2017 年 32 巻 4 号 p. 376-385
2017 年 32 巻 4 号 p. 376-385
Biocontrol agents (BCA) effectively suppress soil-borne disease symptoms using natural antagonistic prokaryotes or eukaryotes. The main issue associated with the application of BCA is that disease reduction effects are unstable under different field conditions. In order to identify potentially effective BCA among several fields, we compared prokaryotic and eukaryotic communities in soil with and without tomato bacterial wilt from three different fields, each of which had the same field management and similar soil characteristics. Soil samples were collected from three fields and two depths because bacterial wilt pathogens were present in soil at a depth greater than 40 cm. We classified soil samples based on the presence or absence of the bacterial phcA gene, a key gene for bacterial wilt pathogenicity and tomato disease symptoms. Pyrosequencing of the prokaryotic 16S rRNA gene and eukaryotic internal transcribed spacer region sequences showed that the diversity and richness of the communities mostly did not correlate with disease symptoms. Prokaryotic and eukaryotic community structures were affected more by regional differences than the appearance of disease. Several prokaryotes and eukaryotes were more abundant in soil that lacked disease symptoms, and eight prokaryotes and one eukaryote of this group were commonly detected among the three fields. Some of these taxa were not previously found in disease-suppressive soil. Our results suggest that several prokaryotes and eukaryotes control plant disease symptoms.
Tomato (Solanum lycopersicum) is one of the most important vegetables in the world, with a global annual yield of approximately 160 million tons (FAO. Statistical database [FAOSTAT], 2014; Available from: http://fatstat.fao.org.). Its production is often prevented by bacterial wilt, a devastating disease caused by Ralstonia solanacearum. This soil-borne pathogen infects more than 200 plant species, e.g., olive, tomato, tobacco, and eggplant, and, thus, causes great losses in agriculture and horticulture (22). Bacterial wilt disease is principally managed by soil fumigation, resistant cultivars, soil amendments, crop rotation, and field sanitation (47). Biocontrol agents (BCA), which control the bacterial wilt pathogen using microbes, have recently been developed (11, 17, 48). One of the serious issues associated with the application of BCA techniques is that their effects are still hindered by soil types, sampling sites, climate conditions, and other factors (12). Therefore, the control of plant disease by BCA is generally site specific (31).
Soils with high antagonist potential lead to the suppression of soil-borne pathogens. These soils, called disease-suppressive soils, have been reported for multiple soil-borne pathogens including those causing Fusarium wilt (29), potato common scab (37), damping-off disease (18), tobacco black root rot (24), and bacterial wilt (41). Soil microbes play important roles in the suppression of plant disease (15, 33, 34). However, the isolation of effective BCA is technically difficult because more than 90% of soil microbes are uncultivable (45). Yin et al. compared bacterial communities in soil that suppressed and conduced Rhizoctonia solani and found effective bacteria for disease control (49). Therefore, a comparison of microbial communities in disease-suppressive and disease-conducive soil may provide insights for identifying BCA for the soil environment. In our previous study, we also compared prokaryotic communities in soil with and without signs of tomato bacterial wilt symptoms, but with pathogens present, and some prokaryotes were specifically detected in soil that did not demonstrate wilt symptoms (27). However, in previous studies, soil samples were only collected from one field. If microbes that are associated with plant disease suppression are detected among several different fields, they may widely control soil-borne pathogens, instead of only at a specific site.
This study aims to reveal differences in microbial communities in soil samples with and without tomato bacterial wilt symptoms collected from three different sampling sites. Although BCA against the bacterial wilt pathogen have been shown with not only bacterial species, but also several eukaryotic species, investigations on eukaryotic communities in disease-suppressive soil are often ignored (14, 35). Prokaryotes in soil at a depth greater than 40 cm are important because R. solanacearum is distributed not only in the plow layer of soil, but also in the hardpan layer (13). We previously revealed that prokaryotic communities differed between soil at depths less than and greater than 40 cm and successfully detected unique prokaryotes at depths greater than 40 cm (27). Therefore, we collected soil samples at two depths, less than and greater than 40 cm, in each field and performed high-throughput sequencing of prokaryotic 16S rRNA genes and eukaryotic internal transcribed spacer (ITS) regions. Each of the fields that we selected to sample were managed identically, but showed the presence and absence of wilt disease. We attempted to find microbes that were specifically detected in soil without disease symptoms among different sampling fields and these specific microbial taxa are BCA candidates to commonly treat bacterial wilt in different regions.
Soil samples were collected from greenhouses of tomato plants located in Hakusan (Ha-field) and Kahoku City (Ka-field), Ishikawa Prefecture and in Inami city, Wakayama Prefecture (Wa-field) in late January 2015. Detailed information on the sampling sites is shown in Table 1. In the Ka- and Ha-fields, greenhouses with (Ha-1 and Ka-1) and without (Ha-2 and Ka-2) disease symptoms adjoined each other. Bacterial wilt symptoms had not appeared in the Ha-2 and Ka-2 fields for at least five years. In Wa-field, one field showed disease symptoms (Wa-1), whereas two did not (Wa-2 and Wa-3), and they were spatially separated. No significant differences were observed in soil types, field management, or soil chemical properties such as pH and electron conductivity (EC) between soil sites that had or lacked bacterial wilt symptoms in each field (P<0.05, t-test). No tomato plants were planted in each field at the sampling time. We collected soil samples from two different depths (20–30 and 40–50 cm) in each field using a core sampler (Gauge Auger DIK-106B; Daiki Rika Kogyo, Saitama, Japan). Five samples were collected from each greenhouse in the Ha- and Ka-fields (a total of 40 samples). In Wa-field, five samples were collected from Wa-1 and Wa-3, and three samples were collected from Wa-2 (a total of 26 samples). Sixty-six samples were collected from all fields and stored at −20°C until used. DNA was extracted from 0.5 g of each soil with an ISOIL for the Beads Beating kit (Nippongene, Tokyo, Japan) following the manufacturer’s instructions. The quantity and purity of DNA was measured using a NanoDrop spectrophotometer (Thermo Fischer Scientific, Waltham, MA, USA) and by visualization on a 0.8% agarose gel in Tris-acetate-EDTA (TAE) buffer.
| Prefecture | Ishikawa | Wakayama | |||||
|---|---|---|---|---|---|---|---|
|
|
| ||||||
| City | Hakusan | Kahoku | Inami | ||||
|
|
|
| |||||
| Field name | Ha-1 | Ha-2 | Ka-1 | Ka-2 | Wa-1 | Wa-2 | Wa-3 |
| Latitude and longitude | 36° 29′ N, 136° 30′ E | 36° 45′ N, 136° 45′ E | 33° 27′ N, 135° 14′ E | 33° 49′ N, 135° 15′ E | 33° 38′ N, 135° 14′ E | ||
| Soil types | Gleysol | Fluvisol | Cambisols | Cambisols | Cambisols | ||
| Pathogenesis | + | − | + | − | + | − | − |
| Sample number | 5 | 5 | 5 | 5 | 5 | 3 | 5 |
| pH | 7.22±0.19 | 7.02±0.14 | 5.67±0.14 | 5.51±0.27 | 7.49±0.33 | 7.39±014 | 7.08±047 |
| EC (mS cm−1) | 0.31±0.07 | 0.31±0.03 | 0.31±0.22 | 0.84±0.17 | 0.88±0.29 | 0.19±005 | 0.21±006 |
The phcA gene, which plays a major role in the regulation of R. solanacearum pathogenicity (38), was amplified from DNA that was extracted from each soil sample as described by Lee et al. (27). Briefly, a two-step nested polymerase chain reaction (PCR) was performed to detect the phcA gene; two primer sets were used for the first step (phcA2981f [5′-TGGATATCGGGCTGGCAA-3′] and phcA4741r [5′-CGCTTTTGCGCAAAGGGA-3′]) and for the second step (phcA3538f [5′-GTGCCACAGCATGTTCAGG-3′] and phcA4209r [5′-CCTAAAGCGCTTGAGCTCG-3′]) (19). Amplification was verified by gel electrophoresis (1.5% agarose in TAE buffer). We classified the three soil types based on phcA gene detection and pathogenesis, according to Lee et al. (27): soil not showing signs of disease, but with phcA (S-soil), soil with diseased tomato plants and phcA (C-soil), and soil not showing bacterial wilt without phcA (unclassified soil).
Amplification of the prokaryotic 16S rRNA gene and eukaryotic ITS region and tag-encoded amplicon pyrosequencingPCR was performed on each sample to amplify the V4 variable region of the 16S rRNA gene using the bacterial and archaeal universal primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGAC-TACVSGGGTATCTAA-3′) (4), and the fungal ITS2 region was amplified using ITS3_KYO2 (5′-GATGAAGAAC GYAGYRAA-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) coupled with the Roche 454 Titanium sequencing adapters (44). The 515F and ITS3_KYO2 primers contained the barcode sequences with a Roche 454-A pyrosequencing adapter (Titanium Lib-L adapters), and the 806R and ITS4 primers had a Roche 454-B adapter. When two or more bands were detected with 1.5% agarose gel electrophoresis, PCR products of approximately 350 bp in length were excised from the gel and purified using a MonoFas DNA purification kit (GL Sciences, Tokyo, Japan) for prokaryotes and eukaryotes. Eleven soil samples were not amplified with the primers for eukaryotes. Each PCR amplicon was cleaned twice using an Agencourt AMPure XP system (Beckman Coulter, Brea, CA, USA) to remove primers and short DNA fragments and then quantified using a Qubit Fluorometer (Invitrogen, Carlsbad, CA, USA). The purified PCR amplicons were combined in equimolar ratios in a single tube for emulsion PCR (emPCR). An emPCR reaction was performed with an approximate ratio of 0.2:1 (amplicon:emPCR beads), and the amplicon sequencing of 16S rRNA genes and ITS sequences was performed on the Roche 454 GS Junior Titanium sequencer using a Lib-L kit (Roche, Branford, CT, USA). Sequencing data have been deposited in the DNA Data Base of Japan (DDBJ) Sequence Read Archive under accession number DRA005842 and DRA005843 for prokaryotes and eukaryotes, respectively.
Data analysisEach raw standard flowgram format (SFF) file was preprocessed in Quantitative Insights Into Microbial Ecology (QIIME) (3). Data from read sequences, quality, flows, and ancillary metadata were analyzed using the QIIME pipeline according to Campisaono et al. (2). Quality filtering consisted of discarding reads <200 bp or >500 bp for 16S rRNA and ITS sequences, excluding homopolymer runs of >6 bp and >6 continuous ambiguous bases, but accepting 1 barcode correction and 2 primer mismatches. Moreover, a mean quality score less than 25 was also a criterion used to remove singleton operational taxonomic units (OTUs) and chimeric sequences for statistical analyses. Denoising was performed using the built-in Denoiser algorithm, and chimera removal and OTU picking were accomplished with USEARCH 61 considering a pairwise identity percentage of 0.97. The taxonomy assignment of each OTU was performed using the RDP (Ribosomal database Project) naïve Bayesian classifier against the Greengenes database and GenBank’s Basic Local Alignments Search Tool (BLAST) for prokaryote and eukaryote sequences, respectively. An OTU-based analysis was performed on pyrotag-based datasets to calculate richness and diversity using the phyloseq R package (1.724) (32). The diversity within each individual sample was estimated using the non-parametric Shannon diversity index and Simpson’s diversity index. The Chao1 estimator and observed OTU numbers were calculated to estimate the richness of each sample. A multivariate analysis of community structure and diversity was performed on pyrotag-based datasets using a weighted UniFrac dissimilarity matrix that was calculated in QIIME, jackknifing (1,000 reiterations) read abundance data at the deepest level possible (3,105 and 542 reads for prokaryotes and eukaryotes, respectively), and using unconstrained ordination by a Principal Coordinate Analysis (PCoA) for prokaryotes. Regarding eukaryotes, dissimilarity tests were performed using the Bray-Curtis dissimilarity index between samples (7). Finally, an indicator species analysis was calculated using the indicspecies R package in prokaryotic and eukaryotic communities (9). The indicator species analysis allows for the identification of species that may be used as an indicator of a site group. We created two patterns of soil groups: one group was divided into samples from soil with or without bacterial wilt symptoms, while the other group was divided based on bacterial wilt symptoms in the sample as well as the soil layer. Each result was merged, and abundant OTUs associated with soil without wilt symptoms were evaluated, rather than those from soil with symptoms (P<0.001). The number of random permutation tests for the calculation of indicator values was 999.
PCR products of phcA were obtained from all fields with disease symptoms, irrespective of soil depth, except for one plot in Wa-field (Wa-1-5) (Table 2). Symptoms associated with R. solanacearum were not observed in five, three, and five plots of the Ha-, Ka-, and Wa-fields, respectively; however, the phcA gene was detected.
| Plot name | Sample number | Soil layer | phcA gene | Disease | Classification* |
|---|---|---|---|---|---|
| Ha-1 | 1 | Up | ++ | − | S-soil |
| Low | ++ | − | |||
| 2 | Up | − | |||
| Low | ++ | − | |||
| 3 | Up | ++ | − | ||
| Low | + | − | |||
| 4 | Up | − | |||
| Low | ++ | − | |||
| 5 | Up | ++ | − | ||
| Low | +++ | − | |||
|
| |||||
| Ha-2 | 1 | Up | ++ | + | C-soil |
| Low | + | + | |||
| 2 | Up | + | + | ||
| Low | ++ | + | |||
| 3 | Up | + | |||
| Low | + | + | |||
| 4 | Up | + | |||
| Low | +++ | + | |||
| 5 | Up | + | |||
| Low | ++ | + | |||
|
| |||||
| Ka-1 | 1 | Up | − | Unclassified soil | |
| Low | − | ||||
| 2 | Up | − | |||
| Low | − | ||||
|
| |||||
| 3 | Up | − | S-soil | ||
| Low | + | − | |||
| 4 | Up | − | |||
| Low | + | − | |||
| 5 | Up | − | |||
| Low | + | − | |||
|
| |||||
| Ka-2 | 1 | Up | + | C-soil | |
| Low | + | + | |||
| 2 | Up | + | |||
| Low | ++ | + | |||
| 3 | Up | + | |||
| Low | + | + | |||
| 4 | Up | + | |||
| Low | +++ | + | |||
| 5 | Up | + | |||
| Low | + | + | |||
|
| |||||
| Wa-1 | 1 | Up | + | + | C-soil |
| Low | + | + | |||
| 2 | Up | + | + | ||
| Low | + | + | |||
| 3 | Up | + | + | ||
| Low | + | ||||
| 4 | Up | + | |||
| Low | + | + | |||
|
| |||||
| 5 | Up | + | Unclassified soil | ||
| Low | + | ||||
|
| |||||
| Wa-2 | 1 | Up | − | ||
| Low | − | ||||
| 2 | Up | − | |||
| Low | − | ||||
|
| |||||
| 3 | Up | − | S-soil | ||
| Low | + | − | |||
|
| |||||
| Wa-3 | 1 | Up | + | − | S-soil |
| Low | ++ | − | |||
| 2 | Up | + | − | ||
| Low | + | − | |||
| 3 | Up | ++ | − | ||
| Low | + | − | |||
| 4 | Up | + | − | ||
| Low | − | ||||
|
| |||||
| 5 | Up | − | Unclassified soil | ||
| Low | − | ||||
Soils were classified based on phcA gene detection and pathogenesis (see Materials and Methods). We selected 5 S-soil and 5 C-soil samples in Ha-field, 3 S-soil and 5 C-soil samples in Ka-field, and 5 S-soil and 4 C-soil samples in Wa-field for further investigation (Table 2). Unclassified soil samples were not investigated further. We did not investigate the Wa-1-5 plot; this plot had tomato plants with disease symptoms, but no phcA in the field soil, suggesting that R. solanacearum infected above-ground parts of the tomato plant to cause wilt symptoms.
Diversity and richness in S- and C-soilsPyrosequencing yielded 402,910 sequences in 54 samples for prokaryotes and 74,226 sequences in 45 samples for eukaryotes (Supplemental Table S1). These sequences were clustered into 8,240 OTUs and 1,369 OTUs for prokaryotes and eukaryotes, respectively. No significant differences were observed in prokaryotic diversity or richness between S-soil and C-soil samples, except for the lower layer of Ha-field (Table 3). In this field, the OTU number and Chao 1 richness estimator were lower in C-soil than in S-soil.
| Ha-field | Ka-field | Wa-field | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
| ||||||
| S-soil | C-soil | S-soil | C-soil | S-soil | C-soil | |||
| Prokaryotes | Shannon | Upper | 0.99±0.001 | 0.99±0.001 | 0.98±0.03 | 0.99±0.001 | 0.84±0.2 | 0.97±0.02 |
| Lower | 0.99±0.01 | 0.99±0.001 | 0.99±0.01 | 0.97±0.01 | 0.97±0.02 | 0.94±0.05 | ||
| Simpson | Upper | 5.87±0.07 | 5.96±0.04 | 5.35±0.77 | 5.7±0.13 | 4.24±1.65 | 5.12±0.27 | |
| Lower | 5.82±0.22 | 5.62±0.15 | 5.65±0.3 | 5.01±0.26 | 5.06±0.28 | 4.35±0.7 | ||
| OTU number | Upper | 671±28 | 658±22 | 586±145 | 659±47 | 406±179 | 471±36 | |
| Lower | 675±48* | 575±44 | 636±60 | 517±96 | 471±25 | 348±65 | ||
| Chao1 | Upper | 1287±57 | 1195±66 | 1268±306 | 1390±111 | 704±299 | 798±56 | |
| Lower | 1308±85* | 1061±69 | 1314±126 | 1093±202 | 840±91 | 614±123 | ||
|
| ||||||||
| Eukaryotes | Shannon | Upper | 2.93±0.63 | 2.91±0.53 | 2.81±0.14 | 3.73±0.1* | 2.27±0.39 | 1.81±0.03 |
| Lower | 3.17±0.64 | 2.13±0.77 | 2.83±0.09 | 3.57±0.32* | 2.41±1.07 | 2.14±0.12 | ||
| Simpson | Upper | 0.84±0.13 | 0.85±0.07 | 0.87±0.02 | 0.93±0.01* | 0.77±0.06 | 0.66±0.09 | |
| Lower | 0.89±0.05 | 0.71±0.23 | 0.85±0.03 | 0.94±0.03* | 0.74±0.25 | 0.81±0.02 | ||
| OTU | Upper | 104±23 | 104±12 | 170±32 | 265±40* | 68±32 | 53±17 | |
| Lower | 164±129 | 63±12 | 178±23 | 172±46 | 72±36 | 32±25 | ||
| Chao1 | Upper | 122±32 | 113±25 | 189±43 | 261±27 | 90±34 | 63±15 | |
| Lower | 143±79 | 84±40 | 193±36 | 223±26 | 97±56 | 65±16 | ||
Asterisks represent a pair of means with significantly higher values than those of the other soil type in each field (t-test, P<0.05).
Previous studies indicated that bacterial diversity and richness correlate with the suppression of plant pathogens (11, 29). A study by van Elsas et al. revealed a negative correlation between the diversity of soil microbiotas and survival of pathogenic microbes (46). They explained that the underlying mechanisms of diversity-invasiveness relationships may involve competition for the utilization of limited resources. Other studies indicated that the composition of indigenous bacterial populations is simpler in plant disease-conducive than in -suppressive soil (17, 40).
Regarding eukaryotes, Shannon and Simpson’s indices were significantly higher in C-soil than in S-soil for the upper and lower layers in Ka-field (Table 3). OTU numbers were higher in C-soil than in S-soil for the upper layer in Ka-field. However, no significant differences were observed in eukaryotic richness or diversity between S-soil and C-soil. Fungal diversity is higher or lower in soil treated with bio-fertilizer, which suppresses plant disease more than conventional soil, depending on previous studies (25, 30, 36). Fu et al. (2017) showed that the application of a bio-fertilizer that suppresses banana Fusarium wilt led to greater bacterial richness and diversity, whereas fungal diversity and richness did not appear to correlate with the incidence of this disease (11).
In the present study, no significant differences were observed in the diversity and richness of prokaryotes or eukaryotes between S-soil and C-soil in most fields. Therefore, the relationships among microbial diversity, richness, and disease symptoms remain unclear.
Microbial community composition and structure in S-soil and C-soilFig. 1 shows the prokaryotic and eukaryotic community compositions of upper- and lower-layer soil samples in the three fields. Proteobacteria represented between 26 and 53% of the prokaryotic species in all fields (Fig. 1A, Supplemental Table S2). Their representation was significantly higher in S-soil than in C-soil in both layers of Ha-field and in the lower layer of Ka-field (t-test, P<0.05). Firmicutes, Acidobacteria, Chloroflexi, and Bacteroidetes were the next most dominant prokaryotic phyla in most cases. Firmicutes were significantly higher in C-soil in the upper layer of Ka-field, Acidobacteria were more abundant in the lower layer of Ka-field and upper layer of Wa-field, and no significant differences were observed in Chloroflexi among S-soil and C-soil in all fields.
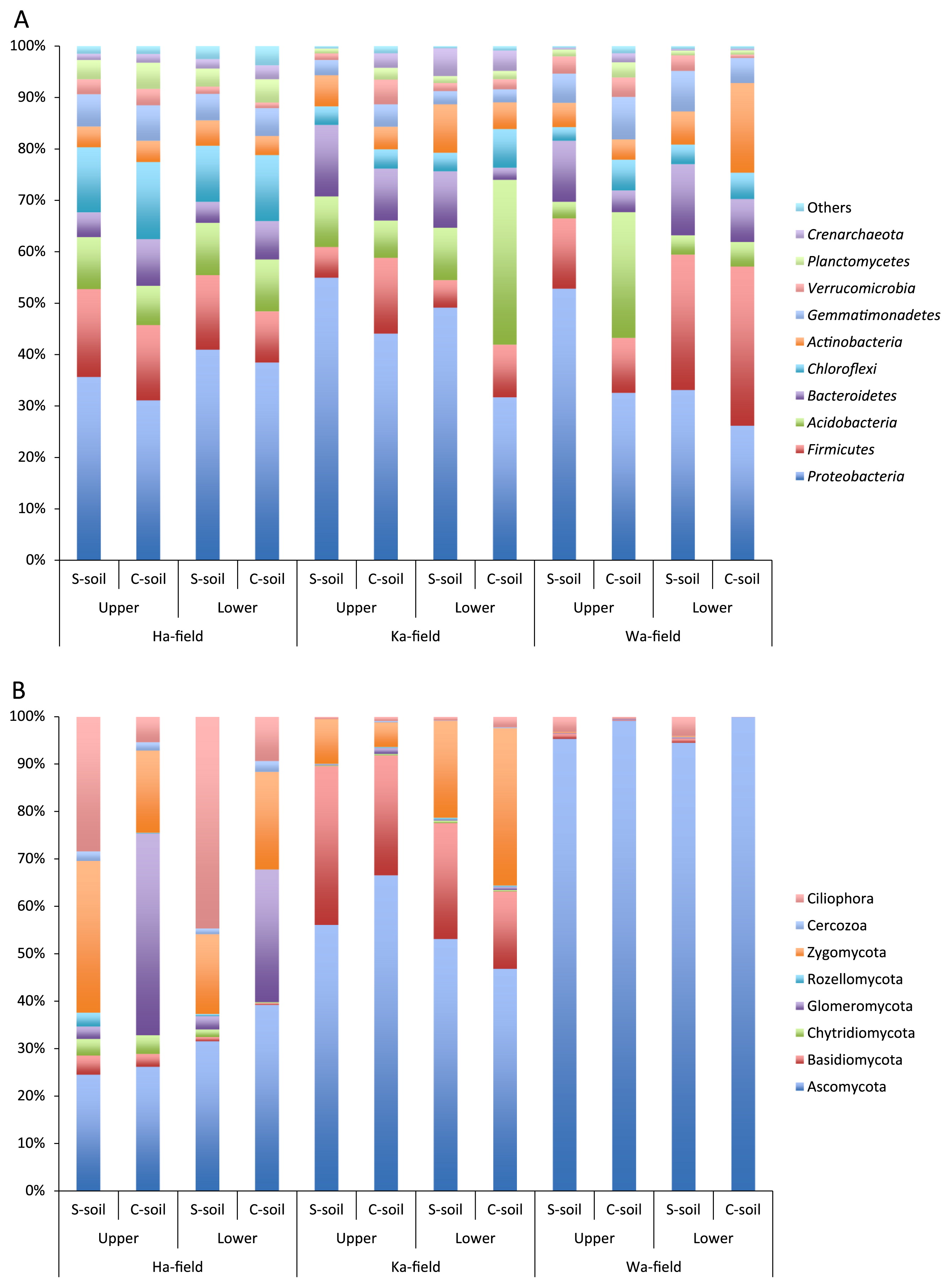
Relative abundance of prokaryotic (A) and eukaryotic (B) phyla in S-soil and C-soil.
Regarding eukaryotic communities, Ascomycota accounted for more than 90% of the relative abundance in Wa-field, whereas they occupied approximately 25 to 67% in the Ha- and Ka-fields (Fig. 1B, Supplemental Table S2). Basidiomycota represented 16 to 33% of eukaryotic abundance in Ka-field, and Zygomycota occupied 17 to 33% of eukaryotic abundance in Ha-field and the lower layer of Ka-field. In Ha-field, Cilliphora levels were significantly higher in S-soil than in C-soil, while Glomeromycota were less abundant in S-soil than in C-soil, irrespective of soil depth. The levels of other phyla were not significantly different between S-soil and C-soil in each field. These results suggest that specified microbes of prokaryotes and eukaryotes in S-soil were not commonly detected among the three fields at the phylum level.
PCoAs based on weighted-UniFrac and Bray-Curtis distance analyses showed that prokaryotic and eukaryotic communities were roughly separated along the sampling fields (Fig. 2). Regarding prokaryotes, communities in S-soil were similar with those in C-soil in Ha-field. In Ka-field, communities in S-soil differed from those in C-soil regardless of the soil layer, whereas those in the upper layer only differed in Wa-field (Fig. 2). Communities in C-soil in the upper layer of Wa-field grouped together with those in Ha-field. Similarly, communities in C-soil in the upper layer of Ka-field were closely related to those in Wa-field. We previously compared soil prokaryotic community structures between S-soil and C-soil using the same statistical method (27). The relative abundance of each prokaryotic phylum was not significantly different between S-soil and C-soil, whereas community structures in the upper soil layer were distinctly classified as different between S-soil and C-soil. Soil samples were collected from the same greenhouse; therefore, a difference in the relative abundance of each prokaryote was not detected at the phylum level. Eukaryotic community structures were more clearly differentiated among sampling fields than soil classification based on the signs of bacterial wilt (Fig. 2). Prokaryotic and eukaryotic community structures were affected more by regional differences than the appearance of disease.
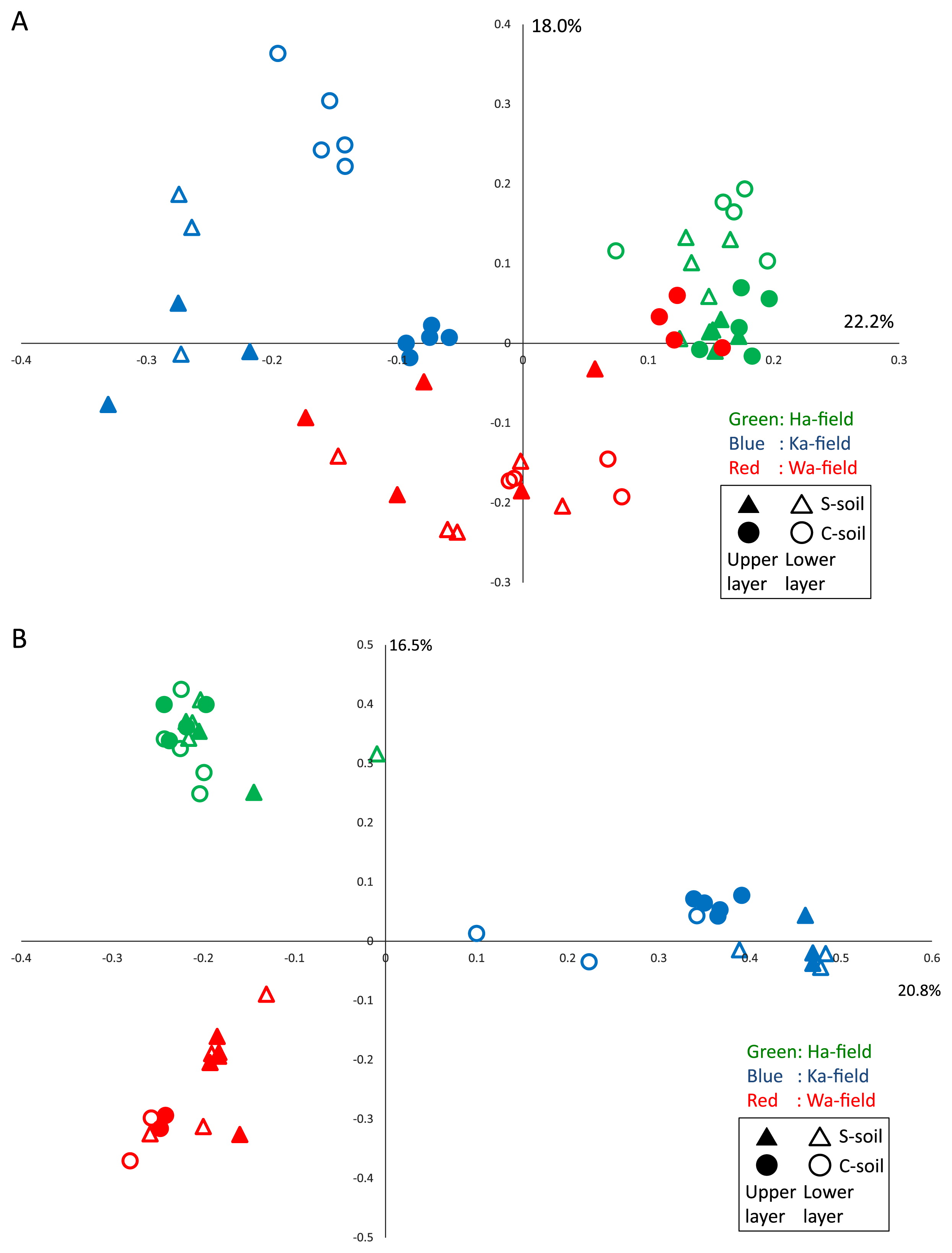
A UniFrac-weighted principal component analysis of prokaryotic (A) and Bray-Curtis principal component analysis of eukaryotic (B) communities in S-soil and C-soil. Closed triangle, S-soil in the upper layer. Open triangle, S-soil in the lower layer. Closed circle, C-soil in the upper layer. Open circle, C-soil in the lower layer. Green plots: Ha-field, Blue plots: Ka-field, Red plots: Wa-field.
A total of 230, 151, and 123 OTUs of prokaryotes were significantly more abundant in S-soil than in C-soil in the Ha-, Ka-, and Wa-fields, respectively (Table 4, Supplemental Table S3). Regarding S-soil-abundant prokaryotic OTUs, Proteobacteria were mainly detected in both layers in all three fields. Bacteroidetes OTUs were the second most abundant, having higher numbers in the Ka- and Wa-fields, and most of them belonged to Sphingobacteriia. Acidobacteria OTUs were commonly detected as the second most abundant group in the upper and lower layers of soil in Ha-field, whereas an Acidobacteria OTU was not detected in the lower layer of Ka-field or the upper layer of Wa-field.
| Domain | Phylum | Ha-field | Ka-field | Wa-field | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
| |||||||||
| All | Upper | Lower | All | Upper | Lower | All | Upper | Lower | |||
| Prokaryotes | Bacteria | Proteobacteria | 22 | 21 | 25 | 21 | 10 | 5 | 13 | 5 | 4 |
| Bacteroidetes | 3 | 4 | 6 | 16 | 3 | 6 | 16 | 3 | 4 | ||
| Acidobacteria | 15 | 4 | 23 | 11 | 2 | 0 | 2 | 0 | 2 | ||
| Firmicutes | 9 | 10 | 11 | 7 | 0 | 4 | 2 | 2 | 2 | ||
| Actinobacteria | 4 | 2 | 7 | 11 | 1 | 2 | 2 | 0 | 2 | ||
| Chloroflexi | 6 | 5 | 6 | 0 | 0 | 1 | 0 | 1 | 1 | ||
| Planctomycetes | 4 | 2 | 5 | 1 | 0 | 0 | 0 | 0 | 2 | ||
| Verrucomicrobia | 2 | 1 | 3 | 2 | 0 | 0 | 4 | 2 | 0 | ||
| Gemmatimonadetes | 1 | 1 | 3 | 0 | 0 | 1 | 2 | 0 | 2 | ||
| Chlamydiae | 3 | 3 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | ||
| Others | 9 | 6 | 13 | 0 | 2 | 3 | 0 | 1 | 1 | ||
|
| |||||||||||
| Eukaryotes | Fungi | Ascomycota | 4 | 0 | 18 | 8 | 2 | 2 | 2 | 0 | 3 |
| Basidiomycota | 1 | 1 | 2 | 11 | 1 | 2 | 0 | 1 | 0 | ||
| Mucoromycota | 0 | 1 | 1 | 5 | 1 | 1 | 0 | 0 | 0 | ||
| Zoopagomycota | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ||
| Unidentified Fungi | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | ||
|
| |||||||||||
| Protists | Ciliophora | 5 | 0 | 15 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Cercozoa | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Adenophorea | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||
|
| |||||||||||
| Viridiplantae | Streptophyta | 6 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Chlorophyta | 4 | 0 | 7 | 2 | 0 | 0 | 0 | 0 | 0 | ||
Nine, fifteen, and twenty-seven OTUs were shared between the Ha- and Ka-fields, Ha- and Wa-fields, and Ka- and Wa-fields, respectively (Table 5). They mainly belonged to Proteobacteria (27 OTUs), Bacteroidetes (9 OTUs), Firmicutes (6 OTUs), and Actinobacteria (4 OTUs). Twenty-four out of 27 OTUs that were shared between the Ka- and Wa-fields belonged to Proteobacteria or Bacteroidetes and were not differentiated by the soil layers. Seven OTUs were commonly detected as significantly abundant OTUs from S-soil in all three sampling fields. Among them, the OTUs of Sphingobacteriaceae and Dokdonella were commonly detected from the upper and lower soil layers in the Ha- and Ka-fields and in the Ha- and Wa-fields, respectively. Sphingomonas spp. belonging to Sphingomonadaceae are known to suppress soil-borne diseases and produce plant hormone-like compounds that promote plant growth (23, 24, Yang, H., J. Niu, J. Tao, et al. 2016. The impacts of different green manure on soil microbial communities and crop health. Agric Sci Agronomy. Preprint. Available from: 2016090056. doi: 10.20944/preprints201609.0056.v1). However, the abundance of Dokdonella-like bacteria has been positively correlated with Fusarium disease rates in Chinese fields (17, 28, 39). OTUs in candidate division WPS-1, Rhodocyclaceae, and Aquihabitans were mainly detected from the upper layer of all fields. Azoarcus and Azospira, as Rhodocyclacea members, are generally involved in nitrogen cycling in soil environments. Bacteria involved in soil nitrogen cycling affect the suppression of plant diseases, and their release of nitrogen influences the microbial community structure (8, 16). An Aquihabitans species was recently isolated from a freshwater environment, and its ecology has yet to be examined in detail (21). An OTU in Gemmatimonas was detected in the lower layer in all three fields. Gemmatimonas members are reported to be more abundant bacteria in suppressive soil for Fusarium wilt, and are difficult to isolate due to their slow growth rate (6). An OTU in Caldicoprobacter (belonging to Clostridia) was detected uniquely as an obligate anaerobic bacterium in all fields, and was frequently detected in the lower soil layer. Caldicoprobacter is a spore-forming, non-motile, and xylanolytic bacterium that was isolated from sheep feces (50). Taylor and Guy showed that bacteria related to Clostridium, in combination with Bacillus, were effective for controlling the pathogenic fungus Peniophora sacrata (43).
| Detected field | OTU number | Ha-field | Ka-field | Wa-field | Phylum | Class | Closest relatives | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
| ||||||||
| Up | Low | Up | Low | Up | Low | |||||
| All | OTU8949 | + | + | + | + | candidate division WPS-1 | ||||
| OTU2469 | + | + | + | + | Proteobacteria | Betaproteobacteria | Rhodocyclaceae | |||
| OTU17222 | + | + | + | + | Actinobacteria | Actinobacteria | Aquihabitans | |||
| OTU6877 | + | + | + | + | + | Proteobacteria | Alphaproteobacteria | Sphingomonadaceae | ||
| OTU5062 | + | + | + | + | + | Proteobacteria | Betaproteobacteria | Dokdonella | ||
| OTU424 | + | + | + | + | Gemmatimonadetes | Gemmatimonas | Gemmatimonas | |||
| OTU411 | + | + | + | Firmicutes | Clostridia | Caldicoprobacter | ||||
|
| ||||||||||
| Ha & Ka | OTU24779 | + | + | + | Actinobacteria | Actinobacteria | Streptosporangium | |||
| OTU15276 | + | + | + | Firmicutes | Bacilli | Cohnella | ||||
| OTU19407 | + | + | + | Acidobacteria | Acidobacteria_Gp6 | Acidobacteria_Gp6 | ||||
| OTU13095 | + | + | + | Chlamydiae | Chlamydiia | Chlamydiales | ||||
| OTU10784 | + | + | + | candidate division WPS-1 | ||||||
| OTU14695 | + | + | + | Proteobacteria | Alphaproteobacteria | Phenylobacterium | ||||
| OTU18851 | + | + | + | Proteobacteria | Gammaproteobacteria | Gammaproteobacteria | ||||
| OTU20848 | + | + | Firmicutes | Clostridia | Romboutsia | |||||
| OTU14704 | + | + | Proteobacteria | Alphaproteobacteria | Rhodospirillales | |||||
|
| ||||||||||
| Ha & Wa | OTU22759 | + | + | + | + | Firmicutes | Clostridia | Lachnospiraceae | ||
| OTU24142 | + | + | + | + | Proteobacteria | Alphaproteobacteria | Alphaproteobacteria | |||
| OTU170 | + | + | + | + | Verrucomicrobia | Subdivision3 | ||||
| OTU15704 | + | + | Planctomycetes | Planctomycetaceae | Planctomycetaceae | |||||
| OTU5215 | + | + | + | Actinobacteria | Actinobacteria | Micromonosporaceae | ||||
| OTU14625 | + | + | + | Bacteroidetes | ||||||
| OTU21179 | + | + | + | Firmicutes | Clostridia | Clostridium | ||||
| OTU8063 | + | + | + | Bacteroidetes | Sphingobacteriia | Chitinophagaceae | ||||
| OTU18274 | + | + | + | Gemmatimonadetes | Gemmatimonadetes | Gemmatimonas | ||||
| OTU20912 | + | + | + | Proteobacteria | Alphaproteobacteria | Altererythrobacter | ||||
| OTU11041 | + | + | + | Proteobacteria | Betaproteobacteria | |||||
| OTU12798 | + | + | Verrucomicrobia | Subdivision3 | ||||||
| OTU23592 | + | + | Candidatus Saccharibacteria | |||||||
| OTU14570 | + | + | Firmicutes | Bacilli | Bacillales | |||||
| OTU13239 | + | + | Planctomycetes | Planctomycetia | Pirellula | |||||
|
| ||||||||||
| Ka & Wa | OTU18820 | + | + | + | + | Bacteroidetes | Sphingobacteriia | Pedobacter | ||
| OTU13685 | + | + | + | + | Bacteroidetes | Sphingobacteriia | Flavisolibacter | |||
| OTU450 | + | + | + | + | Bacteroidetes | Flavobacteriia | Flavobacterium | |||
| OTU7089 | + | + | + | + | Proteobacteria | Alphaproteobacteria | Sphingomonas | |||
| OTU16801 | + | + | + | + | Proteobacteria | Gammaproteobacteria | Rheinheimera | |||
| OTU10791 | + | + | + | + | Proteobacteria | Betaproteobacteria | Achromobacter | |||
| OTU15731 | + | + | + | + | Verrucomicrobia | Opitutae | Opitutus | |||
| OTU10170 | + | + | + | Proteobacteria | Gammaproteobacteria | Gammaproteobacteria | ||||
| OTU19779 | + | + | + | Proteobacteria | Alphaproteobacteria | Devosia | ||||
| OTU24775 | + | + | + | Bacteroidetes | Flavobacteriia | Ohtaekwangia | ||||
| OTU7082 | + | + | + | Proteobacteria | Alphaproteobacteria | Bradyrhizobiaceae | ||||
| OTU1098 | + | + | + | Proteobacteria | Betaproteobacteria | Betaproteobacteria | ||||
| OTU12616 | + | + | Proteobacteria | Alphaproteobacteria | Alphaproteobacteria | |||||
| OTU52 | + | + | + | Bacteroidetes | Flavobacteriia | Flavobacterium | ||||
| OTU3636 | + | + | + | Proteobacteria | Alphaproteobacteria | Devosia | ||||
| OTU1963 | + | + | + | Bacteroidetes | Sphingobacteriia | Flavisolibacter | ||||
| OTU19716 | + | + | + | Bacteroidetes | Sphingobacteriia | Terrimonas | ||||
| OTU8299 | + | + | + | Proteobacteria | Betaproteobacteria | Cupriavidus | ||||
| OTU3281 | + | + | + | Proteobacteria | Gammaproteobacteria | Pseudohaliea | ||||
| OTU10190 | + | + | + | Proteobacteria | Alphaproteobacteria | Bosea | ||||
| OTU21838 | + | + | + | Proteobacteria | Alphaproteobacteria | Dongia | ||||
| OTU22451 | + | + | + | Proteobacteria | Alphaproteobacteria | Rhizomicrobium | ||||
| OTU16175 | + | + | + | Proteobacteria | Gammaproteobacteria | Rhodanobacter | ||||
| OTU16117 | + | + | + | Proteobacteria | Betaproteobacteria | Ramlibacter | ||||
| OTU13358 | + | + | + | Proteobacteria | Alphaproteobacteria | Ensifer | ||||
| OTU7168 | + | + | Actinobacteria | Actinobacteria | Pseudonocardineae | |||||
| OTU18310 | + | + | Acidobacteria | Acidobacteria_Gp3 | Acidobacteria_Gp3 | |||||
Sphingomonadaceae and Rhodocyclaceae were commonly detected in our previous study (27) and from S-soil in the present study. These bacteria have the potential to be effective BCA among several fields. Our previous study also uniquely detected several prokaryotic OTUs from S-soil that may be involved in nitrogen cycling bacteria from S-soil. Moreover, we also commonly found the anaerobic bacteria, Caldicoprobacter and unidentified Anaerilineae in S-soil in the present and previous studies. They may play important roles in the suppression of bacterial wilt.
Specific eukaryotes detected in soil without symptomsEighty, thirty-nine, and six OTUs of eukaryotes were significantly more abundant in S-soil than in C-soil in the Ha-, Ka-, and Wa-fields, respectively (Table 4, Supplemental Table S4). Among them, the main phylum that was specifically detected in S-soil in all fields was Ascomycota. Basidiomycota was dominant in Ka-field, and members of protists and Viridiplantae were mainly detected in Ha-field. Eleven and nine OTUs were shared between the Ha- and Ka-fields and Ha- and Wa-fields, respectively, whereas one OTU was commonly detected in the Ka- and Wa-fields and another OTU was shared in all fields. Twelve out of 22 OTUs were closely related to Ascomycota. Other OTUs belonged to Basidiomycota (3 OTUs); Mucoromycota (2 OTUs) and unidentified fungi (1 OTU) of the fungal phyla; Ecdysozoa (1 OTU), belonging to Metazoa; and Streptophyta (2 OTU) and Chlorophyta (1 OTU), belonging to Viridiplantae (Table 6). Several OTUs that were abundant in S-soil from more than two fields were identified as BCA in the previous study. Plectosphaerella, Epicoccum, Drechslerella, and Clonostachys were identified as BCA for cyst nematodes, the brown rot of peach, reniform nematodes, and damping off of carrot caused by Alternaria, respectively (1, 5, 20, 26). In a previous study, Glomus versiforme, Pythium oligandrum, Gigaspora margarita, G. mosseae, Scutellospora sp., and the lichen Parmotrema tinctorum were identified as BCA against R. solanacearum, and Trichoderma, Gliocladium, Penicillium, and Acremonium spp. are also well-known BCA species for several plant diseases (45, 51). However, these eukaryotes were not found in the present study. Fusarium tricinctum was commonly abundant in S-soil in all fields, and members of the genus of Fusarium are well-known plant pathogens (Table 6). Pratylenchus members are well-known root-lesion nematodes for banana, and plant parasitic nematodes appear to increase the severity of bacterial wilt (10, 42). However, no disease symptoms were observed on tomato plants in S-soil in the fields in spite of the detection of these fungal pathogens.
| Detected field | OTU name | Ha-field | Ka-field | Wa-field | Kingdom | Division | Closest relatives | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
| ||||||||
| Up | Low | Up | Low | Up | Low | |||||
| All | OTU28 | + | + | + | Fungi | Ascomycota | Fusarium tricinctum | |||
|
| ||||||||||
| Ha & Ka | OTU40 | + | + | + | Viridiplantae | Chlorophyta | Chlamydomonas sphagnophila | |||
| OTU989 | + | + | + | Fungi | Ascomycota | Sporendonema purpurascens strain KACC 41227 | ||||
| OTU514 | + | + | + | Fungi | Basidiomycota | Trichosporon sp. ‘shinodae’ culture-collection CBS:9979 | ||||
| OTU307 | + | + | + | Fungi | Basidiomycota | Trichosporon sp. ‘shinodae’ culture-collection CBS:9979 | ||||
| OTU286 | + | + | + | Fungi | Mucoromycota | Umbelopsis vinacea strain CBS 236.82 | ||||
| OTU882 | + | + | + | Fungi | Mucoromycota | Cunninghamella elegans isolate CFR-C10 | ||||
| OTU267 | + | + | Fungi | Ascomycota | Pseudospiromastix tentaculata CBS 184.92 | |||||
| OTU505 | + | + | Fungi | Ascomycota | Epicoccum nigrum strain Zbf-S21 | |||||
| OTU437 | + | + | Fungi | Ascomycota | Zopfiellasp. TMS-2011 voucher BGd10p15-14 | |||||
| OTU278 | + | + | Fungi | Basidiomycota | Coprinopsis semitalis strain CBS291.77 | |||||
| OTU298 | + | + | Fungi | Ascomycota | Drechslerella dactyloides strain 03CQ1-7 | |||||
|
| ||||||||||
| HA & Wa | OTU68 | + | + | + | + | Fungi | Ascomycota | Lecanicillium aphanocladii | ||
| OTU156 | + | + | + | + | Viridiplantae | Streptophyta | Imbribryum blandum | |||
| OTU3 | + | + | + | Viridiplantae | Streptophyta | Imbribryum blandum | ||||
| OTU825 | + | + | + | Fungi | Unidentified FungiEctomycorrhizal fungi isolate ECM 289 | |||||
| OTU795 | + | + | Fungi | Ascomycota | Geomyces sp. 23WI14 1 | |||||
| OTU757 | + | + | + | Fungi | Ascomycota | Plectosphaerella cucumerina isolate Raph-3 | ||||
| OTU152 | + | + | + | Fungi | Ascomycota | Plectosphaerella cucumerina isolate Raph-3 | ||||
| OTU79 | + | + | + | Fungi | Ascomycota | Fusarium sp. strain SAR19 | ||||
| OTU680 | + | + | Metazoa | Ecdysozoa | Pratylenchus goodeyi isolate PgoKL4 clone 4 | |||||
|
| ||||||||||
| Ka & Wa | OTU33 | + | + | Fungi | Ascomycota | Clonostachys rosea | ||||
In the present study, we compared prokaryotic and eukaryotic communities in soil that had or lacked bacterial wilt, and commonly detected specific microbes in S-soil in three different fields. Neither soil prokaryotic and eukaryotic diversity nor richness correlated with the presence or absence of bacterial wilt symptoms. Prokaryotic and eukaryotic community structures were affected more by regional differences than the appearance of disease. A total of 504 and 125 OTUs were more abundant in S-soil for prokaryotes and eukaryotes, respectively. Some OTUs were commonly detected in S-soil in more than two fields, and some of them were well-known plant pathogens. However, since the signs of plant disease were not observed in S-soil, several prokaryotes and eukaryotes may control plant disease symptoms. Future studies are needed in order to elucidate the roles and effectiveness of these microbes as well as the relationship between soil suppressiveness and soil microbes or soil chemical and physical properties.
This work was supported by Cabinet Office, Government of Japan, Cross-ministerial Strategic Innovation Promotion Program (SIP), “Technologies for creating next-generation agriculture, forestry and fisheries” (funding agency: Bio-oriented Technology Research Advancement Institution, NARO). This work was also partially supported by the RIKEN Competitive Program for Creative Science and Technology, a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science, No. 16K14797 (to M.O.), and by the Sasakawa Scientific Research Grant from The Japan Science Society (to C.G.L.).