2022 年 37 巻 4 号 論文ID: ME22078
2022 年 37 巻 4 号 論文ID: ME22078
Psyllids (Hemiptera: Sternorrhyncha: Psylloidea) are plant sap-sucking insects that include important agricultural pests. To obtain insights into the ecological and evolutionary behaviors of microbes, including plant pathogens, in Psylloidea, high-resolution analyses of the microbiomes of nine psyllid species belonging to the family Triozidae were performed using high-throughput amplicon sequencing of the 16S rRNA gene. Analyses identified various bacterial populations, showing that all nine psyllids have at least one secondary symbiont, along with the primary symbiont “Candidatus Carsonella ruddii” (Gammaproteobacteria: Oceanospirillales: Halomonadaceae). The majority of the secondary symbionts were gammaproteobacteria, particularly those of the order Enterobacterales, which included Arsenophonus and Serratia symbiotica, a bacterium formerly recognized only as a secondary symbiont of aphids (Hemiptera: Sternorrhyncha: Aphidoidea). The non-Enterobacterales gammaproteobacteria identified in the present study were Diplorickettsia (Diplorickettsiales: Diplorickettsiaceae), a potential human pathogen, and Carnimonas (Oceanospirillales: Halomonadaceae), a lineage detected for the first time in Psylloidea. Regarding alphaproteobacteria, the potential plant pathogen “Ca. Liberibacter europaeus” (Rhizobiales: Rhizobiaceae) was detected for the first time in Epitrioza yasumatsui, which feeds on the Japanese silverberry Elaeagnus umbellata (Elaeagnaceae), an aggressive invasive plant in the United States and Europe. Besides the detection of Wolbachia (Rickettsiales: Anaplasmataceae) of supergroup B in three psyllid species, a lineage belonging to supergroup O was identified for the first time in Psylloidea. These results suggest the rampant transfer of bacterial symbionts among animals and plants, thereby providing deeper insights into the evolution of interkingdom interactions among multicellular organisms and bacteria, which will facilitate the control of pest psyllids.
Psyllids (Hemiptera: Sternorrhyncha: Psylloidea) are plant sap-sucking insects comprising approximately 4,000 described species worldwide (Burckhardt et al., 2021). Since they feed exclusively on phloem sap, some species transmit plant pathogens, including “Candidatus Liberibacter spp.” (Alphaproteobacteria: Rhizobiales), making them notorious agricultural pests (Grafton-Cardwell et al., 2013; Mora et al., 2021). Furthermore, phloem sap is deficient in some essential nutrients (Ziegler et al., 1975); therefore, psyllids depend on vertically transmitted bacterial mutualists to compensate for these deficiencies. They typically harbor two distinct symbionts (Thao et al., 2000a; Spaulding and von Dohlen, 2001; Sloan and Moran, 2012; Nakabachi et al., 2013b, 2020a, 2020b, 2022; Arp et al., 2014; Hall et al., 2016; Morrow et al., 2017) within an organ called the bacteriome (Nakabachi et al., 2010). “Ca. Carsonella ruddii” (Gammaproteobacteria: Oceanospirillales) (Thao et al., 2000b; Spaulding and von Dohlen, 2001; Sloan and Moran, 2012; Nakabachi et al., 2013b, 2020a, 2020b, 2022; Arp et al., 2014; Hall et al., 2016; Morrow et al., 2017; Nakabachi and Moran, 2022) is the ‘primary symbiont’ that provides the host with essential amino acids (Nakabachi et al., 2006, 2013b, 2020b; Sloan and Moran, 2012). Molecular phylogenetic analyses demonstrated cospeciation between psyllids and Carsonella, resulting from the single acquisition of a Carsonella ancestor by a psyllid common ancestor and its subsequent stable vertical transmission (Thao et al., 2000b; Spaulding and von Dohlen, 2001; Hall et al., 2016; Nakabachi et al., 2020a, 2022). Another bacterial lineage housed in the bacteriome is categorized as a ‘secondary symbiont’, which is phylogenetically diverse among psyllid species and genera, suggesting multiple infections and replacements during the evolution of Psylloidea (Thao et al., 2000a; Spaulding and von Dohlen, 2001; Sloan and Moran, 2012; Hall et al., 2016; Morrow et al., 2017; Nakabachi et al., 2020a, 2022). Secondary symbionts in various insects range from parasites to facultative mutualists (Moran et al., 2008), whereas those in the psyllid bacteriome analyzed to date invariably exhibit the features of obligate mutualists (Sloan and Moran, 2012; Nakabachi et al., 2013b, 2020b; Hall et al., 2016; Morrow et al., 2017). Since these features are characteristic of nutritional symbionts (Moran et al., 2008), secondary symbionts in the psyllid bacteriome are generally considered to have a nutritional basis (Spaulding and von Dohlen, 2001; Sloan and Moran, 2012; Morrow et al., 2017). A unique exception is “Ca. Profftella armatura” (Gammaproteobacteria: Burkholderiales) found in psyllids of the genus Diaphorina (Psyllidae: Diaphorininae) (Nakabachi et al., 2013b, 2020a, 2020b; Dan et al., 2017), the main role of which appears to be protecting the holobiont (the host plus symbionts) from natural enemies (Nakabachi et al., 2013b, 2020b; Nakabachi and Fujikami, 2019; Nakabachi and Okamura, 2019; Yamada et al., 2019; Tanabe et al., 2022). In addition to these bacteriome-associated obligate mutualists, psyllids may harbor various secondary symbionts of a facultative nature, including Wolbachia (Alphaproteobacteria: Rickettsiales), Rickettsia (Alphaproteobacteria: Rickettsiales), Rickettsiella (Gammaproteobacteria: Diplorickettsiales), and Diplorickettsia (Gammaproteobacteria: Diplorickettsiales), which cause systemic infections in host insects (Spaulding and von Dohlen, 2001; Sloan and Moran, 2012; Arp et al., 2014; Chu et al., 2016; Jain et al., 2017; Kruse et al., 2017; Morrow et al., 2017; Chu et al., 2019; Nakabachi et al., 2020a, 2022). Moreover, similar to other hemipteran insects (Nakabachi and Ishikawa, 1997, 1999; Nakabachi et al., 2003, 2005, 2014; Moran et al., 2008; Kikuchi, 2009; Nikoh and Nakabachi, 2009; Tamborindeguy et al., 2010; Shigenobu et al., 2010; Nakabachi, 2015; Uchi et al., 2019), recent studies revealed that not only interactions between host psyllids and symbiotic microbes, including those associated with the bacteriome, facultative symbionts, and plant pathogens (Nakabachi et al., 2006, 2010, 2013a; Sloan et al., 2014; Dan et al., 2017; Nakabachi and Fujikami, 2019), but also interactions among these bacterial populations are important for psyllid biology and host plant pathology (Nakabachi et al., 2013a, 2020a, 2022; Chu et al., 2016; Dan et al., 2017; Jain et al., 2017; Kruse et al., 2017; Chu et al., 2019; Killiny, 2022; Tanabe et al., 2022). Therefore, elucidating the microbiomes in various psyllid lineages, which reflect the ecological and evolutionary behaviors of bacterial populations in Psylloidea, will guide strategies to better control pest species.
Psyllids are currently classified into seven extant families: Aphalaridae, Calophyidae, Carsidaridae, Liviidae, Mastigimatidae, Psyllidae, and Triozidae (Burckhardt et al., 2021). After Psyllidae, Triozidae is the second largest family consisting of 70 genera with more than 1,000 species (Burckhardt et al., 2021). This family encompasses important agricultural pests, including the African citrus psyllid Trioza erytreae, the potato/tomato psyllid Bactericera cockerelli, and the carrot psyllids Bactericera trigonica and Trioza apicalis (Grafton-Cardwell et al., 2013; Mora et al., 2021). T. erytreae transmits “Ca. Liberibacter africanus” (CLaf, Alphaproteobacteria: Rhizobiales), which causes huanglongbing or citrus greening, the most destructive disease of citrus plants (Rutaceae) (Grafton-Cardwell et al., 2013). B. cockerelli, B. trigonica, and T. apicalis transmit “Ca. Liberibacter solanacearum” (CLso), the pathogen of severe diseases in important agricultural crops belonging to the families Solanaceae and Apiaceae, including potato, tomato, pepper, tobacco, carrot, and celery (Mora et al., 2021). Although several high-throughput amplicon-sequencing analyses have been performed on the microbiomes of Triozidae, the target psyllids were biased towards the most devastating pest B. cockerelli (Nachappa et al., 2011; Arp et al., 2014; Morrow et al., 2017). A recent study analyzed 20 Triozidae species (Kwak et al., 2021); however, the analysis was performed using 1) limited numbers of specimens; 12 species were examined with only a single individual, making the analysis less reliable, 2) primers unsuitable to detect symbionts with AT-rich genomes, and 3) an analytical method that clusters sequence reads with a similarity threshold of 97%, resulting in an analysis with a lower resolution.
In the present study, we performed amplicon analyses based on Illumina sequencing of 16S rRNA genes to assess the microbiomes of nine Triozidae species collected in Japan (Table 1) using 1) ten pooled individuals for each species, 2) primers to detect genomes with a wider variety of GC contents, and 3) a method to resolve sequence variants (SVs) down to the level of single-nucleotide differences.
| Species | Sampling site | Collection date | Host plant |
|---|---|---|---|
| Baeoalitriozus swezeyi (Crawford) | Kijoka, Ogimi, Okinawa Is., Ryukyus, Japan (26.705 N 128.145 E) | April 20, 2009 | Diospyros ferra (Ebenaceae) |
| Epitrioza mizuhonica Kuwayama | Nishikawachi, Reihoku, Kumamoto Pref., Amakusa-shimoshima Is., Kyushu, Japan (32.538 N 130.106 E) | May 15, 2010 | Elaeagnus umbellata (Elaeagnaceae) |
| Epitrioza yasumatsui Miyatake | Mitsu, Akitsu-chô, Higashihiroshima city, Hiroshima Pref., Honshu, Japan (34.330 N 132.823 E) | June 20, 2016 | Elaeagnus umbellata (Elaeagnaceae) |
| Leptynoptera sulfurea Crawford | Kabira, Ishigaki Is., Ryukyus, Japan (24.461 N 124.143 E) | May 2, 2000 | Calophyllum inophyllum (Calophyllaceae) |
| Pauropsylla triozoptera Crawford | Mt. Nekumatidi-dake, Ogimi, Okinawa Is., Ryukyus, Japan (26.682 N 128.138 E) | April 20, 2009 | Ficus ampelas (Moraceae) |
| Stenopsylla nigricornis (Kuwayama) | Nobeoka city, Miyazaki Pref., Kyushu, Japan (32.563 N 131.613 E) | March 24, 2000 | Symplocos kuroki (Symplocaceae) |
| Trioza camphorae Sasaki | Ideno, Minamihata, Imari city, Saga Pref., Kyushu, Japan (33.313 N 129.929 E) | April 5, 2012 | Cinnamomum camphora (Lauraceae) |
| Trioza cinnamomi (Boselli) | Hayasaki, Kuchinotsu-chô, Minamishimabara city, Nagasaki Pref., Kyushu, Japan (32.600 N 130.186 E) | April 23, 2015 | Cinnamomum tenuifolium (Lauraceae) |
| Trioza machilicola Miyatake | Koba, Obama-chô, Unzen city, Nagasaki Pref., Kyushu, Japan (32.698 N 130.227 E) | April 30, 2015 | Machilus thunbergii (Lauraceae) |
The adults of nine psyllid species belonging to the family Triozidae were collected from their host plants at various locations in Japan (Table 1). Insect samples were stored in acetone (Epitrioza yasumatsui, Trioza cinnamomi, and Trioza machilicola) or 99.5% ethanol (the other species) at –20°C until DNA extraction. After surface sterilization with ethanol, DNA was extracted from the whole bodies of pooled individuals of five adult males and five adult females of each psyllid species using the DNeasy Blood & Tissue Kit (Qiagen). The quality of the extracted DNA was assessed using a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific) and its quantity was evaluated using a Qubit 2.0 Fluorometer with the Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific).
Construction and sequencing of amplicon librariesBacterial populations in psyllids were analyzed using the MiSeq system (Illumina), as previously described (Nakabachi et al., 2020a, 2022). In brief, amplicon PCR was performed using DNA extracted from psyllids, KAPA HiFi HotStart ReadyMix (KAPA Biosystems), and the primer set 16S_341Fmod (5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGYYTAMGGRNGGCWGCAG-3′) and 16S_805R (5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′) targeting the V3 and V4 regions of the 16S rRNA gene. Both primers were based on the instructions by Illumina (Illumina, 2013), whereas 16S_341F was modified (underlined) with original CC, C, and G being replaced by the mixed bases YY (C or T), M (A or C), and R (A or G). This modification has been shown to increase the sensitivity of detecting symbionts with AT-rich genomes, including Carsonella, without reducing sensitivity for those with GC-rich genomes (Nakabachi et al., 2022). Dual indices and Illumina sequencing adapters were attached to the amplicons with index PCR using Nextera XT Index Kit v2 (Illumina). The libraries were combined with PhiX Control v3 (Illumina), and 250 bp of both ends were sequenced on the MiSeq platform (Illumina) with MiSeq Reagent Kit v2 (500 cycles; Illumina).
Computational analysis of bacterial populationsOutput sequences were imported into the QIIME2 platform (version 2022.2) (Bolyen et al., 2019) and processed as previously described (Nakabachi et al., 2020a, 2022). Briefly, after removing primer sequences, the denoising and joining of paired-end reads and the removal of low-quality or chimeric reads were performed using the dada2 plugin (Callahan et al., 2016). The q2-feature-classifier plugin (Bokulich et al., 2018) was trained using the V3 and V4 regions of the 16S rRNA gene sequences (Silva 138 SSURef NR99) (Glöckner et al., 2017). Dereplicated amplicon reads were then classified, to which taxonomic information was assigned using the trained q2-feature-classifier. The sequence variants (SVs) obtained were manually checked by performing BLASTN searches against the National Center for Biotechnology Information non-redundant database (Camacho et al., 2009).
Phylogenetic analysis of detected bacteriaPhylogenetic analyses of SVs were performed as previously described (Nakabachi et al., 2020a, 2022). In brief, after SVs were aligned to related sequences using SINA (v1.2.11) (Pruesse et al., 2012), phylogenetic trees were inferred by the maximum likelihood (ML) method using RAxML (version 8.2.12) (Stamatakis, 2014). The GTR+Γ model was used without partitioning of the data matrix, with 1,000 bootstrap iterations (options -f a -m GTRGAMMA -# 1000).
Data availabilityNucleotide sequence data are available in the DDBJ/EMBL/GenBank databases under the accession numbers DRR403084–DRR403092 (MiSeq output) and TAAC01000001–TAAC01000048 (dereplicated sequence variants).
MiSeq sequencing of amplicon libraries yielded 43,775–90,039 pairs of forward and reverse reads for the nine psyllid species. The denoising and joining of paired-end reads along with the removal of low-quality or chimeric reads resulted in 17,395–77,671 non-chimeric high-quality reads (Supplementary Table S1). The dereplication of these reads resulted in 206 independent sequence variants (SVs), among which 44 accounted for >1% of total reads (Supplementary Table S2). We focused on these 44 SVs because filtering with a threshold of 1% was shown to be among the most effective and accurate methods to remove potential contaminants (Karstens et al., 2019). SVs with a relative abundance of less than 1% were collectively categorized as ‘others’ in Fig. 1, which accounted for 0% (T. machilicola)–3.6% (E. yasumatsui) of total reads in each psyllid species (Supplementary Table S2). Extremely simple bacterial communities of this type have been reported for sternorrhynchan insects with a bacteriome, including aphids, whiteflies, and other psyllid species (Nachappa et al., 2011; Russell et al., 2013; Jing et al., 2014; Overholt et al., 2015; Morrow et al., 2017; Meng et al., 2019; Nakabachi et al., 2020a, 2022; Kwak et al., 2021). Taxonomic classification by QIIME2 (Supplementary Table S2) followed by independent BLAST searches and phylogenetic analyses showed that all nine psyllid species possess distinct lineages of Carsonella (Fig. 1). In the maximum likelihood (ML) tree, the Carsonella sequences identified in the present study formed a moderately supported clade (bootstrap: 64%) with those of psyllids belonging to the family Psyllidae (Fig. 2), which is consistent with the host psyllid phylogeny inferred by mitochondrial and nuclear data analyses (Burckhardt et al., 2021). Two types each of Carsonella sequences were observed in Baeoalitriozus swezeyi and Stenopsylla nigricornis (Fig. 1 and 2, Supplementary Table S2). In B. swezeyi, SV5 (59.4% of reads) and SV40 (1.8% of reads) were 99.8% identical (Supplementary Table S2). In S. nigricornis, SV6 (43.8% of reads) and SV41 (1.3% of reads) were also 99.8% identical (Supplementary Table S2). Although the dada2 plugin corrects sequencing errors during the denoising process (Callahan et al., 2016), SV40 and SV41 may have been derived from PCR/sequencing errors because they accounted for only small percentages of the reads. Previous studies detected only a trace amount of Carsonella reads (Nachappa et al., 2011; Morrow et al., 2017; Kwak et al., 2021), whereas the present study, which used primers with increased sensitivity to AT-rich symbiont genomes, detected a large percentage of Carsonella reads (Fig. 1 and Supplementary Table S2), more precisely reflecting actual populations (Nakabachi et al., 2020a, 2022).

Composition of bacterial populations in psyllids of the family Triozidae. The relative abundance of Illumina reads belonging to the assigned bacterial taxa are shown.

Maximum likelihood phylogram of Carsonella. A total of 427 unambiguously aligned nucleotide sites of 16S rRNA genes were subjected to the analysis. On each branch, bootstrap support values of >50% are shown. Designations other than those for outgroups refer to psyllid hosts. Families and subfamilies (if applicable) of the host psyllids are shown in brackets. Sequences from this study are shown in bold. DDBJ/EMBL/GenBank accession numbers for sequences are provided in parentheses. The bar represents nucleotide substitutions per position. The outgroups were Ca. Portiera aleyrodidarum, the primary symbiont of the whitefly Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea), and a gammaproteobacterium symbiont of the weevil Metapocyrtus yonagunianus (Coleoptera: Curculionidae).
Besides Carsonella, all nine psyllids analyzed in the present study possessed at least one other symbiont (Fig. 1).
Leptynoptera sulfurea has Halomonadaceae symbionts other than CarsonellaQIIME2 assigned SV32 and SV38, which accounted for 3.1 and 1.9% of L. sulfurea reads, respectively, to Carnimonas (Gammaproteobacteria: Oceanospirillales: Halomonadaceae) (Fig. 1 and 3, Supplementary Table S2). These SVs were 97.9–99.5% identical to the sequences of Carnimonas nigrificans (NR029342), the type species of the genus, and its relatives isolated from arthropod hosts (KT029591 and JQ950499). In the ML tree, these sequences formed a moderately supported clade (bootstrap: 73%) (Fig. 3). Although Carsonella also belongs to this family (Oceanospirillales: Halomonadaceae) (Spaulding and von Dohlen, 2001; Sloan et al., 2014), sequence identity between these SVs and Carsonella was low (<80%). Moreover, the clade of Carnimonas symbionts excluded a well-supported clade (bootstrap: 89%) of Carsonella and “Ca. Portiera aleyrodidarum”, the primary symbiont of whiteflies (Hemiptera: Sternorrhyncha: Aleyrodoidea) (Fig. 3), indicating that the lineages newly identified in the present study are distantly related to Carsonella.
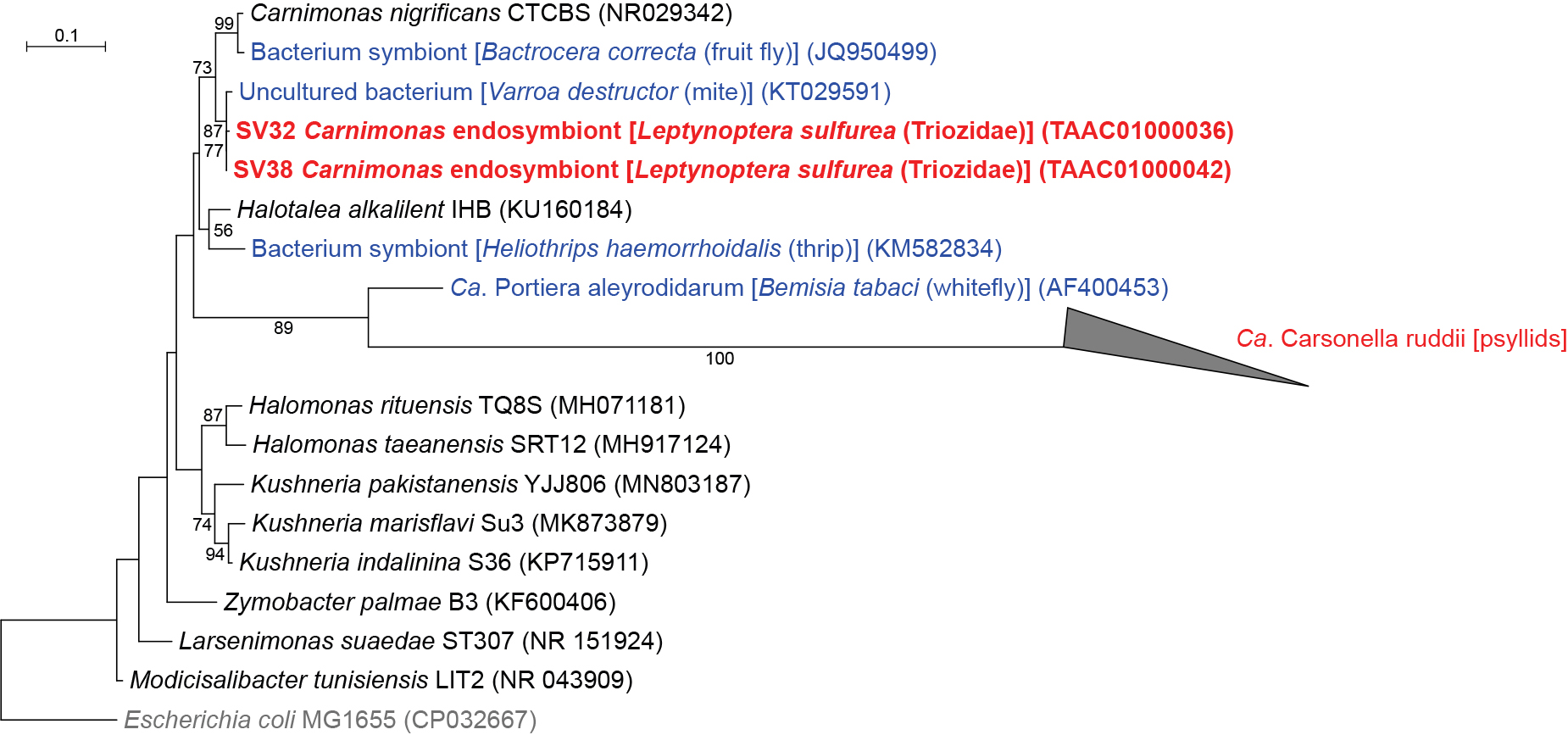
Maximum likelihood phylogram of bacteria belonging to Halomonadaceae. A total of 427 unambiguously aligned nucleotide sites of 16S rRNA genes were subjected to the analysis. On each branch, bootstrap support values of >50% are shown. The scale bar indicates substitutions per site. The triangle represents the collapsed clade of Carsonella shown in Fig. 2. Regarding symbiotic bacteria, host organisms are shown in brackets. Symbionts of animals other than psyllids are shown in blue. Symbionts of psyllids are shown in red. Sequences from this study are shown in bold. DDBJ/EMBL/GenBank accession numbers are provided in parentheses. Escherichia coli was used as an outgroup.
Among the 44 main SVs obtained in the present study, 37 corresponded to gammaproteobacteria, among which 23 belonged to the order Enterobacterales (Supplementary Table S2). Enterobacterales is a group of bacteria that encompasses a large fraction of intimate insect symbionts, including those associated with the bacteriome (Moran et al., 2008). Enterobacterales bacteria identified in the present study included Arsenophonus, Serratia symbiotica, and several lineages with ambiguous phylogenetic placements (Fig. 1 and 4, Supplementary Table S2).

Maximum likelihood phylogram of Enterobacterales. A total of 427 unambiguously aligned nucleotide sites of 16S rRNA genes were subjected to the analysis. On each branch, bootstrap support values of >50% are shown. The scale bar indicates substitutions per site. Regarding symbiotic bacteria, host organisms are shown in brackets. Symbionts of animals other than psyllids are shown in blue. Symbionts of psyllids are shown in red. Sequences from this study are shown in bold. DDBJ/EMBL/GenBank accession numbers are provided in parentheses. Carsonella was used as an outgroup.
Five SVs corresponding to distinct Arsenophonus lineages were detected in four of the nine Triozidae species: B. swezeyi, E. yasumatsui, S. nigricornis, and T. machilicola (Fig. 1 and 4, Supplementary Table S2). SV2 was shared by all 4 species (31.7% of B. swezeyi reads, 11.5% of E. yasumatsui reads, 36.8% of S. nigricornis reads, and 16.1% of T. machilicola reads), and 100% identical to Arsenophonus symbionts detected in various insect lineages, including aphids, whiteflies, louse flies, and the psyllid species Cardiaspina tenuitela (Aphalaridae: Spondyliaspidinae) (KY428657) and Glycaspis brimblecombei (Aphalaridae) (EU043378). In addition to SV2, one more distinct SV each for Arsenophonus was observed in these psyllids (SV29: 6.7% of B. swezeyi reads, SV27: 6.1% of E. yasumatsui reads, SV19: 13.4% of S. nigricornis reads, and SV44: 2.9% of T. machilicola reads). These SVs were 98.8% (SV27)–99.8% (SV2) identical to Arsenophonus nasoniae (CP038613), the type species of Arsenophonus found in the parasitoid wasp Nasonia vitripennis (Hymenoptera: Pteromalidae), and 97.9% (SV27)–98.8% (SV2) identical to “Ca. Arsenophonus triatominarum” (DQ508185) found in the assassin bug Triatoma rubrofasciata (Hemiptera: Reduviidae). These sequences formed a robustly supported clade (bootstrap: 100%) in the ML tree (Fig. 4). Although Arsenophonus shows a wide range of associations from parasitic to obligately mutualistic to host insects (Nováková et al., 2009), its ecological role in psyllids currently remains unknown.
S. symbiotica and its relativesFour SVs found in Epitrioza mizuhonica and one in E. yasumatsui corresponded to the sequence of S. symbiotica, known as a prevalent secondary symbiont of aphids (Perreau et al., 2021) (Fig. 1). SV22, SV23, SV26, and SV37, which accounted for 13.3, 11.6, 10.1, and 2.2%, respectively, of E. mizuhonica reads, and SV14, accounting for 18.5% of E. yasumatsui reads, were 98.6–99.5% identical to a single sequence of S. symbiotica (e.g. AB522706) (Fig. 4 and Supplementary Table S2). This reference sequence was derived from various aphid lineages, including Acyrthosiphon pisum, Aphis fabae, Aphis gossypii, Cinara pinikoraiensis, Cinara ponderosae, and Trama caudata (all Aphididae). The SVs described above and S. symbiotica sequences from aphids formed a robustly supported clade (bootstrap: 97%) together with SVs previously detected in another psyllid species Cacopsylla coccinea (Psyllidae: Psyllinae) (Nakabachi et al., 2022) (Fig. 4). This result supports the recently proposed hypothesis that S. symbiotica is prevalent in psyllids (Nakabachi et al., 2022), although it was formerly recognized only as a secondary symbiont of aphids. Since S. symbiotica was shown to enter plants and cause new infections in aphids, host plants are likely media for the intra- and inter-specific horizontal transmission of this bacterium (Perreau et al., 2021).
SV22, SV23, and SV26 were 98.6% (SV23 vs SV26)–99.3% (SV22 vs SV23) identical to one another. Similarities in nucleotide sequences and read frequencies (Fig. 1 and Supplementary Table S2) implied that SVs corresponded to multiple copies of the 16S rRNA gene in a single S. symbiotica genome. This is consistent with a previous finding showing that the genomes of several S. symbiotica strains encoded more than a single copy of the 16S rRNA gene (Perreau et al., 2021), which is in contrast to primary symbionts with an extremely streamlined genome encoding only a single copy (Nakabachi et al., 2006, 2013b, 2020b; Moran et al., 2008). A similar case was previously observed in C. coccinea described above (Nakabachi et al., 2022). Although the ecological role of S. symbiotica widely varies depending on aphid lineages (Perreau et al., 2021), its role in psyllids is currently unknown and, thus, warrants further study.
Additionally, the present study detected other lineages that are closely related to S. symbiotica. Five more SVs (SV20 from E. mizuhonica, SV21, SV28, SV42, and SV43 from E. yasumatsui) joined the above-described robustly supported clade with S. symbiotica (Fig. 4). However, we refrained from referring to the corresponding bacteria as S. symbiotica because their sequence identity with S. symbiotica was below the generally used arbitrary species threshold of 97% (94.6–96.5% for SV20, SV28, and SV43: Serratia endosymbionts) or genus threshold of 94.5–95% (92.1–92.3% for SV21 and SV42: Enterobacterales endosymbionts) (Yarza et al., 2014; Barco et al., 2020).
Other Enterobacterales symbionts with uncertain identitiesSV34, which accounted for 2.3% of S. nigricornis reads (Supplementary Table S2), was closely related to Sodalis endosymbionts (Fig. 4). It was 93.7% identical to the sequence of the type species Sodalis glossinidius (AP008232), a secondary symbiont of the tsetse fly Glossina morsitans (Diptera: Glossinidae). The sequence was 92.7–93.9% identical to those of Sodalis endosymbionts from various insects, including the other psyllid species Cacopsylla burckhardti (TAAB01000016), C. kiushuensis (Psyllidae: Psyllinae) (TAAB01000030), and Cardiaspina maniformis (Aphalaridae: Spondyliaspidinae) (KY428659). Although SV34 formed a moderately supported clade (bootstrap: 78%) with these sequences (Fig. 4), we refrained from assigning the corresponding bacterium to Sodalis and only referred to it as an Enterobacterales endosymbiont because its sequence identity was below the genus threshold (Yarza et al., 2014; Barco et al., 2020), and family names are fluid in Enterobacterales (Parks et al., 2018). SV1 (62.7% of T. camphorae reads), SV33 (1.8% of T. camphorae reads), SV15 (63.2% of T. machilicola reads), SV3 (77.2% of Pauropsylla triozoptera reads), SV36 (2.0% of P. triozoptera reads), and SV4 (48.5% of T. cinnamomi reads) formed a cluster with a secondary endosymbiont of Trioza magnoliae (Triozidae) (AF077607) in the ML tree (Fig. 4). However, this branching pattern was only poorly supported (bootstrap: <50%), and their sequence identity with those of bacteria with a genus name was low (<94.5%). Therefore, the bacteria for these SVs were also named Enterobacterales endosymbionts (Fig. 4). SV11 (27.8% of L. sulfurea reads) deeply branched in the Enterobacterales tree (Fig. 4), and was closely related to “Enterobacteriaceae” bacteria, including symbionts of the other psyllid species Epiacizzia kuwayamai (Psyllidae: Macrocorsinae) (TAAB01000023) (86.5% identical), C. biwa (Psyllidae: Psyllinae) (TAAB01000001) (85.6% identical), and C. kiushuensis (Psyllidae: Psyllinae) (TAAB01000027) (85.6% identical). Since these sequence identities were above the arbitrary order threshold of 82.0%, but below the family threshold of 86.5% (Yarza et al., 2014), the bacterium for SV11 was also referred to as an Enterobacterales endosymbiont (Fig. 4).
T. camphorae has DiplorickettsiaNon-Enterobacterales gammaproteobacteria detected in the present study were Carsonella (Oceanospirillales: Halomonadaceae), the newly identified Halomonadaceae symbionts described above, and Diplorickettsia (Diplorickettsiales: Diplorickettsiaceae) (Fig. 1 and 5). SV25, which was derived from 7.0% of T. camphorae reads (Supplementary Table S2), was 98.8% identical to the sequence of Diplorickettsia massiliensis 20B (NR_117407), the type species detected in the European sheep tick Ixodes ricinus (Arachnida: Acari: Ixodidae), 98.6% identical to the sequence of Diplorickettsia sp. (TAAA01000038) recently found in the psyllid species Psylla morimotoi (Psyllidae: Psyllinae) (Nakabachi et al., 2022), 97.9% identical to the sequence of Diplorickettsia sp. (TAAA01000010) found in the psyllid species Diaphorina cf. continua (Psyllidae: Diaphorininae) (Nakabachi et al., 2020a), 97.9% identical to that of Diplorickettsia sp. MSebKT1 (AB795342) detected in the leafhopper Macrosteles sexnotatus (Hemiptera: Auchenorrhyncha: Cicadellidae), and 97.7% identical to Diplorickettsia sp. NS15 (JN606082) found in human nasal samples. In the ML tree, SV25 formed a well-supported clade (bootstrap: 80%) with these Diplorickettsia spp. (Fig. 5). D. massiliensis was observed in I. ricinus and patients with suspected tick-borne diseases, suggesting that this bacterium is a human pathogen (Subramanian et al., 2012). Diplorickettsia lineages were then unexpectedly detected in the plant sap-sucking insects M. sexnotatus in Japan (Ishii et al., 2013), D. cf. continua in Corsica (Nakabachi et al., 2020a), and P. morimotoi in Japan (Nakabachi et al., 2022). The present study adds another example of Diplorickettsia in Psylloidea. Collectively, the present results and previous findings suggest that Diplorickettsia is prevalent in various sap-sucking insects. Although their host plants are not shared among M. sexnotatus (Poaceae and Fabaceae), D. cf. continua (Thymelaeaceae), P. morimotoi (Rosaceae), and T. camphorae (Lauraceae), further studies are warranted to establish whether plants are also infected with Diplorickettsia. Since limited information is currently available on the functions of Diplorickettsia in host arthropods, the physiological and ecological effects of Diplorickettsia on psyllids need to be examined in more detail.

Phylogenetic position of Diplorickettsia lineages inferred by the maximum likelihood method. A total of 427 unambiguously aligned nucleotide sites of 16S rRNA genes were subjected to the analysis. On each branch, bootstrap support values of >50% are shown. The scale bar indicates substitutions per site. Regarding symbiotic bacteria, host organisms are shown in brackets. Symbionts of animals other than psyllids are shown in blue. Symbionts of psyllids are shown in red. The sequence from the present study is shown in bold. DDBJ/EMBL/GenBank accession numbers are provided in parentheses. Escherichia coli was used as an outgroup.
Analyses detected “Ca. Liberibacter europaeus” (CLeu, Alphaproteobacteria: Rhizobiales) for the first time in E. yasumatsui, which feeds on Elaeagnus umbellata (Elaeagnaceae), known as the Japanese silverberry or autumn olive (Fig. 1 and Supplementary Table S2). SV17, which accounted for 16.9% of E. yasumatsui reads, was 100% identical to the sequence of CLeu previously detected in C. pyri (Psyllidae: Psyllinae) (FN678792) and D. cf. continua (Psyllidae: Diaphorininae) (TAAA01000007) (Nakabachi et al., 2020a), and 99.8% identical to the sequence of CLeu recently detected in Anomoneura mori (Nakabachi et al., 2022).
CLeu is a close relative of CLaf, “Ca. L. asiaticus”, and “Ca. L. americanus”, which are pathogens of the devastating greening disease in citrus (Rutaceae) (Grafton-Cardwell et al., 2013), and CLso, the pathogen causing serious diseases in solanaceous and apiaceous crops as described above (Mora et al., 2021). CLeu was detected in various psyllids from various locations: Cacopsylla spp. (Psyllidae: Psyllinae) in Italy and Hungary, Arytainilla spartiophila (Psyllidae: Psyllinae) in New Zealand and the U.K. (Tannières et al., 2020), D. cf. continua (Psyllidae: Diaphorininae) in Corsica island (Nakabachi et al., 2020a), and A. mori in Japan (Nakabachi et al., 2022). CLeu was also detected from rosaceous plants and the Scotch broom Cytisus scoparius (Fabaceae), which are host plants of Cacopsylla spp. and Ar. spartiophila, respectively (Tannières et al., 2020). The presence of CLeu is associated with pathological symptoms in the Scotch broom (Tannières et al., 2020). The present study adds another example of CLeu from the psyllid species E. yasumatsui in Japan. Further studies are required to clarify whether the host plant E. umbellata, which is distantly related to previously known infected plants, is also infected with CLeu and if infection causes disease symptoms. Since E. umbellata is an aggressive invasive plant in the United States and Europe, if CLeu causes a disease in E. umbellata, CLeu transmitted by E. yasumatsui may potentially be exploited as a biological herbicide.
First detection of Wolbachia supergroup O in PsylloideaAnalyses identified five SVs corresponding to distinct lineages of Wolbachia (Alphaproteobacteria: Rickettsiales) (Fig. 1 and 6, Supplementary Table S2). Wolbachia are rickettsial bacteria that are distributed in a wide variety of arthropods and nematodes (Werren et al., 2008), the strains of which are currently classified into supergroups A–Q (Lindsey et al., 2016). Supergroups A and B are the most common supergroups infecting arthropods, and all Wolbachia strains previously detected in psyllids belonged to supergroup B (Spaulding and von Dohlen, 2001; Sloan and Moran, 2012; Arp et al., 2014; Jain et al., 2017; Morrow et al., 2017; Chu et al., 2019; Nakabachi et al., 2020a, 2022). In contrast, SV10, which accounted for 25.9% of T. cinnamomi reads (Supplementary Table S2), was 100% identical to the sequence of Wolbachia belonging to supergroup O, which was detected in two aphid species, Kaburagia rhusicola (MT554837) and Schlechtendalia chinensis (MT554838) (Ren et al., 2020). The ML analysis placed the sequence within a robustly supported clade (bootstrap: 90%) of Wolbachia supergroup O (Fig. 6). To the best of our knowledge, this is the first detection of Wolbachia supergroup O in Psylloidea. All other Wolbachia strains found in the present study belonged to supergroup B (Fig. 6).

Maximum likelihood phylogram of Wolbachia. A total of 402 unambiguously aligned nucleotide sites of 16S rRNA genes were subjected to the analysis. On each branch, bootstrap support values of >50% are shown. Host organisms are shown in brackets. Symbionts of animals other than psyllids are shown in blue. Symbionts of psyllids are shown in red. The sequence from the present study is shown in bold. DDBJ/EMBL/GenBank accession numbers for sequences are provided in parentheses. Supergroups of Wolbachia are shown in angle brackets. The scale bar represents nucleotide substitutions per position. Liberibacter was used as an outgroup.
The majority of Wolbachia strains manipulate the reproduction of arthropod hosts to boost dissemination (Werren et al., 2008). Due to this ability, Wolbachia are recognized as promising agents to control insect pests by affecting their traits or microbiomes (Brinker et al., 2019). Based on the high infection rates of Wolbachia in pest psyllids worldwide (Spaulding and von Dohlen, 2001; Sloan and Moran, 2012; Arp et al., 2014; Chu et al., 2016, 2019; Morrow et al., 2017; Nakabachi et al., 2020a, 2022) and the suggested interactions between Wolbachia and other symbionts (Chu et al., 2016, 2019; Jain et al., 2017; Kruse et al., 2017; Killiny, 2022), the application of Wolbachia to control pest psyllids and/or plant pathogens is anticipated (Chu et al., 2016, 2019; Kruse et al., 2017). The present results suggest the rampant horizontal transmission of various Wolbachia strains among various insects, including pest psyllids (Fig. 6); therefore, the artificial infection of Wolbachia appears to be feasible in psyllids and will facilitate the exploitation of this bacterial group as a tool to control pest psyllids and/or the plant pathogens they carry.
L. sulfurea has an Enterococcus symbiontSV7, which accounted for up to 40.0% of L. sulfurea reads (Supplementary Table S2), was 100% identical to the sequence of Enterococcus faecalis (Firmicutes: Bacilli: Lactobacillales: Enterococcaceae) found in mammalian animals (e.g. NR 115765), and Enterococcus spp. identified in marine sponges (e.g. MT484183) and insect lineages, including the silk moth Bombyx mori (CP092784), the ant Polyrhachis lamellidens (AP025690), and the flies Bactrocera dorsalis (MK764705) and Hermetia illucens (OK012249) (Akami et al., 2019) (Fig. 7). Although E. faecalis is a common bacterium found in the gut of mammals, including humans, we consider this SV to be derived not from contaminants, but from a stable associate of L. sulfurea because 1) SV7 accounted for a large percentage of L. sulfurea reads, and 2) no other SVs in L. sulfurea were similar to the bacterial sequences found in the animal intestinal flora. Gram-positive E. faecalis (Firmicutes: Bacilli: Lactobacillales: Enterococcaceae), which is distantly related to all other Gram-negative proteobacterial symbionts identified in the present study, has only been recognized as a transient gut resident in insects (Akami et al., 2019). Therefore, the localization and functional role of this bacterium in L. sulfurea need to be examined in more detail.

Maximum likelihood phylogram of bacteria belonging to the order Lactobacillales. A total of 428 unambiguously aligned nucleotide sites of 16S rRNA genes were subjected to the analysis. On each branch, bootstrap support values of >50% are shown. Host organisms are shown in brackets. Bacteria found in insects other than psyllids are shown in blue. The sequence from the present study is shown in bold red. DDBJ/EMBL/GenBank accession numbers for sequences are provided in parentheses. The scale bar represents nucleotide substitutions per position. Bacillus subtilis was used as an outgroup.
The present study identified various bacterial symbionts in nine psyllid species of the family Triozidae. The majority of secondary symbionts were gammaproteobacteria, particularly those of the order Enterobacterales, including Arsenophonus and S. symbiotica. Regarding non-Enterobacterales gammaproteobacteria, Diplorickettsia (Diplorickettsiales: Diplorickettsiaceae), a potential human pathogen, and Carnimonas (Oceanospirillales: Halomonadaceae), a lineage independent of Carsonella within the family Halomonadaceae, were identified. As for alphaproteobacteria, the potential plant pathogen CLeu (Rhizobiales: Rhizobiaceae) was detected for the first time in E. yasumatsui. Since E. yasumatsui feeds on the Japanese silverberry E. umbellata (Elaeagnaceae), which is an aggressive invasive plant in the United States and Europe, the combination of E. yasumatsui and CLeu may potentially be exploited as a biological herbicide for E. umbellata. Wolbachia (Rickettsiales: Anaplasmataceae) strains of supergroup B were identified in three Triozidae species, whereas a lineage belonging to supergroup O was detected in T. cinnamomi, which is the first report of this supergroup in Psylloidea. Moreover, E. faecalis (Firmicutes: Bacilli: Lactobacillales: Enterococcaceae), which has only been recognized as a transient resident in insects, was suggested to constitute a large part of the microbiome in L. sulfurea, implying that this bacterium acquired the status of a stable symbiont. These results provide more detailed insights into the interactions among insects, bacteria, and plants, which may be exploited to facilitate the control of pest psyllids in the future.
Nakabachi, A., Inoue, H., and Hirose, Y. (2022) High-resolution Microbiome Analyses of Nine Psyllid Species of the Family Triozidae Identified Previously Unrecognized but Major Bacterial Populations, including Liberibacter and Wolbachia of Supergroup O. Microbes Environ 37: ME22078.
https://doi.org/10.1264/jsme2.ME22078
This work was supported by the Japan Society for the Promotion of Science (https://www.jsps.go.jp) KAKENHI [grant numbers 26292174 and 20H02998 to AN, and 25850035 to HI], and research grants from the Tatematsu Foundation and Nagase Science and Technology Foundation to AN. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The DNA sequencing facility was supported by the Department of Applied Chemistry and Life Science and the University-Community Partnership Promotion Center in Toyohashi University of Technology.