2017 年 32 巻 4 号 p. 398-401
2017 年 32 巻 4 号 p. 398-401
When soil oxygen levels decrease, some bradyrhizobia use denitrification as an alternative form of respiration. Bradyrhizobium diazoefficiens (nos+) completely denitrifies nitrate (NO3−) to dinitrogen, whereas B. japonicum (nos−) is unable to reduce nitrous oxide to dinitrogen. We found that anaerobic growth with NO3− as the electron acceptor was significantly lower in B. japonicum than in B. diazoefficiens, and this was not explained by the absence of nos in B. japonicum. Our results indicate that the reason for the limited growth of B. japonicum is weak NO3− reduction due to impaired periplasmic nitrate reductase activity, which may rely on posttranscriptional events.
Rhizobia are gram-negative α-proteobacteria with the ability to fix dinitrogen (N2) in symbiosis with leguminous plants (16, 28). Soybean generally accommodates rhizobia from the genera Bradyrhizobium (2). If oxygen levels drop, some bradyrhizobial species use denitrification as alternative respiration and sequentially reduce nitrate (NO3−) to nitrous oxide (N2O) or N2 (5, 11, 15, 19, 29). Bradyrhizobial denitrification starts with the reduction of NO3− to nitrite (NO2−) by the periplasmic nitrate reductase (Nap) encoded by napA in the napEDABC gene cluster (5). The second step is the reduction of NO2− to nitric oxide (NO) by the copper-containing nitrite reductase encoded by nirK (5). The reduction of NO to N2O occurs through a c-type NO reductase, the cytochrome bc complex NorCB encoded by norECBQD (5, 26). N2O is then reduced to N2 by the N2O reductase NosZ encoded in the nosRZDFYLX gene cluster (5).
Bradyrhizobium diazoefficiens (formerly classified into B. japonicum) (9) has a complete set of denitrification genes, whereas B. japonicum lacks the nos gene cluster and releases N2O as the final product of denitrification (12, 14, 19, 24). Therefore, B. diazoefficiens and B. japonicum share the first three steps of denitrification (reduction of NO3− to N2O). Shiina et al. (23) demonstrated that the occurrence of bradyrhizobia with nosZ (nosZ+) or without nosZ (nosZ−) in Japanese soybean fields largely depended on the soil type. Andosol, a volcanic soil composed of porous sediments (3, 25), is significantly dominated by nosZ−, whereas Gleysol, a wetland soil saturated with groundwater, in which the water regime favors low-oxygen conditions (13), is significantly dominated by nosZ+ (23). These findings suggested that B. diazoefficiens (nosZ+) is predominant in Gleysol soils and B. japonicum (nosZ−) in Andosols (23). Saeki et al. (18) recently indicated that the possession of nosZ confers a competitive advantage to B. diazoefficiens in flooded soil; this is consistent with the predominance of nosZ+ bradyrhizobia in Gleysol soils (23). Thus, the aim of the present study was to identify key physiological traits in denitrification that differentiate the distribution of B. diazoefficiens and B. japonicum in soybean fields in Japan.
The Bradyrhizobium strains used in this study are listed in Table S1. Cells were precultured at 30°C for 72 h in HM salt medium (8) supplemented with 0.1% l-(+)-arabinose and 0.25% (w/v) yeast extract. HM medium supplemented with trace metals (20) and 10 mM KNO3 (HMMN medium) was employed in denitrification assays. In growth experiments, precultured cells were inoculated into 34-mL test tubes containing 5 mL HMMN medium. Initial optical density at 660 nm was adjusted to 0.01. Foam stoppers were used for the aerobic treatment and butyl rubber stoppers for the anaerobic and microaerobic treatments. In the anaerobic treatment, the gas phase was replaced with 100% N2 in a vacuum line. In the microaerobic treatment, the gas phase (2% O2 [v/v], N2 balance) was replaced daily. Cells were grown at 30°C with reciprocal shaking at 300 rpm. Dissolved oxygen levels were verified in each treatment with a 5300A Biological Oxygen Monitor (Yellow Springs Instruments, Yellow Springs, OH, USA) (Fig. S1). Growth was monitored daily by measuring the optical density of the cultures at 660 nm; the number of cells was assessed by direct counting with a 20-μm-deep hemocytometer (Sunlead Glass, Saitama, Japan) and an Olympus BX51 Fluorescence Microscope (Olympus, Tokyo, Japan).
In denitrification assays, precultured cells were inoculated into 100-mL vials containing 20 mL of HMMN at an initial optical density (660 nm) of 0.02, and grown anaerobically at 30°C with reciprocal shaking at 100 rpm. Extracellular NO3− concentrations were assessed using a Dionex ICS-1100 Basic Integrated Ion Chromatography System (Thermo Fisher Scientific, Waltham, MA, USA). Prior to injections, each sample was filtered through a Minisart syringe filter (pore size, 0.2 μm; Sartorius, Göttingen, Germany) and diluted with Milli-Q water. In N2O measurements, 0.2 mL of the gas phase was injected into a GC-17A Gas Chromatograph (Shimadzu, Kyoto, Japan) as described previously (19). Methyl viologen–dependent nitrate reductase activity was measured as described by Sánchez and co-workers (21).
The isolation of total RNA, the DNaseI treatment, and cDNA synthesis were performed as described previously (4, 10). Prior to cDNA synthesis, the absence of DNA in DNaseI-treated RNA was confirmed by PCR with the sigA primer pair (Table S2). Relative expression was analyzed by quantitative reverse-transcription PCR in a LightCycler Nano Instrument (Roche, Basel, Switzerland) using FastStart Essential DNA Green Master (Roche) and specific primers for sigA, napA, nirK, and norB (Table S2). The PCR program was set according to the manufacturer’s instructions, and the specificity of PCR amplification was confirmed by a melting-curve analysis. The relative expression of the target genes was calculated by the 2−ΔΔCT method (22) using sigA as an internal control.
The anaerobic growth of B. japonicum USDA 6T and CPAC 15 with NO3− as the electron acceptor was significantly lower than that of B. diazoefficiens USDA 110T and CPAC 7 based on the means of optical density and cell number (Fig. 1A; Fig. S2). However, no significant differences were observed between the growth of B. japonicum and B. diazoefficiens strains under aerobic or microaerobic conditions in the presence of NO3− (Fig. S2). Since B. japonicum lacks the nos gene cluster (14, 24), we investigated whether N2O reductase, encoded by nosZ, supports fast growth by B. diazoefficiens USDA 110T. The growth of the nosZ mutant USDA 110T (USDA 110T-nosZ) was similar to that of wild-type USDA 110T, indicating that the N2O reduction step did not markedly contribute to the difference observed in growth between B. japonicum and B. diazoefficiens under anaerobic NO3−-respiring conditions (Fig. 1A). In terms of bioenergetics, there are few disadvantages for cells failing to perform the final step of N2O reduction (17). In contrast, Saeki et al. suggested that nosZ confers a competitive advantage in flooded soils (18). Soil factors may influence the relevance of the N2O reduction step in bradyrhizobial competition.

Growth of Bradyrhizobium japonicum and B. diazoefficiens. (A) Anaerobic growth of B. japonicum (USDA 6T and CPAC 15) and B. diazoefficiens (USDA 110T, CPAC 7, and USDA 110T-nosZ mutant) in HMMN medium. *Values significantly different from those of B. diazoefficiens USDA 110T (t-test, P<0.05; n=3). (B–D) Average growth profiles of B. diazoefficiens (15 strains) and B. japonicum (11 strains) at the indicated conditions in HMMN medium; error bars indicate SE. *Values significantly different between B. diazoefficiens and B. japonicum (t-test, P<0.001; n=3). Bd, B. diazoefficiens; Bj, B. japonicum.
In order to examine whether the difference in growth extends to the species level, we randomly selected 11 strains of B. japonicum and 15 strains of B. diazoefficiens (Table S2). The phylogenetic tree of these strains is shown in Fig. S3. In the presence of NO3−, the mean growth of B. japonicum strains was significantly lower than that of B. diazoefficiens strains under anaerobiosis (Fig. 1B; Table S3), but not under aerobiosis or microaerobiosis (Fig. 1C and D; Table S3). Thus, low growth appeared to be a general phenomenon in B. japonicum.
In order to compare the steps of denitrification that were common to B. japonicum and B. diazoefficiens (i.e. reduction of NO3− to N2O), we monitored NO3− and N2O concentrations in batch cultures of B. japonicum USDA 6T and CPAC 15 and B. diazoefficiens USDA 110T and CPAC 7 under anaerobic NO3−-respiring conditions. The B. diazoefficiens USDA 110T-nosZ mutant was used as a control equivalent to B. japonicum in terms of the reduction of NO3− to N2O. The three strains of B. diazoefficiens had completely consumed NO3− by day 7 (Fig. 2A). On the other hand, N2O production was observed exclusively in the gas phase of the USDA 110T-nosZ mutant culture (Fig. 2B), which likely reflected the stoichiometric conversion of 2 moles of NO3− to 1 mole of N2O (Fig. 2A and B). The two strains of B. japonicum consumed less NO3− (Fig. 2C) and produced less N2O (Fig. 2D) than the USDA 110T-nosZ mutant. These results indicate that B. japonicum is less capable of reducing NO3− to N2O than B. diazoefficiens.
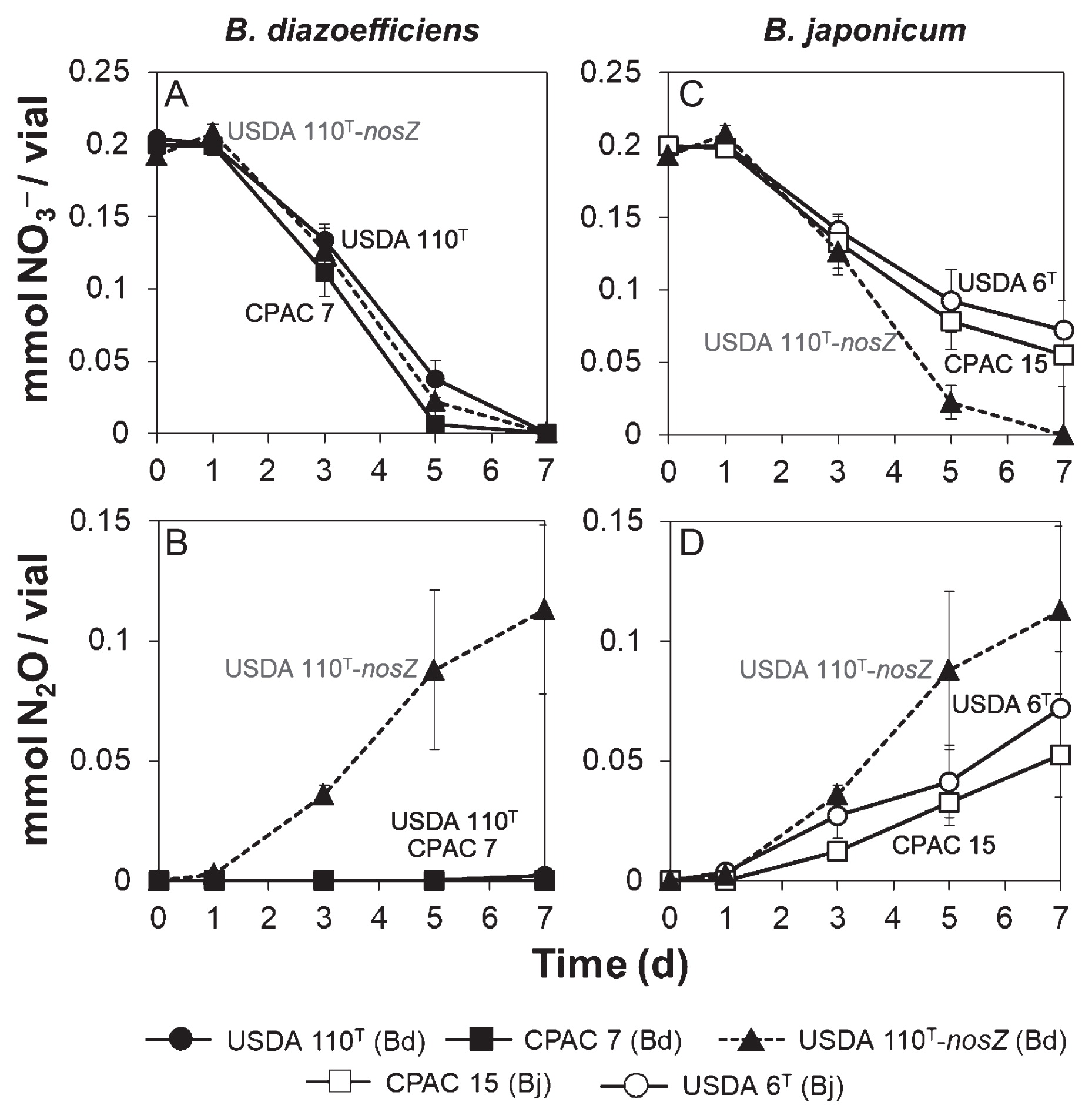
Nitrate (NO3−) consumption (A, C) and nitrous oxide (N2O) production (B, D) by B. diazoefficiens and B. japonicum strains growing under anaerobiosis in HMMN medium. B. diazoefficiens USDA 110T-nosZ is shown as a reference; data are the means of three different starter cultures; error bars indicate SE. Bd, B. diazoefficiens; Bj, B. japonicum.
Nap activity was markedly weaker in B. japonicum (USDA 6T and CPAC 15) than in B. diazoefficiens (USDA 110T, CPAC 7, and USDA 110T-nosZ) under anaerobic NO3−-respiring conditions (Fig. 3A); B. japonicum USDA 6T and CPAC 15 showed no activity at 48 h and low activity at 72 h (Fig. 3A). This may explain the low rates of NO3− consumption and N2O production in B. japonicum (Fig. 2C and D). We found that NapA and NapB (the catalytic and electron-transfer subunits of Nap, respectively) amino acid sequences shared 94–97% identity among USDA 110T, USDA 6T, and CPAC 15 and conserved the motifs involved in catalysis (29), which suggest the functional conservation of Nap in B. japonicum. Thus, the lower Nap activity in B. japonicum may rely on differences during the expression process. We then assessed the napA transcript level in each of the strains relative to that in USDA 110T. Although transcript levels varied among strains, napA expression was not significantly different between B. diazoefficiens and B. japonicum (Fig. 3A). Collectively, these results suggest that the low efficiency for NO3− reduction in B. japonicum relies on posttranscriptional events. Similarly, Sinorhizobium meliloti is unable to grow under anaerobic NO3−-respiratory conditions even though denitrification genes are fully induced (27). In addition, Agrobacterium tumefaciens and Pseudomonas sp. G59 are unable to make an effective switch to denitrification in the absence of oxygen (1, 6).
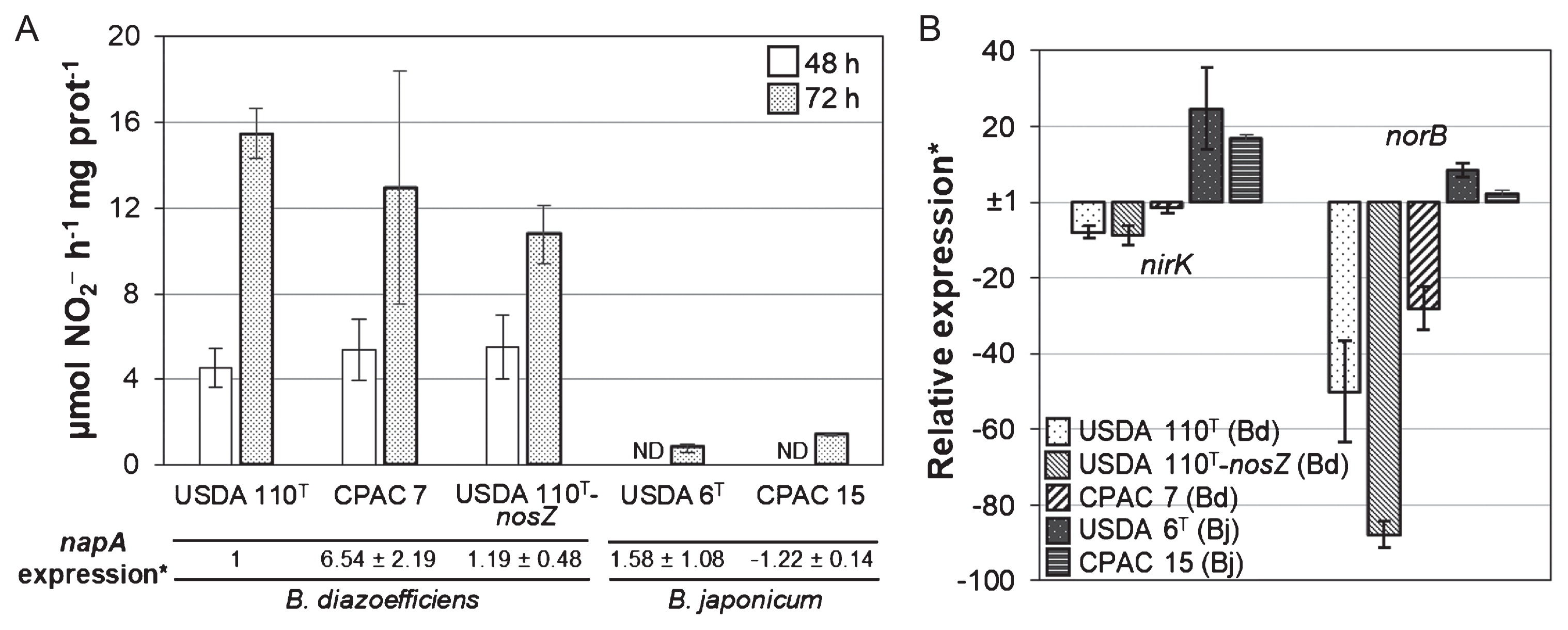
Periplasmic nitrate reductase activity and relative expression of napA, nirK, and norB. (A) Methyl viologen–dependent nitrate reductase activity (top) and relative expression of napA (bottom) in the indicated strains. Activities were measured after an incubation for 48 or 72 h under anaerobiosis in HMMN medium; data are the means±SE of three different starter cultures. The expression of napA was measured at 48 h; data are the means±SE of three independent RNA samples. *napA expression values are relative to that of USDA 110T, which was set at 1. ND, not detected. (B) Relative expression of nirK and norB in the indicated strains; data are the means±SE of three independent RNA samples; *Values are relative to the napA value of each strain. Bd, B. diazoefficiens; Bj, B. japonicum. In A and B, up-regulated (1>2−ΔΔCT) or down-regulated (1>2−ΔΔCT>0) expression is indicated as 1/2−ΔΔCT or −1/2−ΔΔCT, respectively.
We also analyzed nirK and norB transcript levels relative to that of the napA transcript for each strain. The levels of both transcripts were higher in B. japonicum (Fig. 3B), even though B. japonicum reduced less NO3− than B. diazoefficiens (Fig. 2C; Fig. 3A). A possible explanation is that B. japonicum overexpresses nirK and norB to compensate for the lower activity of Nap. This induction may be dependent on the FixLJ–FixK2–NnrR regulatory cascade (5, 7), which controls the expression of denitrification genes in B. diazoefficiens. B. japonicum USDA 6T and CPAC 15 conserved a complete set of these regulatory genes and binding sites for FixK/FNR (fumarate and nitrate reductase regulator) regulators (5) in the promoter regions of napE, nirK, and norC genes (data not shown). However, we cannot exclude the possibility of different regulatory networks in B. japonicum.
Our results show that the low efficiency for NO3− reduction, as a consequence of impaired Nap activity, is the main factor limiting the growth of B. japonicum under anaerobic NO3−-respiring conditions, which cannot be interpreted at the expression level of the genes responsible for denitrification. If confronted with oxygen depletion, the activation of NO3− reduction may be crucial for preventing anoxia entrapment by providing energy for the biosynthesis of the entire denitrification proteome (6, 7, 27). B. japonicum may be less competitive than B. diazoefficiens due to energy depletion under anaerobic conditions, despite sufficient nap expression, which may contribute to the predominance of B. diazoefficiens in Gleysol (23).
This work was supported by a Grant-in-Aid for Scientific Research (A) 26252065 from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by a grant from the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry. A.F.S. was supported by fellowships from the National Council for Scientific and Technological Development-Brazil and the Japan Society for the Promotion of Science.