8(a)). When the suspension was filtered from the cloth side, yeast cells were captured on the cellulose fibers of the lint cloth (Figs. 7(b) and 8(b)). These findings support the high filtration resistance in screen filtration from the PHA side and the lower resistance in depth filtration from the cloth side. Similar phenomena were observed in other screen and depth filtrations using asymmetric membranes [13,23-25].

SEM images of surfaces of PHA-lint cloth composite membranes after filtration of yeast cell suspensions. (a) Filtration from the PHA side; (b) filtration from the cloth side.

SEM images of cross sections near the surfaces of PHA-lint cloth composite membranes after filtration of yeast cell suspensions. (a) Filtration from the PHA side; (b) filtration from the cloth side.
In this study, we developed composite PHA and cellulose membranes. The composite membranes fabricated from 25% PHA solutions in DMF and cellulose lint cloth successfully retained yeast cells on or inside the membrane. The membranes functioned as depth filters when yeast suspensions were filtered from the cloth side, preventing the formation of a filter cake, which causes high filtration resistance in screen filtration. The composite membranes will be sustainable and efficient filter media although the structures of the membranes should be further optimized for different suspensions in food and biochemical processes.