2023 年 38 巻 2 号 論文ID: ME22081
2023 年 38 巻 2 号 論文ID: ME22081
Vegetable soybean (Glycine max [L.]) is mainly consumed in Asian countries, but has recently attracted attention worldwide due to its high nutritional value. We aimed to identify the indigenous rhizobia of vegetable soybean in Yao City, Osaka Prefecture, Japan, and to clarify the relationships between the rhizobial community and soil environmental factors. Soil samples were collected from 12 vegetable soybean cultivation fields under two different conditions (six greenhouses and six open fields) in Yao City with different varieties of vegetable soybean. A total of 217 isolates were obtained from the nodules and clustered into nine operational taxonomic units (OTUs) with 97% homology based on the 16S-23S rRNA internal transcribed spacer (ITS) region. A phylogenetic analysis showed that OTUs were closely related to Bradyrhizobium liaoningense, B. ottawaense, B. elkanii, and other Bradyrhizobium species and were dominant in this order. B. liaoningense was widely found in sampled sites and accounted for 50.7% of all isolates, while B. ottawaense was mostly limited to open fields. This rhizobial community differed from Japanese soybean rhizobia, in which B. diazoefficiens, B. japonicum, and B. elkanii were dominant. These results imply the characteristic differences among host plants or regional specialties. A non-metric multidimensional scaling (NMDS) analysis revealed the significant impact of soil pH and the contents of Ca, Mg, Mn, total nitrogen (TN), and total carbon (TC) on the distribution of rhizobia. B. liaoningense was detected in soils with a neutral pH, and high TN and low Mn contents increased its abundance. The present study provides novel insights into Japanese rhizobia and potentially novel resources for sustainable agriculture.
Nitrogen fixation activity is important for supplying the nitrogen demand of soybean (Glycine max [L.] Merr.). Bradyrhizobium, a Gram-negative bacterium belonging to the Alphaproteobacteria family, is a major symbiont of soybean. The host range of this species is wide and includes important legume crops, such as cowpea (Vigna unguiculata L. Walp.), faba beans (Vicia faba L.), and peanuts (Arachis hypogaea) (Santos et al., 2019). Nitrogen-fixing bacteria, including Bradyrhizobium, fix approximately 200 kg N ha–1 year–1 (Herridge et al., 2008), and between 60 and 80% of the nitrogen required for soybean growth is derived from nitrogen fixation. The number of species in the genus Bradyrhizobium has increased in recent years, revealing its vast genomic diversity (Shamseldin et al., 2017; Avontuur et al., 2019; Ormeño-Orrillo and Martínez-Romero, 2019). In addition to N2 fixation, some Bradyrhizobium species exhibit unique characteristics related to plant growth and the global environment. B. elkanii has been shown to synthesize indole-3-acetic acid (IAA), a compound belonging to the auxin family, and promotes symbiosis with its host plant (Fukuhara et al., 1994; Torres et al., 2018). B. elkanii also synthesizes rhizobitoxine and induces plant chlorosis in soybean (Owens and Wright, 1965). While some species exhibit hydrogenase activity, which increases the efficiency of nitrogen fixation (Black et al., 1994), B. cosmicum and related strains possess photosynthetic genes (Avontuur et al., 2019; Wasai-Hara et al., 2020b). In addition, B diazoefficiens possesses the nitrous oxide reductase gene nosZ, which mitigates the emission of N2O, a known greenhouse gas (Itakura et al., 2013; Akiyama et al., 2016).
Rhizobial diversity in soybean fields in Japan ranges among regions (Saeki et al., 2006). Only B. japonicum, B. diazoefficiens, and B. elkanii have been reported as major indigenous rhizobacteria in soybean fields in Japan. B. japonicum was found to be dominant in northern Japan and B. elkanii in southern Japan (Suzuki et al., 2008; Saeki et al., 2013). In addition, B. japonicum was dominant in Andosol and B. diazoefficiens in Graysol (Shiina et al., 2014). B. elkanii, B. japonicum, B. liaoningense, B. yuanmingense, and Ensifer have frequently been detected as soybean rhizobia in China (Man et al., 2008; Li et al., 2011). B. japonicum and B. diazoefficiens are the dominant species of soybean symbionts in South Africa (Naamala et al., 2016). Environmental factors contributing to genotypic differences in rhizobia include soil temperature, pH, salt, moisture content, and geographic location (Suzuki et al., 2008; Zhang et al., 2011; Adhikari et al., 2012; Saeki and Shiro, 2014; Mason et al., 2017; Yang et al., 2018). Differences have been reported in the compatibility of Rhizobacteria strains with their host plants as well as their growth-promoting effects. Therefore, stronger strains are generally selected as inoculants to increase legume yields. Effective rhizobacteria, such as SEMIA 587 (B. elkanii), SEMIA 5079 (B. japonicum), and SEMIA 5080 (B. diazoefficiens), have been used as inoculants for soybean cultivation in Brazil (Castro-Sowinski et al., 2007; de Souza et al., 2019).
Vegetable soybeans are immature soybeans harvested during the R6 stage (Fehr et al., 1971). They are consumed in various Asian countries and are known as edamame in Japan and maodou in China. Vegetable soybeans have recently been attracting increasing attention due to their high contents of protein, fat, phospholipids, calcium, iron, zinc, vitamins A, C, K, and B, and dietary fiber (Takahashi and Ohyama, 2011; Jiang et al., 2018).
Although the total cultivation area of vegetable soybeans in Osaka Prefecture is limited, average yield is higher than in other regions in Japan (MAFF, 2020). Yao City is the main production area for vegetable soybeans in Osaka Prefecture and vegetable soybeans harvested in this city are marketed as a regional brand called “Yao-Edamame”. Yield fluctuations have been reported in Yao City, and surveys conducted in 2017 and 2018 revealed vegetable soybean yields under greenhouse conditions that ranged between 2.99 and 9.68 t ha–1 (Matsumura et al., 2022). Therefore, it is important to evaluate various aspects, including rhizobial symbiosis, contributing to stable vegetable soybean cultivation.
Many studies have been conducted on the identification and diversity of indigenous rhizobacteria in soybean fields (Santos et al., 1999; Abaidoo et al., 2007; Shiro et al., 2013); however, limited information is currently available on rhizobial diversity in vegetable soybean fields. Therefore, the objectives of the present study were to identify the indigenous rhizobia of vegetable soybeans in multiple fields in Yao City, Osaka Prefecture, Japan, and to compare the diversity of rhizobia among these fields. Moreover, the present study investigated the relationships between the diversity of indigenous rhizobia and environmental factors, such as soil chemical characteristics and the effects of inoculations on plant growth.
Yao City is located in east-central part of Osaka Prefecture (34°62′68″N, 135°60′9″E), Japan, and has a thriving production of vegetable soybeans. Twelve vegetable cultivation sites, including six greenhouse fields (Yaogikita [Ya], Osakabe [Os1], Fukuei-cho [Fu], Yaogihigashi-kita [Yh], Okubo [Ok1], and Kyoukouji [Ky]) and six open fields (Kashimura-cho [Ka], Osakabe [Os2], Onjikita-machi [On], Akegawahigashi-kita [Ak], Okubo [Ok2], and Miyakoduka [Mi]) in Yao City were selected for the present study (Fig. 1). Information on soybean cultivation at each site is shown in Table 1.
Map of Yao City in Osaka Prefecture indicating soil sampling sites. Soil samples were collected from 12 sites (6 greenhouse fields and 6 open fields), marked with black dots.
| Site | Vegetable soybean cultivar | Amount of fertilization (kg ha–1) |
marketable pods yield (t ha–1) |
previous crop | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Latitude (°N) | Longitude (°E) | N | P2O5 | K2O | ||||||
| Greenhouse | Yaogi-kita | Ya | 34.365 | 135.365 | Taisetsu-midori | 168 | 196 | 140 | 8.15 | Burdock |
| Osakabe | Os1 | 34.365 | 135.371 | Green 80 | 67 | 22 | 14 | 8.68 | Burdock | |
| Fukuei | Fu | 34.643 | 135.627 | Taisetsu-midori | 126 | 42 | 27 | 5.59 | Japanese mustard spinach |
|
| Yaogi-higashi | Yh | 34.363 | 135.374 | Taisetsu-midori | 22 | 30 | 13 | 5.13 | Burdock | |
| Okubo | Ok1 | 34.374 | 135.392 | Taisetsu-midori | 16 | 22 | 10 | 8.09 | Burdock | |
| Kyoukouji | Ky | 34.377 | 135.376 | Taisetsu-midori | 73 | 100 | 43 | 7.41 | Burdock | |
| Open field | Kashimura | Ka | 34.365 | 135.373 | Ezo-midori | 168 | 200 | 140 | 7.28 | Burdock |
| Osakabe | Os2 | 34.365 | 135.371 | Ezo-midori | 38 | 13 | 8 | 7.37 | Spinach | |
| Onjikita | On | 34.364 | 135.376 | Ezo-midori | 13 | 4 | 3 | 8.87 | Spinach | |
| Akegawa-higashi | Ak | 34.364 | 135.365 | Ezo-midori | 43 | 14 | 9 | 12.07 | Burdock | |
| Okubo | Ok2 | 34.374 | 135.392 | Ezo-midori | 18 | 24 | 10 | 3.31 | Burdock | |
| Miyakoduka | Mi | 34.362 | 135.372 | Ezo-midori | 68 | 92 | 40 | 7.63 | Green onion | |
Approximately 1 kg of soil was collected from each site in 2017. During the harvesting of vegetable soybeans, soil samples were collected near the plant base between 1 to 10 cm from the soil surface. Soil samples collected from five points in each field were composited into one sample. Soil subsamples were thoroughly mixed and air-dried before being subjected to chemical analyses.
pH (H2O) and electrical conductivity (EC) were measured sequentially with a glass electrode (Horiba F-53 and Horiba DS-51, respectively) using a 1:5 (w/v) suspension of soil and distilled water. Nitrate-N (NO3–-N) concentrations were measured using the CATALDO method (Cataldo et al., 1975). Available phosphate (P) was extracted using the Truog–P method, and its concentration was measured using the molybdenum blue colorimetric method. To assess the concentrations of soil exchangeable cations, we extracted potassium (K), calcium (Ca), magnesium (Mg), and manganese (Mn) using ammonium acetate (pH 7.0) and measured their contents using a polarized Zeeman atomic absorption spectrophotometer (ZA3000; Hitachi). Total carbon (TC) and total nitrogen (TN) contents were measured using the dry combustion method (CN coder MT-700, Yanagimoto). Similarly, soil samples were also collected before vegetable soybean cultivation, and soil chemistry data were obtained as described above. Some soil data before cultivation were previously reported by Matsumura et al. (2022).
Isolation of indigenous rhizobiaTo isolate indigenous rhizobia, the vegetable soybean varieties cv. Taisetsu-midori and cv. Ezo-midori were used as host plants for greenhouse and open-field soils, respectively. Taisetsu-midori and Ezo-midori are common varieties in Yao City in each type of cultivation field. Plants were grown without fertilizer in pots filled with soil (470–500 g) collected from each site. Five seeds were surface sterilized with 0.5% sodium hypochlorite for two min, rinsed five times with sterilized water, and then sown in each pot. The pots were covered with plastic bags to prevent contamination. After emergence, seedlings were thinned to accommodate two plants pot–1. Plants were grown in a greenhouse without temperature control, and sterile water was supplied to the soil as needed. When plants reached the V4–V5 developmental stage, 20 nodules plant–1 were randomly collected from the roots. Nodules were surface sterilized by immersion in 70% ethanol and 0.5% sodium hypochlorite solution for 1 min. Nodules were carefully crushed in sterile water using toothpicks. The liquid obtained was then applied to yeast mannitol agar (YMA) (agar, 15 g; mannitol, 10 g; yeast extract, 0.4 g; KH2PO4, 0.5 g; MgSO4·7H2O, 0.2 g; NaCl, 0.1 g; distilled water, 1 L; pH 6.8) medium and incubated at 28°C for 7 days. Bacterial colonies were repeatedly streaked onto YMA medium to obtain pure cultures.
Identification of indigenous rhizobial isolatesColonies were selected with sterilized loops and placed directly into PCR tubes as DNA templates. An approximately 900-bp internal transcribed spacer (ITS) region between the 16S and 23S rRNA genes from the colonies obtained was amplified using colony PCR with primers from the 3′-end of 16S rRNA (FGPS1490: 5′-TGCGGCTGGATCACCTCCTT-3′) and the 5′-end of 23S rDNA (FGP132′: 5′-CCGGGTTTCCCCATTCGG-3′) (Laguerre et al., 1994). PCR amplification was performed using Gflex (Takara Bio). The reaction mixture was incubated at 94°C for 5 min, followed by 38 cycles at 94°C for 30 s, 55°C for 30 s, and 72°C for 2 min, and then at 72°C for 10 min.
PCR-amplified products were purified using the Fast Gene TM gel/PCR extraction kit (NIPPON Genetics). Sanger sequencing was performed using primers (FGPS1490 and FGP132) from Eurofins Genomics.
After checking the sequence electropherogram, samples containing contamination and/or sequencing errors were removed. Clustering was conducted using CD-Hit-est (Huang et al., 2010) with a threshold of 97%, and representative sequences were used for a phylogenetic analysis. A phylogenetic tree was constructed by MEGA X software (Kumar et al., 2018, version 10.2.6, https://www.megasoftware.net/) using the neighbor-joining method with conserved regions of the representative sequences. A clustering analysis with a threshold of 100% was also performed to establish whether isolates were identical, and isolates from the same plant host with 100% homology in the ITS sequence were defined as close isolates.
ITS sequences were deposited in the NCBI GenBank database under accession numbers ON500436 to ON500498 and OP038215. Draft genomes for representative strains of OTU1-4 were identified by shotgun sequencing using DNBSEQ-G400 (MGI Tech) at Bioengineering Lab. Genome sequences were deposited in the DDBJ database under BioSample accession numbers SAMD00570452 to SAMD00570455. Based on this genome information, average nucleotide identity (ANI) values were calculated (FastANI, Jain et al., 2018) and a detailed phylogenic analysis was conducted using the 31 AMPHORA genes identified (Wu and Eisen, 2008). Detailed methods for the genome analysis are described in the supplemental materials.
Inoculation effects of rhizobial isolates on vegetable soybean growthAn inoculation test using an isolate selected from each OTU was conducted to evaluate its effects on the early growth of vegetable soybeans. Plant boxes were filled with 250 mL sterilized vermiculite and 150 mL B&D liquid medium (Broughton and Dilworth, 1971). Soil pH and EC after the application of B&D medium were 7.0 and 0.1 dS m–1, respectively. This experiment consisted of ten treatments for the nine test isolates (YHC11 [OTU1], YFG21 [OTU2], YHB32 [OTU3], YHC43 [OTU4], YHC52 [OTU5], YHF61 [OTU6], YFL71 [OTU7], YFH81 [OTU8], and YHA91 [OTU9], see Table S1) and a non-inoculation (NI) treatment with five replicates. Test isolates were incubated in yeast mannitol broth (YMB) (mannitol, 10 g; yeast extract, 0.4 g; KH2PO4, 0.5 g; MgSO4·7H2O, 0.2 g; NaCl, 0.1 g; distilled water, 1 L; pH 6.8) at 28°C with reciprocal shaking at 120 rpm for 7 days. The rhizobial solution was washed, and optical density at 660 nm (OD660) was adjusted to approximately 0.3. The seeds of vegetable soybeans (cv. Ezo-midori) were surface-sterilized by immersion in 0.5% sodium hypochlorite (NaClO) for 1 min and then washed three times with sterilized distilled water. At sowing, seeds were inoculated with a diluted solution of each test isolate (1 mL seed–1). Five inoculated or non-inoculated seeds were sown in each plant box using a sterilized spoon and then thinned to one plant at the stage of primary leaf development. After thinning, the soil surface was covered with plastic wrap to prevent contamination. Plants were grown under the following controlled environmental conditions: temperature, 28°C; photoperiod, 16 h light/8 h dark; light intensity, 220 μmol m–2s–1, and sterilized distilled water supplied as needed. After 22 days, plants approximately at the V4 stage were harvested and the dry weights of the shoot, root, and nodules were measured after drying at 70°C for 48 h.
The total number of nodules (NN) plant–1 was counted before nodule drying.
Data analysisWe used the Shannon–Wiener index (H’) and Simpson index (D) to estimate the diversity of bradyrhizobial communities. H’ shows diversity in consideration of species richness in the community, and D indicates species dominance in the community. H’ and D were calculated using the following equations (Mpepereki and Wollum, 1991; Ricotta and Szeidl, 2006).
H’=–Σ (Pi log Pi)
D=1–ΣPi^2
Pi is the fraction of individuals belonging to the ith species.
Additionally, relationships between these diversity indexes, soil characteristics, the occupancy of rhizobial operational taxonomic units (OTUs), and the yield of vegetable soybeans were evaluated using Pearson’s correlation. A non-metric multidimensional scaling (NMDS) analysis was performed with the ‘metaMDS’ function to elucidate whether environmental variables affected rhizobial distributions. The ‘envfit’ function in ‘vegan’ was used to examine the relationships between OTU compositions and the soil and chemistry. The NMDS analysis was conducted by R software using ‘vegan’ package 2.6-2 (Oksanen et al., 2022). In the inoculation examination, results were subjected to Tukey’s test and Dunnett’s test comparing the NI treatment at 5% significance after an analysis of variance (ANOVA). Statistical analyses were performed using IBM SPSS Statistics version 26 (IBM).
A total of 217 pure rhizobial isolates were obtained from 12 soybean cultivation fields in Yao City. The results of the BLAST analysis using 16S–23S ITS sequencing showed that all isolates belonged to the genus Bradyrhizobium. Based on a cluster analysis of the ITS region, isolates were classified into nine OTUs with 97% homology (Fig. 2) and 31 genotypes with 100% homology (Table S1, 6 genotypes in OTU1, 5 in OTU2, 4 in OTU3, 8 in OTU4, 2 in OTU 5, 3 in OTU6, and 1 in OTU7-9).
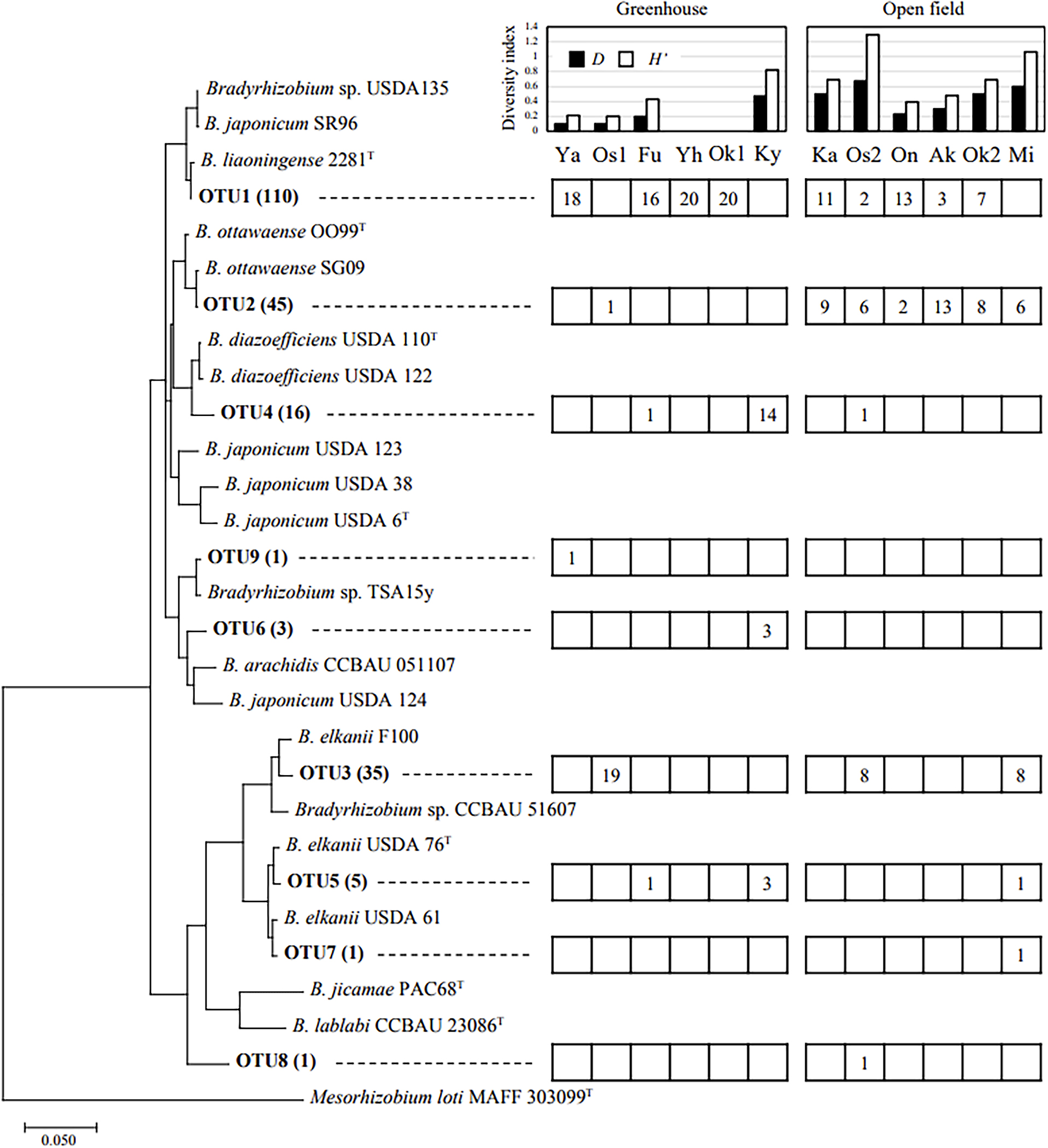
Phylogenic tree of isolates based on ITS sequences. OTU was clustered with a threshold of 97%. The numbers in parentheses show the total number of isolates included in each OTU. The right boxes show the number of isolates in each field.
Genome Average Nucleotide Identity (ANI) values calculated between representative strains of OTU1 (YHC11), OTU2 (YFG21), OTU3 (YHB32), and OTU4 (YHC43) and closely related strains showed that each isolate belonged to B. liaoningense, B. ottawaense, B. elkanii, and B. diazoefficiens, respectively (Table S2). A detailed phylogenic analysis based on AMPHORA genes supported this classification (Fig. S1).
The most commonly detected species in Yao City was OTU1 (50.7%), closely related to B. liaoningense, followed by OTU2 (20.7%), closely related to B. ottawaense (Fig. 2). OTU1 was distributed regardless of whether the sites were in greenhouses or open fields. On the other hand, OTU2 was abundant in all open fields, but was limited in greenhouse fields. OTU3 (16.1%), closely related to B. elkanii F100, was detected in three fields. Site Os1, with a high dominance of OTU3, was the only greenhouse field in which Green 80 was cultivated instead of Taisetsu-Midori (Table 1). OTU4 (7.4%), closely related to B. diazoefficiens USDA 110T and USDA 122, was distributed in three sites. The percentage of OTU4 at site Ky was high. OTU5 (2.3%), closely related to B. elkanii USDA 76T, was distributed at three sites, but was less dominant at all sites. OTU6 (1.4%), closely related to B. arachidis, was less dominant, being detected in only three isolates. OTU7 (0.5%), closely related to B. elkanii USDA 61, OTU8 (0.46%), without closely related isolates, and OTU9 (0.46%), closely related to Bradyrhizobium sp. TSA15y, were distributed in only one of the sites. OTU8 comprised a single isolate, YFH81, and the BLAST search showed the highest homology to the B. japonicum strain SR96 (Fig. 2), but at a low value (91.4%), and its exact classification remains unclear.
Using the number of each bradyrhizobial OTU, we calculated two diversity indexes, Simpson (D) and Shannon–Wiener (H’) in each site (Fig. 2). The highest D was found at site Os2 (0.67), followed by site Mi (0.60). The lowest D value (0) was found at sites Yh and Ok1, where only OTU1 was isolated. Similar results were obtained for the diversity index H’, with the highest H’ at site Os2 (1.29), followed by site Mi (1.06), and the lowest H’ (0) at sites Yh and Ok1. The correlation analysis revealed no correlations between each diversity index and soil chemistry (data not shown).
The marketable pod yield of vegetable soybeans ranged between 5.13 and 8.68 t ha–1 among the greenhouse fields surveyed (Matsumura et al., 2022) and between 3.31 and 12.07 t ha–1 among the open fields (Table 1), revealing a large yield variation among the fields. In the present study, no correlations were observed between vegetable soybean yield and rhizobial OTU occupancy or diversity indexes.
Relationship between the distribution of each OTU and soil chemistrySoil chemistry data at the harvesting of vegetable soybeans are shown in Table 2. Overall, soil pH was higher in the open fields, while EC and the content of Mg were higher in greenhouse fields. Regarding the other factors tested, large differences were noted among fields regardless of whether they were open or greenhouse fields.
| Site | pH (H2O) |
EC | NO3-N | P2O5 | K2O | CaO | MgO | Mn | TC | TN | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (dS m–1) | (mg kg–1) | (mg g–1) | |||||||||||
| Greenhouse | Ya | 6.6 | 0.4 | 121.8 | 2,968.7 | 405.2 | 2,265.2 | 576.8 | 4.4 | 1.66 | 0.18 | ||
| Os1 | 6.0 | 0.1 | 17.9 | 219.9 | 181.1 | 896.0 | 205.1 | 21.8 | 0.81 | 0.05 | |||
| Fu | 6.3 | 0.8 | 90.2 | 1,544.6 | 52.3 | 2,976.4 | 366.7 | 2.4 | 1.08 | 0.14 | |||
| Yh | 6.7 | 0.7 | 28.7 | 1,496.5 | 98.0 | 3,427.2 | 369.8 | 11.7 | 1.08 | 0.12 | |||
| Ok1 | 6.6 | 0.7 | 84.1 | 2,977.0 | 124.0 | 3,014.2 | 423.1 | 6.8 | 1.63 | 0.18 | |||
| Ky | 5.3 | 0.1 | 41.8 | 130.5 | 54.1 | 5,471.2 | 665.5 | 37.7 | 0.73 | 0.07 | |||
| Open field | Ka | 7.3 | 0.1 | 68.7 | 3,317.8 | 199.9 | 1,638.0 | 384.3 | 2.5 | 1.21 | 0.12 | ||
| Os2 | 6.6 | 0.1 | 10.2 | 313.3 | 172.6 | 1,289.4 | 252.9 | 11.6 | 1.04 | 0.04 | |||
| On | 6.9 | 0.1 | 43.3 | 1,636.2 | 49.4 | 2,564.8 | 155.7 | 5.1 | 0.85 | 0.08 | |||
| AK | 7.0 | 0.1 | 121.8 | 4,397.0 | 87.9 | 2,459.8 | 158.9 | 3.0 | 1.13 | 0.11 | |||
| Ok2 | 6.5 | 0.1 | 26.0 | 2,367.3 | 42.3 | 2,198.0 | 157.1 | 7.7 | 1.40 | 0.10 | |||
| Mi | 6.0 | 0.2 | 1.0 | 2,880.3 | 181.0 | 1,940.4 | 146.9 | 49.6 | 1.12 | 0.10 | |||
| Average | 6.5 | 0.3 | 54.6 | 2,020.8 | 137.3 | 2,511.7 | 321.9 | 13.7 | 1.14 | 0.11 | |||
The NMDS ordination of rhizobial communities showed a low stress level (0.043), indicating a good representation of OTU compositions (Fig. 3). The NMDS ordination did not show a clear separation of sites according to the cultivation method or field type. There was a trend for sites to cluster together according to OTU abundance, for example, a high OTU1 group (sites Ya, Fu, Yh, Ok1, and On) and high OTU2 group (sites Ka, Ak, and Ok2). Three sites, Os2, Mi, and Os1, in which OTU3 closely related to B. elkanii F100 was detected, positioned right side, while site Ky, in which OTU4 closely related to B. diazoefficiens was identified, was clearly separated to upper side.

Non-metric multidimensional scaling (NMDS) ordination of rhizobial community structures (circles) and soil sampling sites (triangles). Arrows represent vectors of significant environmental variables explaining the ordination (P<0.05).
Ten soil chemical factors that fit as vectors in the NMDS analysis showed that pH and the contents of Ca, Mg, Mn, TC, and TN significantly contributed to the distribution of OTUs (Fig. 2 and Table 3). Important factors related to the distribution of OTUs were soil pH (P=0.003, R2=0.765) and the content of Ca (P=0.004, R2=0.760) (Table 3). The main species OTU1 was detected in soils with a neutral pH and high TN and TC contents. The next main species OTU2 was found under various soil conditions. In contrast, OTU3 and OTU4 were identified in slightly acidic soils with a higher Mn content. OTU1, OTU4, OTU5, and OTU6 were detected in soils with high Ca and Mg contents. The minor species, OTU7 and OTU8 were positioned near OTU3 and OTU9 near OTU1.
| Variable | R2 | P value |
|---|---|---|
| pH | 0.765 | 0.003 |
| EC | 0.422 | 0.074 |
| NO3-N | 0.429 | 0.080 |
| P2O5 | 0.386 | 0.101 |
| K2O | 0.083 | 0.694 |
| CaO | 0.760 | 0.004 |
| MgO | 0.529 | 0.031 |
| Mn | 0.741 | 0.007 |
| TC | 0.486 | 0.048 |
| TN | 0.622 | 0.008 |
The NMDS ordination of rhizobial OTUs and soil chemistry before vegetable soybean cultivation showed that pH and the contents of Ca, Mg, Mn, and P significantly contributed to the distribution of OTUs (Fig. S1). These results were similar to those of the NMDS analysis using soil collected at harvesting (Fig. 3).
Inoculation examinationVegetable soybeans (cv: Ezo-midori) were inoculated with nine representative isolates obtained from the 97% homology classification, and the following phenotypes were evaluated at the V4 stage. Shoot dry weights (SDWs) significantly differed among the treatments (Fig. 4A). Growth-promoting effects on shoot growth varied among the isolates: the growth of vegetable soybeans inoculated with YFG21 (OTU2), THC52 (OTU5), and YFL71 (OTU7) was significantly better than those with the NI treatment. On the other hand, no significant differences were observed between the non-inoculated control and inoculations with YHC11 (OTU1), YHB32 (OTU3), YHC43 (OTU4), YHF61 (OTU6), YFH81 (OTU8), and YHA91 (OTU9) (Fig. 4A). Root dry weights (RDWs) were slightly lower with inoculations than with the NI treatment. The inoculation with YHC43 (OTU4) significantly inhibited root growth (Fig. 4B). Nodule dry weights (NDWs) varied among the isolates, particularly those inoculated with YFH81 (OTU8), which were significantly higher than those inoculated with the other test isolates, except for YFG21 (OTU2) and YHC52 (OTU5) (Fig. 4C). NN did not significantly differ among the test isolates; however, YFL71 (OTU7) formed the most nodules and YFG21 (OTU2) the fewest (Fig. 4D). Some isolates, such as YFG21 (OTU2), had low NN, but high NDWs, and the size of the nodules formed by the inoculated isolates varied. In comparisons of OTU1 and OTU3, the dominant isolates in Yao City, the nodule size of YFG21 (OTU2) was larger than that of YHC11 (OTU1) or YHB32 (OTU3).

Effects of each test strain inoculation on shoot dry weight (SDW) (A), root dry weight (RDW) (B), nodule dry weight (NDW) (C), and the number of nodules (NN) (D) of vegetable soybeans under controlled conditions. Values are averages (n=5)±SE (error bars). Averages followed by different letters are significantly different at P<0.05 between the inoculation treatment analyzed using Tukey’s test, after an analysis of variance (ANOVA), and averages followed by * are significantly different from the non-inoculation (NI) treatment at P<0.05 analyzed using Dunnett’s test.
In the present study, indigenous rhizobacteria, such as B. liaoningense, B. ottawaense, B. elkanii, and B. diazoefficiens, were isolated from vegetable soybean fields in Yao City. B. liaoningense and B. ottawaense were highly distributed in the surveyed fields. B. liaoningense was detected in both greenhouse and open fields, whereas B. ottawaense was mostly restricted to open fields (Fig. 2).
B. liaoningense, which had the highest dominance in vegetable soybean cultivation fields, was originally isolated from soybean fields in Liaoning and other provinces in China (Xu et al., 1995; Zhang et al., 2011). It was also isolated from alkaline-saline soils and was reportedly tolerant of high pH (Appunu et al., 2008; Li et al., 2011). In the present study, B. liaoningense was detected in fields with neutral soil (pH 6.3–7.3), and was absent from those with acidic soil (pH 5.3–6.0), suggesting that it is not suited to thrive in acidic soils. In the NMDS analysis (Fig. 3) fitting significant soil chemical properties (Table 2), the abundance of B. liaoningense was slightly higher in fields with high TN soils, particularly in greenhouse fields at sites Ya, Fu, Yh, and Ok1. At B. liaoningense-dominant sites, soil EC was higher than the average. In contrast, a high Mn content appeared to negatively affect the abundance of B. liaoningense. Based on previous studies and the present results, B. liaoningense may be adapted to more fertile soils under neutral to high pH conditions during vegetable soybean cultivation.
B. ottawaense, the second most dominant species (Fig. 2), was originally isolated from soybean fields in Ottawa, Canada (Yu et al., 2014). It has been also isolated from soybean and peanut cultivation fields in China, and the distribution of B. ottawaense has been reported to positively correlate with available nitrogen and the TN content in soil (Yan et al., 2017; Shao et al., 2020). In the present study, B. ottawaense was detected under various soil nutrient conditions: soil pH (6.0–7.3), soil nitrate (1.0–121.8 mg kg–1), available phosphate (219.9–4397.0 mg kg–1), K2O (42.3–199.9 mg kg–1), Mn (2.5–49.6 mg kg–1), and TN (0.04–0.12 mg g–1), suggesting that it may widely adapt to different soil environments.
B. elkanii, reportedly the main indigenous Bradyrhizobium species in Japan, was isolated from three sites (Os1, Os2, and Mi) (Fig. 2). These three sites had low soil EC, TN contents, and nitrate-N concentrations, which suggested the preference of B. elkanii for soils with low fertility. Previous studies reported that B. elkanii was dominant in acidic soils (Zhang et al., 2011; Suzuki et al., 2014). Since the surveyed fields in the present study generally had neutral soil, soil characteristics other than pH may have affected the distribution of B. elkanii. B. elkanii was previously found to be dominant in fields with low soil fertility (Herrmann et al., 2014), which is consistent with the present results.
OTU4, closely related to B. diazoefficiens, was mostly limited to the Ky site. In Japan, B. diazoefficiens USDA110 appeared to be dominant in reductive soils with low permeability, such as rice paddy conversion fields (Shiina et al., 2014; Saeki and Shiro, 2014; Saeki et al., 2017). However, site Ky is a recently developed upland with soil dressing, not an upland rice paddy conversion field. As a major feature, site Ky had the lowest soil pH (5.3) among the fields surveyed. Tu et al. (2021) isolated acid-tolerant rhizobial bacteria closely related to B. diazoefficiens USDA 110T from soybean nodules in Taiwan, and identified them as potential inoculants to induce the nodulation of soybeans in strongly acidic soils. These findings are consistent with the present results; therefore, OTU4 closely related to B. diazoefficiens may be dominant in site Ky.
The closely related isolate of B. elkanii, OTU5 was also detected in fields with low fertility, similar to OTU3. Furthermore, site Ky, from which OTU5 was isolated, had a significantly higher Ca content in its soil than in the other study plots, suggesting that B. elkanii prefers Ca-rich soils. Previous studies (Zhang et al., 2022) suggested that B. arachidis was isolated from acidic soils and was more likely to be distributed in soils with low fertility, which is consistent with the soil chemistry characteristics of the Ky site, from which OTU6 closely related to B. arachidis was isolated. Since only one strain was detected in OTU7, OTU8, and OTU9, this cannot be definitively discussed in the present study.
Inoculation effects of indigenous Bradyrhizobium in Yao CityIn the present study, no correlations were observed between the farmer’s yield and the occupancy rate of each rhizobial OTU. However, since different farmers in different fields cultivate vegetable soybeans using different methods, difficulties are associated with obtaining accurate data. We herein investigated the effects of B. liaoningense (YHC11[OTU1]), B. ottawaense (YFG21 [OTU2]), B. elkanii (YHB32 [OTU3], YHC52 [OTU5], and YFL71 [OTU7]), B. diazoefficiens (YHC43 [OTU4]), B. arachidis (YHF61 [OTU6]), Bradyrhizobium sp. (YHA91 [OTU9]), and YFH81 (OTU8) on the early growth of vegetable soybean cv. Ezo-midori. The results obtained showed that shoot growth was significantly greater with some rhizobia (YFG21 [OTU2], YHC52 [OTU5], and YFL71 [OTU7]) than with the non-inoculated treatment, but was limited in early growth evaluations (Fig. 4). Therefore, further studies are needed to examine the effects of each test isolate on the yield of vegetable soybeans under the same field conditions.
Unique rhizobial communities in vegetable soybeans in Yao CityThe rhizobial communities identified in the present study are unique from those described in previous studies conducted on soybean fields in Japan (Saeki et al., 2006; Saeki et al., 2013) because B. liaoningense and B. ottawaense were dominant (Fig. 2). B. ottawaense was only reported to have been isolated from the roots of sorghum in Japan (Wasai-Hara et al., 2020a), and no studies showed that B. liaoningense was dominant in soybean nodules. Therefore, to the best of our knowledge, the present study is the first to isolate B. liaoningense and B. ottawaense from soybean roots in Japan. Interestingly, B. ottawaense was detected almost exclusively in open field soils, while B. liaoningense was identified in both greenhouse and open field soils. Although geographic location has been reported to affect the distribution of Bradyrhizobium (Shiro et al., 2013), these differences were less effective in the present study because the surveyed fields were located in a very small area (Fig. 1 and Table 1). Temperature sensitivity may affect the dominance of these rhizobia because vegetable soybeans were grown between February and May in greenhouses and between April and August in open fields in Yao City. Zhang et al. (2003) and Suzuki et al. (2014) reported that the nodulation and competitiveness of Bradyrhizobium were affected by soil temperature, and tolerance of low temperatures varied among rhizobial species. However, an incubation test conducted under low temperatures showed no evidence of B. liaoningense, which was dominant in greenhouse fields, being tolerant of low temperatures, suggesting that the low temperature tolerance of each indigenous rhizobium did not affect the distribution of Bradyrhizobium in Yao City (Fig. S2). Although crop rotation may affect indigenous rhizobia, burdock was cultivated as a previous crop in most fields (Table 1) indicating little effect on differences in the dominance of B. liaoningense and B. ottawaense.
Other differences between greenhouse and open field cultivation is the vegetable soybean cultivar, with ‘Taisetsu-Midori’ mainly being used for greenhouse cultivation and ‘Ezo-Midori’ for open field cultivation. The effector proteins of rhizobia and the Rj and NNL1 genes in host plants have been shown to affect compatibility (Yang et al., 2010; Sugawara et al., 2018; Zhang et al., 2021). Further studies with a gene analysis of host-controlled nodulation are needed to clarify the mechanisms underlying compatibility between vegetable soybeans and B. liaoningense and B. ottawaense. Furthermore, rhizobial invasion from external sources may be possible because farmers often use mountain soil as soil dressing in Yao City. Although it was unclear whether soil dressing was performed in the surveyed fields, it may have contributed to the difference in dominance between B. liaoningense and B. ottawaense.
Potential agricultural use of major indigenous Bradyrhizobium in Yao CityPrevious studies reported that Ensifer melitoi, Rhizobium leguminosarum, and B. diazoefficiens possess nosZ-coding N2O reductase, and B. diazoefficiens may mitigate soil N2O emissions during the growth and post-growth periods of soybean crops on a field scale (Itakura et al., 2013; Torres et al., 2014; Akiyama et al., 2016; Catherine et al., 2022). B. ottawaense was also recently shown to possess nosZ (Mania et al., 2019; Wasai-Hara et al., 2020a). Sustainable agricultural technology with a low environmental impact is highly expected in the near future. The results of the genome analysis confirmed that YHC11 (OTU1) and YFG21 (OTU2), which are closely related to B. liaoningense and B. ottawaense, also possess nosZ. By focusing on vegetable soybean fields, the present study provides novel insights into the Japanese rhizobial flora and new materials for agriculture with less of an environmental impact.
Minakata, C., Wasai-Hara, S., Fujioka, S., Sano, S., and Matsumura, A. (2023) Unique Rhizobial Communities Dominated by Bradyrhizobium liaoningense and Bradyrhizobium ottawaense were Found in Vegetable Soybean Nodules in Osaka Prefecture, Japan. Microbes Environ 38: ME22081.
https://doi.org/10.1264/jsme2.ME22081
We would like to thank the vegetable soybean farmers in Yao City for their cooperation with this study. The present study was supported in part by a grant from the Takano Life Science Research Foundation, JA Bank Osaka Shinren, and Grants-in-Aid for JSPS Fellows Grant Numbers [20J12228] and [22J01397].