2017 年 32 巻 4 号 p. 390-393
2017 年 32 巻 4 号 p. 390-393
Archaeal communities in mineral soils were compared between a boreal forest in Finland and cold-temperate forest in Japan using 16S rRNA gene-targeted high-throughput sequencing. In boreal soils, Thaumarchaeota Group 1.1c archaea predominated and Thaumarchaeota Group 1.1a-associated and Group 1.1b archaea were also detected. In temperate soils, Thaumarchaeota Group 1.1a-associated and Group 1.1b archaea were dominant members at the subsurface, whereas their dominancy was replaced by Thermoplasmata archaea at the subsoil. An analysis of the ammonia monooxygenase subunit A gene of Archaea also indicated the distribution of Thaumarchaeota Group 1.1a-associated and Group 1.1b archaea in these soils.
Archaea are now widely accepted to play important roles in the carbon and nitrogen cycles in various ecosystems (6, 8, 14). A large part of the mesophilic amoA-harboring Archaea of the phylum Thaumarchaeota found in subsoils adapted ammonia oxidation under acidic conditions or low ammonia availability (2, 11). A larger number of the archaeal amoA copy has been detected than bacterial amoA genes (12), and ammonium-oxidizing Archaea have been found in several soil environments, including forests and agricultural lands (5). Some strains within Thaumarchaeota Group 1.1a, Group 1.1a-associated, and Group 1.1b were directly shown to be ammonia oxidizers (8, 11, 16, 17), whereas evidence for ammonia oxidization by members in Group 1.1c has not yet been obtained (10). The abundance of Thaumarchaeota Group 1.1c and Thermoplasmata, which are generally found in hot environments, was found to increase with the depth of temperate acidic soil, which is a loamy sand with a pH of 3.8–4.3, in Germany (7). Furthermore, pyrosequencing-based investigations demonstrated that Archaea, particularly Thaumarchaeota, are abundant significantly in a mineral horizon (10–20 cm) than an organic horizon (0–10 cm) in a planted Norway spruce forest in France (18). However, limited information is currently available on archaeal communities in acidic forest soils inside the Arctic Circle in Finland. Weedon et al. previously reported that, in contrast to the fluctuations observed in bacteria, those in soil mesophilic ammonia-oxidizing Archaea (AOA) were rarely affected by temperature increases, and this may be related to the levels of water-soluble organic carbon (WSOC) (20). However, the relationship between archaeal community structures and WSOC concentrations has not yet been elucidated in detail. Therefore, we herein investigated archaeal community structures in mineral soils from two different types of forest beds: the mineral horizons of a mixed conifer forest edge in Finland inside the Arctic Circle, in which the litter supply and its decomposition are limited and slow, and a planted larch forest in northern Japan, in which a large amount of litter is supplied and rapidly decomposed. In the present study, we focus on archaeal communities in the soils of mineral horizons collected from boreal and temperate forests in order to provide insights into archaeal community distributions among different forest sites (Fig. 1).
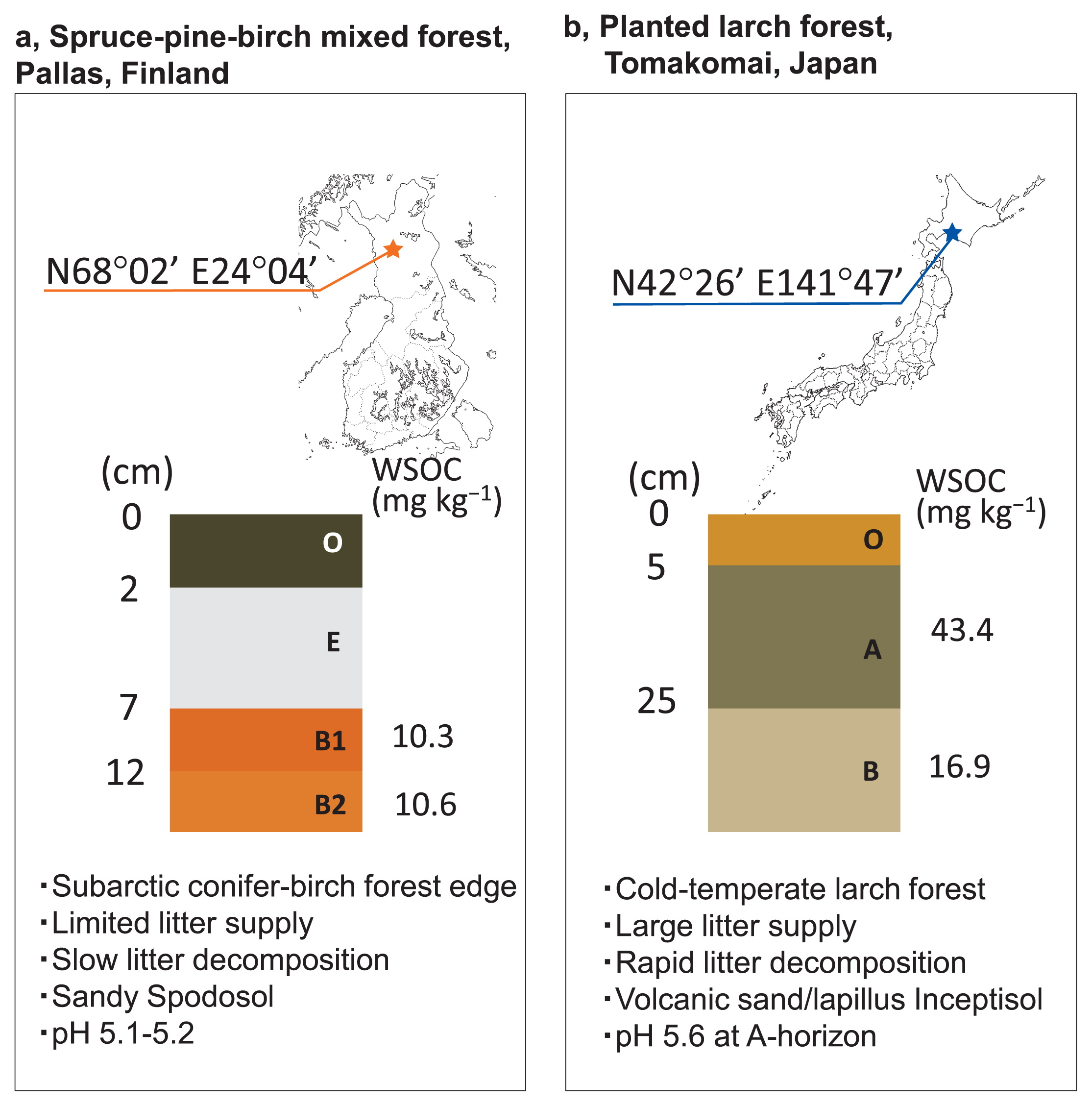
Location and soil profiles of two sampling sites in the conifer-birch forest edge, Finland, and cold-temperate volcanic lapilli, Japan. (a) The mixed conifer-birch forest edge, containing sparsely grown pine, spruce, and birch in a moderate slope with a smaller litter supply, opened toward a hilly heathland. (b) The planted larch forest supplied a large mass of leaf litter on the forest bed as a thick organic topsoil layer (O-horizon).
The subsoil of the boreal forest was collected from Pallas National Park, Finland, which is located inside the Arctic Circle (68°02′N, 24°04′E, altitude 514 m). B1- and B2-horizon soils were collected. Their pH values were 5.12 and 5.20, respectively, while WSOC were 10.3 and 10.6 mg kg−1, respectively. The subsoil of a cold-temperate forest was collected from Tsutamori Forest Park in Tomakomai, Japan (42°39′N, 141°47′E, altitude 25 m), which is a planted larch forest (>40 years old) established on a volcanic sand/lapilli bed. A- and B-horizon soils were collected. Their pH values were 5.64 and 6.58, respectively, while WSOC were 43.4 and 16.9 mg kg−1, respectively. Full methods for pH and WSOC measurements are described in Supplementary Information.
The archaeal communities in the Pallas B1- and B2-horizons of soils and Tomakomai A- and B-horizons of soils, which were analyzed using a high-throughput short-read sequencer, were dominated by the phyla Thaumarchaeota and Euryarchaeota (Fig. 2). The major thaumarchaeal reads, except for those of the marine group (MG), were observed, while most of the euryarchaeal reads were identified as those of class Thermoplasmata (3, 4). Thaumarchaeota Group 1.1, previously known as “Finnish Forest Soil Archaea”, is widely distributed under vegetative acidic soil at pH 5 or less (1, 10), and is one of the most abundant groups of soil Archaea in the B1- and B2-horizon soils of Pallas. Thaumarchaeota Group 1.1c is adapted to soils with a relatively low pH and high water content, and some subgroup members also prefer high organic matter and low nitrate concentrations in soils (15). Thaumarchaeota Group 1.1a-associated, including the obligate acidophilic AOA ‘Candidatus Nitrosotalea devanaterra’ (11), dominated in the mineral horizons of soils from the planted larch forest in the Tomakomai site. The A-horizon soil of the site was very rich in Group 1.1a-associated archaea (approximately 50% of the archaeal population in A-horizon soil). In addition, the A-horizon soil of the Tomakomai site was uniquely abundant in another thaumarchaeal group of the Nitrososphaera cluster (Group 1.1b), including the soil AOA Nitrososphaera viennensis (16, 17) (Fig. 2).
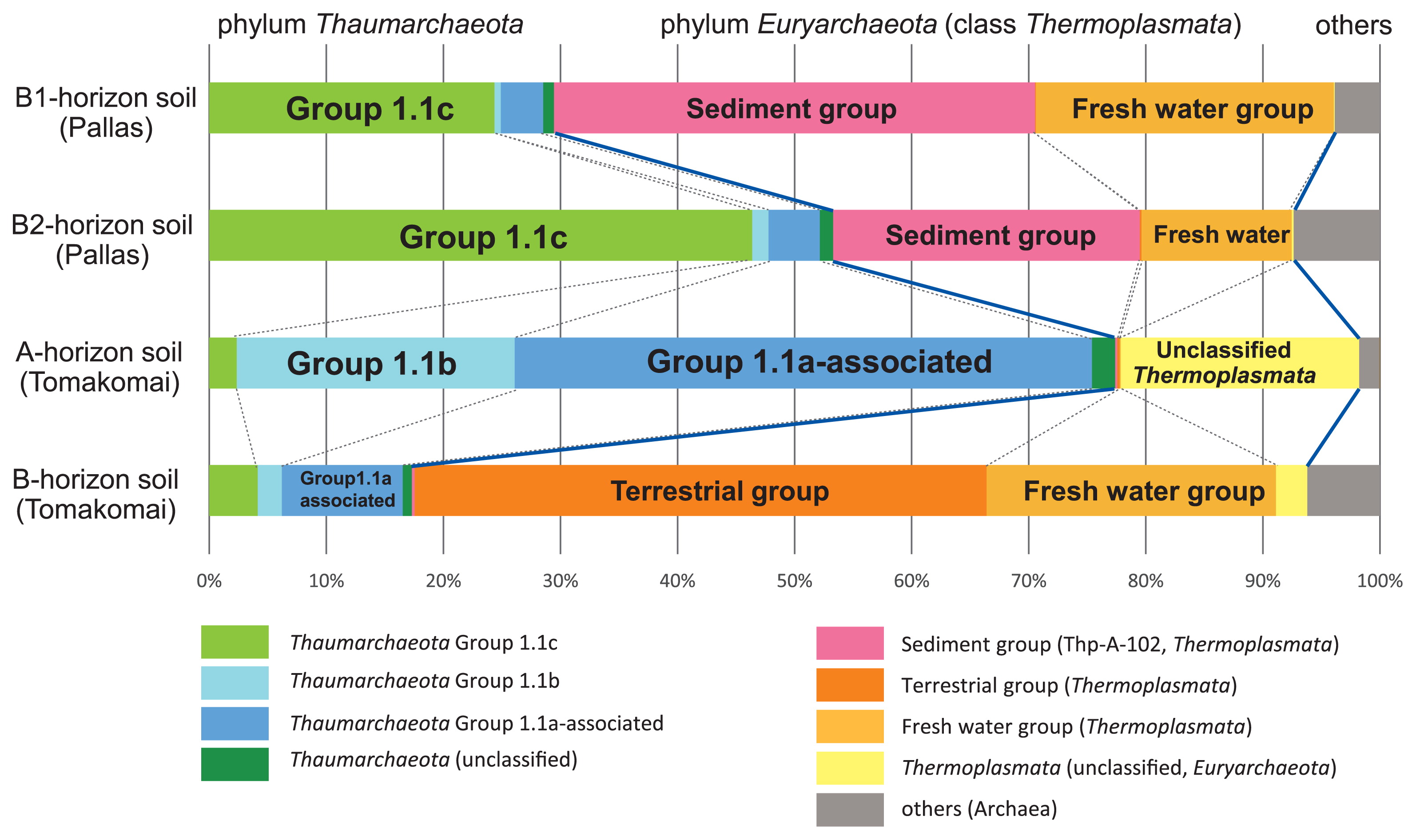
High-throughput analysis of archaeal 16S rRNA gene fragments from Pallas and Tomakomai mineral horizons of soils. The numbers of the mapped reads of the four samples used in this analysis (Pallas B1, Pallas B2, Tomakomai A, and Tomakomai B horizons) were 93732, 211468, 123628, and 209389, respectively. Sequences longer than 370 bp having a quality score of more than 20 without Ns were submitted to the RDP classifier using a bootstrap cut-off of 0.8. Blue solid lines show the boundary between the phylum Thaumarchaeota and class Thermoplasmata of the phylum Euryarchaeota, or between Thermoplasmata and other Archaea.
Members of the class Thermoplasmata were also the abundant archaeal class in these mineral horizon soils. The B1-and B2-horizon soils from Pallas were uniquely rich in Thermoplasmata Thp-A-102 (sediment group), which was initially detected in geothermal spring water in Greece at a depth of 20–30 cm below the surface (9). The population ratios of Thermoplasmata Thp-A-102 in the B1- and B2-horizon soils were 42 and 36%, respectively. In contrast, more than 70% of the sequence from the B-horizon soil of Tomakomai was identified as the Thermoplasmata group, mainly composed of terrestrial and fresh water groups (50 and 26%, respectively), while A-horizon soil contained uniquely unclassified Thermoplasmata. Thus, marked differences were observed in the community structures of the class Thaumarchaeota between these two sites (Fig. 2).
Along with a 16S rRNA gene-based high-throughput analysis for archaeal community structures, the ammonia monooxygenase subunit A (amoA) gene of Archaea from soils was cloned after the separation of amoA gene-targeted PCR-denaturing gradient gel electrophoresis (DGGE) bands. A phylogenetic analysis indicated that two groups of amoA genes derived from Thaumarchaeota Group 1.1a-associated and Group 1.1b co-existed in boreal forest soils and the A-horizon soil of the temperate forest (Fig. 3a, b).

Denaturing gradient gel electrophoresis (DGGE) profile of the partial amoA gene obtained from soil samples, and phylogeny of amoA base sequences. a) DGGE profile of amoA for soil DNA extracted directly from each soil sample. Successfully sequenced DNA bands are shown with red arrows. b) Phylogenetic tree with some reference strains. Regarding clustering, we used the neighbor-joining method with 1000 bootstrap replicates. Clones of partial amoA genes (593 bp) are shown by red letters.
Hernández et al. suggested that ammonia oxidizers in newly emergent volcanic sand/lapillus soil are pioneers that promote the development of carbon and nitrogen sinks necessary for biodiversity enrichment in the soil ecosystem (3). Hence, Thaumarchaeota Group 1.1a-associated and Group 1.1b dominant in A-horizon soil at the Tomakomai site may be one of them. This is the first study on the archaeal community structure of volcanic sand/lapillus soil under a planted larch forest in Japan. The composition of amoA genes may serve as a clear indicator of the unique dynamics of the thaumarchaeal AOA at different sites and depths of mineral soils, reflecting overall local soil conditions and the nitrification rate. Conversely, Thaumarchaeota Group 1.1c in boreal forest soil has already been reported to neither harbor any functional amoA gene nor show evidence of ammonia oxidation (19). Thus, the ecological function of Group 1.1c Thaumarchaeota is poorly understood; however, the abundance of Group 1.1c and the mesophilic groups of euryarchaeal Thermoplasmata may contribute to C mineralization (13).
Although our results highlight only limited members of the microbial community at a few sites (or were even biased due to the soil preservation method stocked at 4°C), the ecological roles of Thaumarchaeota Group 1.1c and other soil Archaea warrant further study as an important research target.
We would like to thank Editage (www.editage.jp) for English language editing. This research was supported by Grants-in-Aid A (20255002 and 26252058 to Y.H.) and B (26304042 to Y.H.) of the Japan Society for the Promotion of Science. The authors confirm no conflict of interest.