2022 年 37 巻 3 号 論文ID: ME22042
2022 年 37 巻 3 号 論文ID: ME22042
Many stinkbugs in the superfamily Coreoidea (Hemiptera: Heteroptera) develop crypts in the posterior midgut, harboring Caballeronia (Burkholderia) symbionts. These symbionts form a monophyletic group in Burkholderia sensu lato, called the “stinkbug-associated beneficial and environmental (SBE)” group, recently reclassified as the new genus Caballeronia. SBE symbionts are separated into the subclades SBE-α and SBE-β. Previous studies suggested a regional effect on the symbiont infection pattern; Japanese and American bug species are more likely to be associated with SBE-α, while European bug species are almost exclusively associated with SBE-β. However, since only a few insect species have been investigated, it remains unclear whether region-specific infection is general. We herein investigated Caballeronia gut symbionts in diverse Japanese, European, and North American populations of a cosmopolitan species, the Western conifer seed bug Leptoglossus occidentalis (Coreoidea: Coreidae). A molecular phylogenetic analysis of the 16S rRNA gene demonstrated that SBE-β was the most dominant in all populations. Notably, SBE-α was rarely detected in any region, while a third clade, the “Coreoidea clade” occupied one fourth of the tested populations. Although aposymbiotic bugs showed high mortality, SBE-α- and SBE-β-inoculated insects both showed high survival rates; however, a competition assay demonstrated that SBE-β outcompeted SBE-α in the midgut crypts of L. occidentalis. These results strongly suggest that symbiont specificity in the Leptoglossus-Caballeronia symbiotic association is influenced by the host rather than geography, while the geographic distribution of symbionts may be more important in other bugs.
Recent studies revealed that insect gut microorganisms play a pivotal role in the evolution and environmental adaptation of insects. Gut microorganisms provide essential nutrients, digest indigestible food materials, and/or degrade phytotoxins and insecticides (Kikuchi et al., 2012; Engel and Moran, 2013; Salem et al., 2014; Sudakaran et al., 2017; Itoh et al., 2018; Moran et al., 2019). Many species of stinkbugs in the superfamily Coreoidea (Hemiptera: Heteroptera) develop numerous crypts at the posterior part of the midgut, wherein specific Caballeronia symbionts (previously included in the genus Burkholderia) densely proliferate - generally as a single species - until almost full occupation of the luminal space (Kikuchi et al., 2005, 2011a; Olivier-Espejel et al., 2011; Garcia et al., 2014; Kuechler et al., 2016; Takeshita and Kikuchi, 2017; Ohbayashi et al., 2019b; Acevedo et al., 2021; Hunter et al., 2022). Caballeronia gut symbionts play important roles in their hosts, such as the recycling of metabolic waste materials and providing essential amino acids and vitamins, thereby enhancing the growth and fecundity of stinkbugs (Kikuchi et al., 2007; Kikuchi and Fukatsu, 2014; Ohbayashi et al., 2019a). A particularity of this mono-species symbiosis is the horizontal transmission of symbionts. Hatchlings are symbiont-free and acquire Caballeronia symbionts from soil during the early instar stages (Kikuchi et al., 2007, 2011b; Ohbayashi et al., 2019b). This implies not only that insects depend on efficient and stringent selection mechanisms to sort environmental bacteria in order to give access to Caballeronia symbionts only (Ohbayashi et al., 2015; Itoh et al., 2019; Kikuchi et al., 2020), but also that the geographic distribution of symbiont species may be a factor influencing the outcome of symbiosis (Ohbayashi et al., 2019b).
The genus Burkholderia was initially separated from Pseudomonas Group II in 1992 (Yabuuchi et al., 1992) into a heterogeneous taxonomic group of more than 100 bacterial species (Eberl and Vandamme, 2016). In a recent reclassification of this Burkholderia “sensu lato” taxonomic group, six new genera (Paraburkholderia, Caballeronia, Robbsia, Mycetohabitans, Pararobbsia, and Trinickia) have been proposed next to the genus Burkholderia sensu strico (Sawana et al., 2014; Dobritsa and Samadpour, 2016; Beukes et al., 2017; Lopes-Santos et al., 2017; Estrada-de Los Santos et al., 2018; Lin et al., 2020). Caballeronia is also called the Stinkbug-associated Beneficial and Environmental (SBE) group of Burkholderia, which is divided into two subgroups, group-α (SBE-α) and group-β (SBE-β).
A previous survey of Caballeronia symbionts in stinkbugs revealed region-specific infection with species of the two subgroups: SBE-α was more likely to be detected in Japanese and American stinkbug species of Coreoidea (Kikuchi et al., 2005, 2011b; Olivier-Espejel et al., 2011; Garcia et al., 2014; Kuechler et al., 2016; Ohbayashi et al., 2019b; Acevedo et al., 2021; Hunter et al., 2022), and SBE-β in European species of Coreoidea (Kuechler et al., 2016; Ohbayashi et al., 2019b). However, since only a few insect species have been investigated, and even fewer species from a wide geographic distribution, it remains unclear whether region-specific infection is general. The mechanisms underlying region-specific infection have not yet been elucidated, but may be influenced by the region-dependent composition of the soil microbiota.
The Western conifer seed bug Leptoglossus occidentalis (Coreoidea: Coreidae) (Fig. 1A), a notorious pest of conifer forests (Lesieur et al., 2014), originates from North America (Heidemann, 1910; Koerber, 1963). This stinkbug has become a serious invading pest worldwide. In 1999, L. occidentalis was found in Europe for the first time in Italy (Taylor et al., 2001) and its population has been rapidly expanding in recent years throughout Europe (Dusoulier et al., 2007; Malumphy et al., 2008; Fent and Kment, 2011; Gapon, 2013; Lesieur et al., 2018; van der Heyden, 2019) and other more distant regions, such as North Africa (Ben Jamaa et al., 2013; Gapon, 2015). It has also spread to South America (Faúndez et al., 2017) and Asia (Ishikawa and Kikuhara, 2009; Ahn et al., 2013). In Japan, L. occidentalis was initially collected in Tokyo in 2008 (Ishikawa and Kikuhara, 2009) and, similar to Europe, it has since rapidly spread to almost all areas of Japan, including the Tohoku and Kyushu districts (Tsuru et al., 2020). Therefore, this cosmopolitan species is ideal for clarifying regional effects on the symbiotic association. In the present study, we investigated the diversity of Caballeronia symbionts of L. occidentalis collected in Japan, North America, and Europe in order to confirm whether the geographical origin affects the gut symbionts of the conifer bug. Furthermore, symbiont inoculation tests of insects reared in the laboratory with SBE-α and SBE-β symbionts were conducted to analyze the gut colonization ability and fitness effects of these two Caballeronia subgroups on the host insect.

The conifer bug Leptoglossus occidentalis and its midgut structure. (A) An adult of L. occidentalis on the leaves of a pine tree and (B) its whole midgut structure. The bug shown was reared in the laboratory. Symbiont inoculation was performed by feeding the insect a soil suspension. A symbiont native in the soil colonizes the midgut crypts (M4). Abbreviations of the midgut sections are as follows: M1, midgut first section; M2, midgut second section; M3, midgut third section; M4, midgut fourth section with crypts; M4B, M4 bulb; H, hindgut.
Samples of L. occidentalis used in the present study are listed in Table 1. Regarding bacterial inoculation tests, L. occidentalis was collected in Gif-sur-Yvette, France in 2018 and maintained in cages by feeding on pignolia nuts and distilled water containing 0.05% ascorbic acid (DWA) at 25°C under a long-day regime (16 h light, 8 h dark).
| Country | State/Prefecture | Locality | Collection year | Collector | Symbiont detection | Specimens number | Symbiont isolates/ clones number |
16S rRNA Accession number |
|---|---|---|---|---|---|---|---|---|
| Japan | Kumamoto | Koshi | March, 2021 | R. Hara, K. Matsunaga | Isolating | 3 | 3 | LC713090–LC713092 |
| Yamagata | Yuza | April, 2021 | Y. Hatanaka | Isolating | 4 | 4 | LC713093–LC713096 | |
| Akita | Akita | October, 2018 | K. Takeshita | Isolating | 2 | 2 | LC713097–LC713098 | |
| Akita | Akita | April, 2021 | S. Noriyuki | Isolating | 4 | 4 | LC713099–LC713102 | |
| France | Essonne | Gif-sur-Yvettea | November, 2016 | P. Mergaert | Isolating | 3 | 8 | LC713103–LC713110 |
| Essonne | Gif-sur-Yvettea | November, 2021 | G. Lextrait | Isolating | 6 | 22 | LC713111–LC713132 | |
| Italy | Piedmont | Alessandria | October 2020 | S. Chiesa | Cloning | 2 | 6 | LC713133–LC713138 |
| Spain | Catalonia | Artes | April 2021 | S. Lopez Romero | Cloning | 3 | 12 | LC713139–LC713150 |
| USA | Idaho | Lenore | October 2020 | S. Cook | Cloning | 4 | 31 | LC713151–LC713181 |
| Canada | Nova Scotia | Vaughan | October 2020 | S. Blatt | Cloning | 4 | 13 | LC713182–LC713194 |
| British Col. | Vernon | October 2020 | W. Strong | Cloning | 4 | 15 | LC713195–LC713209 |
a The same collection site
Reared insects were used for inoculation tests with one SBE-α strain and one SBE-β strain. We used strain RPE225 (Kikuchi and Fukatsu, 2014), a green fluorescent protein (GFP)-labeled derivative of B. insecticola (Caballeronia insecticola) strain RPE64, as a typical strain of the SBE-α clade. RPE64 was isolated from the midgut crypts of a Japanese specimen of the bean bug Riptortus pedestris (Coreoidea: Alydidae) (Takeshita et al., 2018). Regarding the SBE-β strain, we selected Caballeronia sp. strain 1876, which was isolated from the midgut crypts of L. occidentalis collected in Gif-sur-Yvette, France in 2016. A GFP-labeled derivative of this SBE-β strain, labeled strain 2482, was constructed by a mini-Tn7 transposon delivery system as previously reported (Kikuchi and Fukatsu, 2014). Insect inoculation tests with these two GFP strains were performed as previously described for other stinkbug species (Ohbayashi et al., 2015; Ohbayashi et al., 2019b). Briefly, the two bacterial strains were pre-cultured in yeast extract and glucose (YG) medium (yeast extract 5.0 g L–1, glucose 4.0 g L–1, and NaCl 1.0 g L–1) containing rifampicin 30 μg mL–1 at 28°C overnight at 180 rpm, and 200 μL of the overnight culture was inoculated into 5 mL fresh YG, incubated at 28°C at 180 rpm for 2 h, and finally diluted to 107 CFU mL–1 in DWA. The cotton pad with DWA was removed from the rearing container with 2nd instar L. occidentalis nymphs, and nymphs were maintained overnight without water to make them thirsty. Symbiont suspensions, prepared as described above, were poured onto new cotton pads and placed into rearing containers. Aposymbiotic nymphs were obtained by placing a cotton pad soaked with DWA only. Containers were maintained as described above until later analyses.
Fluorescence microscopy observationsThe infection status of the inoculated nymphs was confirmed based on the detection of GFP signals in the dissected midgut crypts of third instar nymphs. Dissections were performed in phosphate-buffered saline (PBS) using fine forceps and scissors under a fluorescent binocular microscope (Leica, MZ FZ III). Pictures of the dissected midguts were taken by a digital camera (Leica, EC3).
Insect survivalThe survival rate of aposymbiotic insects and symbiotic insects infected with either SBE-α strain RPE225 or SBE-β strain 2482 was estimated by regularly observing insect rearing populations (n=27, 13 or 20 insects, respectively) over time until the last adult emergence in the surviving insects. At each observation, the number of alive and dead insects was scored, as well as the number of emerged adults. The survival rate was analyzed by Fisher’s exact test with the Bonferroni correction. The developmental time until adulthood in the aposymbiotic insect sample was removed from the statistical analysis due to a single surviving insect (n=1), and those in SBE-α and SBE-β inoculated insects were analyzed by the Student’s t-test.
Competition assayIn the competition assay, we used red fluorescent protein (RFP) strain RPE525 (SBE-α), a derivative of C. insecticola strain RPE64 (Itoh et al., 2019) and GFP strain 2482 (SBE-β). Exponential phase cells were suspended in DWA and an inoculum containing 107 CFU mL–1 of both strains was prepared from them. The inoculation of insects with the mixed inoculum was performed as described above. At 7 days post inoculation, when insects became 3rd instar nymphs, midgut crypts were dissected as described above. In the microscopy analysis, midgut crypts were observed under a fluorescent microscope (Nikon, Eclipse 80i). Regarding the quantitative assessment of the two strains, midgut crypts were collected in 100 μL of PBS buffer in a 1.5-mL tube and homogenized by a sterilized pestle. The pestle was washed by 400 μL of PBS buffer. The relative number of symbiont cells of GFP and RFP bacteria in the extracts of the midgut crypts and in the bacterial suspension of the inoculum were analyzed by flow cytometry (Beckman Coulter, Cytoflex).
Identification of gut symbionts of L. occidentalisGut symbionts were isolated from the midgut crypts of L. occidentalis individuals collected in Japan and France (Table 1 and Fig. 1) by plating crypt contents on YG agar plates. Growing bacteria were identified by direct sequencing of the 16S rRNA gene, as previously described (Kikuchi et al., 2011a). Since conifer bugs captured in Italy, Spain, USA, and Canada were preserved in 100% ethanol, their dissected midgut crypts were subjected to DNA extraction and a clone library analysis of the 16S rRNA gene, as previously reported (Ohbayashi et al., 2019b). Sequences obtained by the bacterial isolation and clone analysis were assembled by ATSQ software ver. 5.2 (Software Development), followed by manual corrections. The most similar bacterial species/strains were identified by BLAST comparisons. Sequences showing more than 99% identity were assigned to the same operational taxonomical unit (OTU).
Molecular phylogenetic analysis of L. occidentalis gut symbiontsA multiple sequence alignment of the 16S rRNA gene was constructed with MAFFT on the EMBL-EBI server (Li et al., 2015). A molecular phylogenetic tree was generated by the maximum likelihood (ML) method with the removal of gap-including and ambiguous sites and with a bootstrap analysis (1,000 replicates) in MEGA software version 10.1.8 (Kumar et al., 2018; Stecher et al., 2020). We selected the Tamura-Nei model of nucleotide substitutions with gamma distributed and invariant sites (G+I) (Tamura and Nei, 1993).
Nucleotide sequence accession numbersThe nucleotide sequence data of the 16S rRNA gene obtained in the present study have been deposited in the DDBJ/EMBL/GenBank public databases with the accession numbers LC713090–LC713209 (Table 1).
To investigate the diversity of gut symbionts in the conifer bug L. occidentalis, two methods were used depending on the nature of the insect sample. In the bacterial isolation method, 43 symbionts were isolated from the midgut crypts of 22 individuals collected from 4 and 2 populations of Japan and France, respectively (Table 1). In the clone library analysis of the 16S rRNA gene, 17 specimens collected in Italy, Spain, USA, and Canada were used, from which 77 sequences were obtained (Table 1). The 120 assembled sequences were assigned to 11 OTUs based on the 99% sequence identity threshold (Table S1). Seven OTUs (OTU1–OTU7) were identified as members of Caballeronia by a BLAST search. OTU2 and OTU7 represented 69% and 22%, respectively, of the 110 sequences identified as Caballeronia, indicating that OTU2 and OTU7 are the main gut symbionts of L. occidentalis. These dominant OTUs were detected in both the bacterial isolation and cloning methods, which suggests no method-related bias in the identification of symbionts. The remaining four OTUs (OTU8–OTU11), detected only in four individuals of two insect populations, were identified as Rickettsia spp., which is a well-known intracellular secondary symbiont of diverse insects (Kikuchi, 2009). The insect specimen in which Rickettsia clones were identified, yielded a majority of Caballeronia clones (Table S1), indicating that these individuals were also colonized with Caballeronia symbionts.
Phylogenetic placement of Caballeronia symbiontsWe performed a molecular phylogenetic analysis based on the 16S rRNA gene, including sequences of the seven Caballeronia OTUs of L. occidentalis gut symbionts, type strains of Burkholderia sensu lato species (Burkholderia sensu strico, Paraburkholderia, and Caballeronia species), and previously reported Caballeronia gut symbionts of various coreoid insects. Based on phylogenetic divergence, four subclades were identified within the Caballeronia clade: SBE-α, SBE-β, SBE-γ, and Coreoidea clade (Fig. 2). The most dominant OTU2, detected in specimens collected in all countries, was located in SBE-β. SBE-β contained four other OTUs (OTU3, OTU4, OTU5, and OTU6) in addition to OTU2 (Fig. 2). On the other hand, the second most dominant OTU7 was placed in the Coreoidea clade (Fig. 2). This subclade contained neither environmental isolates nor type species, but formed a monophyletic group with many gut symbionts of Japanese and European coreoid bugs (Fig. 2), suggesting their very specialized nature for symbiosis with stinkbug species. OTU1 was located in the SBE-α clade, known as major gut symbionts of Japanese and American coreoid stinkbugs (Fig. 2 and S1).

Molecular phylogenetic analysis of Caballeronia gut symbionts of the conifer bug Leptoglossus occidentalis. A maximum-likelihood tree was generated based on 1,256 aligned nucleotide sites of the 16S rRNA gene. Numbers at the tree nodes indicate the maximum-likelihood bootstrap values (%) with 1,000 replicates, and bootstrap values of more than 50 are shown. We referred to the nucleotide sequence information reported in previous studies on the Caballeronia gut symbionts of coreoid insects in Japan (Kikuchi et al., 2011a; Kuechler et al., 2016; Ohbayashi et al., 2019b), in America (Olivier-Espejel et al., 2011; Garcia et al., 2014; Acevedo et al., 2021; Hunter et al., 2022), and in Europe (Kuechler et al., 2016; Ohbayashi et al., 2019b). The subtree of the SBE-α group is compressed. An uncompressed subtree is shown in Fig. S1. Accession numbers in the DNA database (DDBJ/EMBL/GenBank) are shown in square brackets. L. occidentalis gut symbionts are shown in blue with bold case font. Stars: bacterial strains used for symbiont inoculation tests. GS: Gut symbiont.
The detection rates of the three subclades (SBE-α, SBE-β, and the Coreoidea clade) in the world’s populations of conifer bugs are summarized in Fig. 3 and S2 (also see Table S2), which are based on the phylogenetic placement of seven OTUs in the subclades of Caballeronia (Fig. 2). Overall, conifer bugs were almost exclusively associated with SBE-β, while SBE-α was rarely detected (Fig. 3). The Coreoidea clade occupied one fourth of the populations and was frequently detected in the Japanese and European populations, but not in the North American populations of conifer bugs (Fig. 3).

Relative abundance of SBE-α, SBE-β, and Coreoidea clade bacteria among gut symbionts of conifer bugs normalized by one OTU by an individual at the country level. The number of investigated insects in each country is shown in the graphs, and the precise numbers are provided in Table S1 and S2. Relative abundance at the local level is shown in Fig. S2.
SBE-α species have consistently been found in the midgut crypts of 33 stinkbug species of the superfamily Coreoidea (Kikuchi et al., 2011a, 2005; Olivier-Espejel et al., 2011; Garcia et al., 2014; Kuechler et al., 2016; Ohbayashi et al., 2019b; Acevedo et al., 2021; Hunter et al., 2022). However, in the present study, only one out of 120 symbiont isolates or clones from 39 specimens of L. occidentalis were SBE-α (Fig. 3 and Table S1). To confirm whether SBE-α and SBE-β symbionts are capable of colonizing the midgut crypts of the conifer bug, the GFP-labeled strains, C. insecticola strain RPE225 (SBE-α) and Caballeronia sp. strain 2482 (SBE-β) were inoculated into nymphs of L. occidentalis. The SBE-α and SBE-β strains were both capable of colonizing the midgut crypts of L. occidentalis (Fig. 4A, B, D, and E), as indicated by enlarged crypts and the presence of a GFP signal. Moreover, the swollen M4B midgut region, typical for symbiont colonization, also confirmed proper colonization by both strains (Fig. 4A and B). On the other hand, aposymbiotic (uninfected) insects showed small M4 crypts and a narrow M4B region (Fig. 4C and F).
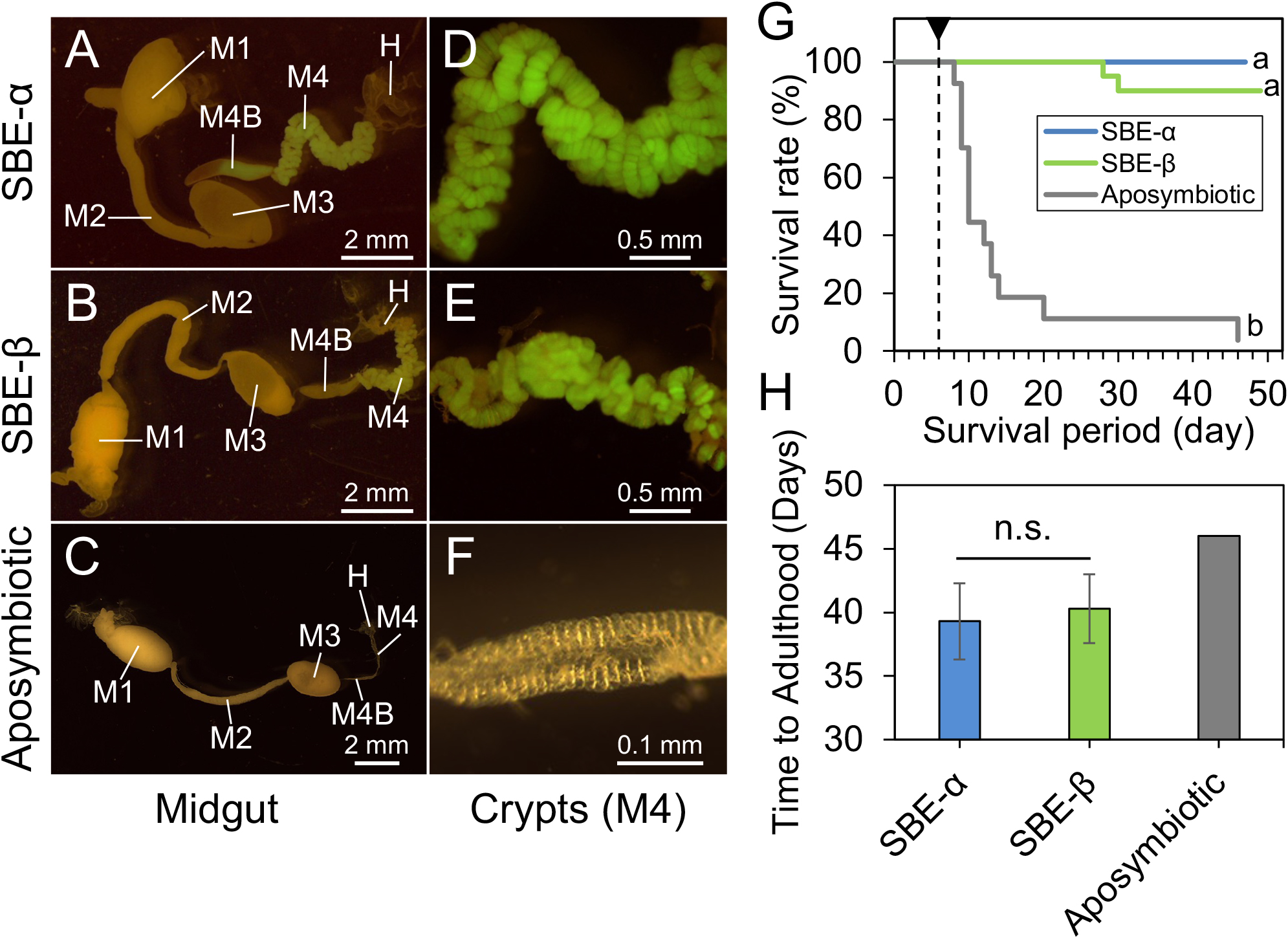
Leptoglossus occidentalis infection with SBE-α and SBE-β strains and their host fitness effects. (A, B, and C) Whole midguts of L. occidentalis at the 3rd instar stage, and (D, E, and F) an enlarged image of midgut crypts. A dissected midgut inoculated with (A and D) an SBE-α GFP strain, with (B and E) an SBE-β GFP strain, and without (C and F) any inoculant (aposymbiotic). An abbreviation of the midgut section is as shown in Fig. 1. (G) Survival rates of L. occidentalis inoculated with SBE-α (blue line, n=13) and SBE-β (green, n=20), and without any inoculant (aposymbiotic: gray, n=27). The survival period was followed from hatching to the last adult emergence. A black arrow and dotted line indicate symbiont infections at 6 days post-hatching. Different letters indicate significant differences (P<0.0001, Fisher’s exact test with the Bonferroni correction). (H) Developmental time from hatching to adulthood in conifer bugs inoculated with SBE-α (blue bar, n=13) and SBE-β (green, n=18), and without any inoculant (aposymbiotic: gray line, n=1, i.e. the only surviving insect in the experiment). The mean±SD is shown. n.s. indicates no significant difference (Student’s t-test).
We then investigated the survival of aposymbiotic and SBE-α- or SBE-β-inoculated conifer bugs. Aposymbiotic bugs showed high mortality during their development (Fig. 4G). The majority of insects died during the 2nd to 3rd instar (Fig. 4G). In contrast, most nymphs inoculated with SBE-α or SBE-β strains survived and reached adulthood (Fig. 4G; survival percentage [adult/total investigated insects]=3.7% [1/27] in aposymbiotic insects; 100% [13/13] in SBE-α-inoculated insects; 90% [18/20] in SBE-β-inoculated insects). The developmental times from hatching to adult emergence were 46, 39±3, and 40±3 days (mean±SD) in aposymbiotic, SBE-α-, and SBE-β-inoculated L. occidentalis, respectively (Fig. 4H). Survival rates significantly differed between symbiotic (inoculated with SBE-α or SBE-β) versus aposymbiotic insects, while no significant differences were observed in survival rates or developmental times between SBE-α- and SBE-β-inoculated L. occidentalis (Fig. 4G and H).
On the other hand, a competition assay, in which nymphs were inoculated with an equal mixture of the SBE-α and SBE-β strains, demonstrated that SBE-β significantly outcompeted SBE-α in the midgut crypts of L. occidentalis (Fig. 5). Collectively, these results strongly suggest that the Caballeronia symbiont makes a very large positive contribution to the survival and development of L. occidentalis, and that SBE-α has a sufficient ability to colonize midgut crypts to give the same fitness effects to L. occidentalis as SBE-β. Nevertheless, microbe-microbe competition in the midgut crypts of L. occidentalis may contribute to the observed predominance of SBE-β.

Competition assay of RFP-labeled SBE-α and GFP-labeled SBE-β strains in midgut crypts of Leptoglossus occidentalis. (A) The midgut crypts of L. occidentalis at 7 days post-infection (dpi), infected with an equal mixture of both strains. A merged GFP and RFP image is shown. (B) Relative abundance, determined by flow cytometry, of GFP and RFP strains at the inoculum and at the midgut crypts at 7 dpi (n=10). The abundance of GFP and RFP strains at the midgut crypts is significantly different (P<1×10–10, the Student’s t-test). Fluorescent microscopic images and the relative abundance of GFP and RFP strains in 10 individual insects are shown in Fig. S3. Note that both strains resulted in 100% infection when used in control mono-infections (Fig. S4).
The present study revealed that although conifer bug specimens are associated with genetically diverse Caballeronia (SBE-Burkholderia) symbionts, members of the subclade SBE-β were dominant in all of the investigated Japanese, European, and North American populations of the conifer bug, and the Coreoidea clade was also frequently found in Japanese and European insects (Fig. 2 and 3, Table S1 and S2). Previous studies reported that Japanese and American species of the stinkbug superfamily Coreoidea were more likely to harbor symbionts belonging to the SBE-α subclade (Kikuchi et al., 2011a; Ohbayashi et al., 2019b); however, we herein demonstrated that SBE-α was rarely detected in the conifer bug, even in Japanese and American populations (Fig. 3, Table S1 and S2). Based on this broad survey of this cosmopolitan species, we concluded that infection was not affected by geographic origins; therefore, it is more likely to be influenced by selection mechanisms in the host insect.
Experimental inoculation tests revealed no significant differences in colonization ability or fitness effects on the host bug between SBE-α and SBE-β symbionts (Fig. 4). However, the competition assay showed that SBE-β outcompeted SBE-α in the midgut crypts of L. occidentalis, possibly resulting in the low detection rate of SBE-α in the conifer bug (Fig. 3, Table S1 and S2). Infection specificity between the bean bug host and Caballeronia symbiont was demonstrated to be largely influenced by the native symbiont’s colonization competitiveness in the midgut. In midgut crypts in co-infection experiments, Caballeronia symbionts always outcompeted the non-symbiont species Paraburkholderia and Pandoraea, which were fully capable of colonizing crypts in the absence of competition species (Itoh et al., 2019). The present study is the first to report competition-based selection in the stinkbug midgut between species of different SBE clades. Since competition-based mechanisms have not yet been elucidated in detail, further studies are warranted.
Among the Caballeronia gut symbionts of L. occidentalis, the second most dominant OTU7 formed a monophyletic group, the Coreoidea clade, with gut symbionts of other Coreoid species, including Cletus rusticus, Plinachtus bicoloripes, Hygia lativentris, Molipteryx fuliginosa, Acanthocoris sordidus (Kikuchi et al., 2011a), Coreus marginatus (Ohbayashi et al., 2019b), and Dicranocephalus albipes (Kuechler et al., 2016). The Coreoidea clade does not include environmental isolates/clones or any named species of Caballeronia, it only consists of gut symbionts of Coreoidea (Fig. 2), strongly suggesting that these symbiont strains are highly specific to the insect group. To date, two strains—one from A. sordidus and the other the OTU7 clone from L. occidentalis described herein—have been isolated as culturable symbionts of this clade. Further studies are needed to reveal the future genomic and physiological features of these Coreoidea clade symbionts in order to clarify why these Coreoidea clade members are specific to this insect group. The intercontinental infection pattern of the Coreoidea clade (Fig. 3) is notable from the viewpoint of evolution. Since L. occidentalis originated from North America (Heidemann, 1910; Koerber, 1963) and recently invaded European and Asian countries, we speculate that L. occidentalis was originally associated with SBE-β and may have become symbiotic with the Coreoidea clade as its distribution expanded.
Caballeronia symbionts make a very large positive contribution to the fitness of the conifer bug (Fig. 4G), similar to that reported for other coreoid stinkbugs, including C. marginatus, L. zonatus, L. phyllopus, and R. pedestris (Kikuchi et al., 2007, 2011a; Ohbayashi et al., 2019b; Hunter et al., 2022). In R. pedestris, our previous transcriptomic study revealed that Caballeronia provided the host with essential amino acids and vitamins by recycling host metabolic waste materials, such as sulfate, allantoin, and urea (Ohbayashi et al., 2019a). These findings suggest that Caballeronia symbionts critically complement these essential nutrients lacking in conifer seeds in L. occidentalis, resulting in high mortality in aposymbiotic nymphs in L. occidentalis. In contrast, aposymbiotic insects in R. pedestris showed retarded growth, a smaller body size, and lower fecundity than symbiotic insects, although these aposymbiotic nymphs were nevertheless able to develop to adulthood with a high survival rate (Kikuchi et al., 2007; Kikuchi and Fukatsu, 2014). Feeding on soybean seeds with high nutritional value may provide sufficient, albeit non-optimal, nutrition for the development and survival of aposymbiotic R. pedestris. Caballeronia symbionts may play more important metabolic roles for hosts that are feeding on nutritionally poor non-leguminous plants, such as L. occidentalis.
Rickettsia was detected in the American and Canadian populations of L. occidentalis (Table S1). Although some members are human pathogens, most members of the genus Rickettsia are facultative intracellular symbionts in many arthropods (Perlman et al., 2006), and these symbionts are maintained by vertical transmission. Since Rickettsia is a reproductive manipulator that causes male-killing and parthenogenesis in many insects (Perlman et al., 2006), a similar function needs to be considered in the North America populations of the conifer seed bug. The bacterial group has rarely been detected from stinkbug species, except for some species of Miridae (Chang and Musgrave, 1970; Caspi-Fluger et al., 2012; Dally et al., 2020), and a broader survey is required to clarify the prevalence of Rickettsia in heteropteran insects.
The present study on L. occidentalis is the first to demonstrate that the specificity of the Caballeronia symbiont is influenced by host species rather than biogeography. The symbiont’s competitiveness in the gut symbiotic organ appears to be pivotal for specificity. Additionally, differences in fitness effects on host bugs between species, as shown in L. zonatus and L. phyllopus (Hunter et al., 2022), may be involved in specificity. To elucidate the mechanisms underlying host-symbiont specificity in stinkbugs in more detail, the following two points need to be clarified in the future. The worldwide distribution of Caballeronia in soil needs to be analyzed because microbial geography is critical in animals that are closely associated with environmentally transmitted beneficial microorganisms. In addition, further experimental inoculation assays, particularly in vivo competition assays, are crucial for confirming and understanding the mechanisms underlying host-microbe specificity in each stinkbug species.
Ohbayashi, T., Cossard, R., Lextrait, G., Hosokawa, T., Lesieur, V., Takeshita, K., et al. (2022) Intercontinental Diversity of Caballeronia Gut Symbionts in the Conifer Pest Bug Leptoglossus occidentalis. Microbes Environ 37: ME22042.
https://doi.org/10.1264/jsme2.ME22042
We thank R. Hara, K. Matsunaga, Y. Hatanaka, S. Noriyuki, S. Chiesa, S. Lopez Romero, S. Cook, S. Blatt, A. Ravenscraft, M. Hunter, and W. Strong for insect sampling, and J. Lachat and A. Yokota for their assistance with insect rearing. The present study has benefited from the Imagerie-Gif core facility supported by l’Agence Nationale de la Recherche (ANR-11-EQPX-0029/Morphoscope, ANR-10-INBS-04/FranceBioImaging; ANR-11-IDEX-0003-02/Saclay Plant Sciences). This study was supported by a JSPS-CNRS Bilateral Open Partnership Joint Research Project and the CNRS International Research Project “Ménage à Trois” to YK and PM, by the French national research agency (ANR) grant ANR-19-CE20-0007 and a Saclay Plant Sciences research grant to PM, and by the JSPS Research Fellowship for Young Scientists (20170267 and 19J01106) to TO, by the MEXT KAKENHI (18KK0211 to YK and TH; 20H03303 to YK and K. Takeshita), and the Moonshot project JPNP18016, commissioned by the New Energy and Industrial Technology Development Organization (NEDO), to TO and K. Tago.