2023 年 38 巻 2 号 論文ID: ME23006
2023 年 38 巻 2 号 論文ID: ME23006
Hydrogen peroxide (H2O2) inhibits microbial growth at a specific concentration. However, we previously isolated two environmental bacterial strains that exhibited sensitivity to a lower H2O2 concentration in agar plates. Putative catalase genes, which degrade H2O2, were detected in their genomes. We herein elucidated the characteristics of these putative genes and their products using a self-cloning technique. The products of the cloned genes were identified as functional catalases. The up-regulation of their expression increased the colony-forming ability of host cells under H2O2 pressure. The present results demonstrated high sensitivity to H2O2 even in microbes possessing functional catalase genes.
Two Comamonadaceae bacteria, Rhodoferax sp. OS-1 and Curvibacter sp. OS-4 were aerobically isolated from freshwater samples as microbes that were highly sensitive to H2O2-embedded agar medium (Watanabe et al., 2022). Their colony-forming ability on agar medium was completely inhibited in the presence of 7.2 μM H2O2 (Watanabe et al., 2022). Common agar growth medium contains approximately 15 μM H2O2 (Tanaka et al., 2014). This concentration does not affect the growth of common laboratory strains, such as Escherichia coli, Pseudomonas putida, and Bacillus subtilis, but was shown to markedly inhibit the growth of environmental microbes, including OS-1 and OS-4 (Watanabe et al., 2022).
H2O2 is endogenously generated by aerobic microbes during aerobic respiration, and is produced by E. coli at a rate of 10–15 μM s–1 (Seaver and Imlay, 2001). Intracellular H2O2 is immediately degraded by various enzymes, including catalase (Seaver and Imlay, 2001; Mishra and Imlay, 2012; Sen and Imlay, 2021), because the accumulation of 0.5 μM of intracellular H2O2 inhibits microbial growth (Sen and Imlay, 2021). Strains OS-1 and OS-4 both grow aerobically, and a genome sequencing analysis of these strains revealed that they possessed at least one putative catalase gene despite exhibiting high sensitivity to H2O2 (Watanabe et al., 2023). We hypothesized that if the putative catalase genes in OS-1 and OS-4 were properly expressed and translated products were functional, the colony-forming ability of both strains on agar media may be enhanced. However, the functions of these genes and their products remain unclear. High sensitivity to H2O2 may occur if (1) the putative catalase gene is a structural pseudogene with critical mutations (e.g., a frameshift mutation) that hinder the formation of the proper amino acid sequence, (2) putative catalase is not expressed in the gene or its expression is insufficient to detoxify H2O2 from agar medium, or (3) the translated product does not function as a catalase enzyme. The colony-forming abilities of mutant strains of Escherichia, Salmonella, Shewanella, and Yersinia species lacking genes for the degradation of H2O2 were previously shown to be reduced (Ezraty et al., 2014; Shi et al., 2015; Wan et al., 2017; Li and Imlay, 2018; Wan et al., 2021).
The present study investigated the sequences and expression of catalase genes in H2O2-sensitive environmental strains. These genes were cloned and examined to confirm gene functionality and elucidate the relationship between catalase activity and colony-forming ability on artificial agar media.
Since both strains are highly sensitive to H2O2, their putative catalase genes were initially suspected to be structural pseudogenes; therefore, these genes were re-sequenced and their adequacy was analyzed. Based on a genome sequencing analysis (Watanabe et al., 2023), strain OS-1 was found to possess two putative catalase genes (locus tags of os1_07570 and os1_37390), while strain OS-4 harbored one gene (locus tag of os4_08060). Putative catalase genes in the OS-1 and OS-4 genomes were intact open reading frames (ORF) with proper start and stop codons, and no frameshift mutations were detected. The predicted amino acid sequences of these enzymes showed sequence similarities to previously reported catalases; these genes were less likely to be structural pseudogenes. A BLASTp (NCBI) analysis revealed that the highest amino acid sequence identity of os1_07570 was 83.72% to the putative catalase gene of Rhodoferax sp. OV413, that of os1_37390 was 91.55% to the putative catalase gene of Rhodoferax sp. OV413, and that of os4_08060 was 96.19% to the putative catalase gene of Rhodoferax aquaticus. The amino acid sequences of these genes and the catalase genes with previously reported activities (e.g., E. coli catalases) were utilized to construct the phylogenetic tree shown in Fig. 1. Each of the catalases from both strains showed the closest relationship with those identified in the genomes of Rhodoferax spp.; however, the enzymatic activities of the putative catalases of Rhodoferax spp. were not experimentally confirmed. In the tree, one OS-1 catalase (os1_37390) and OS-4 catalase (os4_08060) clustered with KatG enzymes that encode bifunctional catalase-peroxidase. Another OS-1 catalase (os1_07570) clustered with KatE enzymes that encode monofunctional catalases. Therefore, we designated these catalase genes as katGos1 and katE os1 for strain OS-1 catalases and katGos4 for strain OS-4 catalase. The expression of these catalase genes was evaluated using a reverse transcription polymerase chain reaction (PCR). Their expression was not confirmed in wild-type OS-1 or OS-4 colonies, indicating that neither of these genes were expressed or their expression was below the detection level of the method used (data not shown).
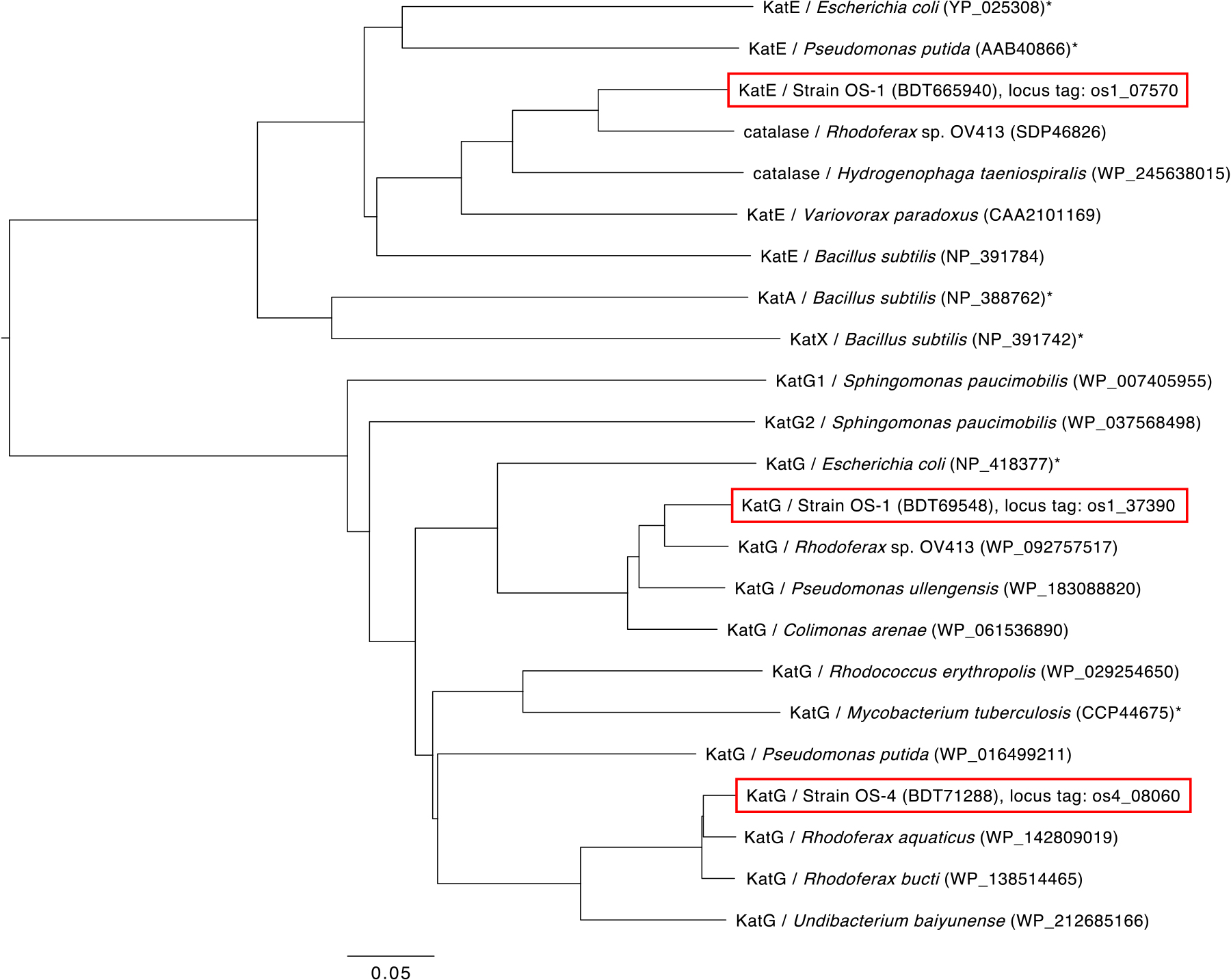
Phylogenetic tree of catalases based on their predicted amino acid sequences. The catalases present in OS-1 and OS-4 are shown in the red frame. Catalases experimentally tested for their activities are marked with an asterisk “*”. The scale bar indicates 0.05 substitutions per amino acid position. Protein ID numbers are shown in parentheses after the host name.
Catalase genes were cloned to confirm their enzymatic activities. The putative catalase genes of OS-1 and OS-4 were amplified from the genomic DNA of OS-1 or OS-4 using PrimeSTAR GXL DNA Polymerase (Takara) with the following primers:
katGos1: forward primer; 5′-AGGAGACATTACATATGACCACCGAAGCCAAATGC-3′, reverse primer; 5′-CCTTGGATCCCTCGAGTTACGCCAGATCGAACCG-3′,
katEos1: forward primer; 5′-AGGAGACATTACATATGACCCCCACCGCTACCCAA-3′, reverse primer; 5′-CCTTGGATCCCTCGAGCTATGCAGGTACAGTGGC-3′, and
katGos4: forward primer; 5′-AGGAGACATTACATATGACTACTGAAGCCAAATGCC-3′, reverse primer; 5′-CCTTGGATCCCTCGAGTCACACGAGGTCGAAGCG-3′. PCR conditions were as follows: the initial denaturation of DNA at 94°C for 1 min, followed by 30 cycles at 98°C for 10 s and at 68°C for 1 min kb–1, with a final extension at 68°C for 3 min. Only the protein-coding region of each gene was amplified and gene fragments were separately inserted into a plasmid vector using an In-Fusion cloning kit (Takara), followed by the confirmation of “PCR error-free” by sequencing. Since the environmental isolates OS-1 and OS-4 have no previously established host–vector system, the broad-host-range vector pHRP308 (RSF1010 replicon) (Parales and Harwood, 1993) was used with the following modifications. The gentamycin-selective marker gene of pHRP308 was replaced with a kanamycin marker gene, and the lacZ gene was removed, yielding the vector, pCPKM. The plasmids with catalase gene inserts were named pOS1katG (harboring katGos1), pOS1katE (harboring katEos1), and pOS4katG (harboring katGos4). In addition, functional catalase genes from E. coli were used as a positive control (Loewen et al., 1993; Singh et al., 2008). The katG and katE genes were PCR-amplified from the genomic DNA of E. coli K-12, with the same PCR conditions as the amplification of other catalases, but using the following primers:
katG: forward primer; 5′-GCTAAGGAAGCTAAAATGAGCACGTCAGACGATATC-3′, reverse primer; 5′-TATGTTGCGACATTACAGCAGGTCGAAACGG-3′ and
katE: forward primer; 5′-CGACCTGCTGTAATGTCGCAACATAACGAAAAGAAC-3′, reverse primer; 5′-ACTGCCTTAAAAAAATCAGGCAGGAATTTTGTCAATC-3′.
The amplified katG and katE gene fragments were tandemly connected and inserted into the pCPKM vector, and the plasmid was named pECkatGE. To enhance the probability of gene expression, a bacterial consensus promoter (TTGACA-17 nt-TATAAT) and Shine-Dalgarno sequence (AGGAGA) were located upstream of the cloned genes on each plasmid (Fig. S1). These plasmids were introduced into strains OS-1 and OS-4 by electroporation. Electrocompetent cells were prepared as follows: OS-1 and OS-4 cultured in liquid peptone-yeast-glucose (PYG) medium (Tanaka et al., 2014) were collected by centrifugation (1,500×g at 4°C), cell pellets were washed twice with sterilized 10% glycerol, and cells were finally re-suspended in 10% glycerol. Using the Gene Pulser Xcell Electroporation System with the following parameters: exponential protocol, voltage: 1–1.6 kV, resistance: 400 Ω (Bio-Rad), the pOS1katG and pOS1katE plasmids were introduced into OS-1 cells and pOS4katG into OS-4 cells, while pECkatGE (positive control) and the blank vector pCPKM (negative control) were introduced into OS-1 and OS-4 cells.
To confirm cellular catalase activity, the wild-type and transformed strains of OS-1 and OS-4 were examined using the conventional catalase test. Bacterial colonies grown aerobically on solid medium were transferred onto a glass slide, and 30% commercially available H2O2 was dropped on the top. The degradation of H2O2 was assessed by observing the formation of oxygen bubbles (Fig. 2). Wild-type OS-1 and OS-4 and their blank vector transformants did not form bubbles (Fig. 2A, B, F, and G), whereas the transformants harboring cloned catalase genes rapidly formed bubbles after the application of H2O2 droplets (Fig. 2C, D, E, H, and I).

Detection of cellular catalase activities of OS-1, OS-4, and their transformants. Strains used in the panels; wild-type OS-1 (A), OS-1+pCPKM (B), OS1+pECkatGE (C), OS-1+pOS1katG (D), OS-1+pOS1katE (E), Wild-type OS-4 (F), OS-4+pCPKM (G), OS-4+pECkatGE (H), and OS-4+pOS4katG (I). Images were taken right after 20 μL of 30% H2O2 was dropped on the top of smeared colonies.
These results indicate that the broad-host-range vector used was successfully maintained and also that E. coli catalase was functionally expressed in these strains (Fig. 2C and H). Furthermore, the results of self-cloning experiments demonstrated that the catalases produced in OS-1 and OS-4 were functionally active enzymes (Fig. 2D, E, and I).
The colony-forming abilities of transformed OS-1 and OS-4 were examined by cultivating them on agar medium. Wild-type strains and their transformants were initially cultured in PYG liquid medium and diluted until OD600 reached 0.1, followed by a 10-fold serial dilution to 10–7 in sterile distilled water. After each dilution, 10 μL was spotted on the surface of PYG solid medium embedded with different H2O2 concentrations. The preparation of solid medium and the measurement of H2O2 concentrations were performed as described in our previous study (Watanabe et al., 2022). After an incubation at 20°C for 6 days, the growth of each strain was evaluated. On the plate containing 0.9 μM H2O2, strains OS-1 and OS-4 both showed similar growth among wild-type, blank vector, and catalase gene-containing transformants up to the 10–6 or 10–5 dilution (Fig. 3A). Regarding strains OS-1 and OS-4, the growth of wild-type and blank vector transformants on the plate with 13.3 μM H2O2 was only observed on the first spot. E. coli catalase transformants exhibited growth up to the 10–3 dilution spot, and self-cloning transformants showed growth up to the 10–5 dilution spot (Fig. 3B). The colony-forming units (CFU) of wild-type strains growing on the agar plate containing 13.3 μM H2O2 decreased to approximately 10–5 order of magnitude compared to the CFU of strains growing on the agar plate containing 0.9 μM H2O2. Self-cloning transformants maintained the original CFU count even in the presence of 13.3 μM H2O2. Furthermore, the original CFU count of the self-cloning transformants was maintained in the presence of 47.9 μM H2O2 (data not shown). Collectively, these results demonstrated that endogenous catalase expression and activity were crucial for colonization.

Growth of OS-1, OS-4, and their transformants on PYG agar plates containing 0.9 μM (A) or 13.3 μM (B) H2O2. Strains grown aerobically in PYG liquid medium were collected and re-suspended to obtain an OD600 of 0.1 (dilution 100), and the cell suspension was serially diluted and 10 μL of each dilution was spotted. Images were taken after an incubation at 20°C for 6 days.
Although putative catalase genes were identified in the genomes of OS-1 and OS-4, their mRNA expression was not detected. To elucidate the function of the gene products, we self-cloned these catalase genes. The present study is the first to successfully self-clone the catalase genes of environmental H2O2-highly sensitive isolates using a broad-host-range plasmid vector, and we demonstrated that the products expressed were functional catalases. In addition, the results obtained herein indicate that the function and activity of a gene cannot be predicted by its presence in the genome, even for well-known and studied genes. Since the up-regulated expression of self-cloned catalase genes markedly increased the colony-forming abilities of the two strains (Fig. 3), we showed, for the first time, that H2O2 in the agar plate was the main component that inhibited colony formation by strains OS-1 and OS-4, and the insufficient expression of the catalase genes was one of the causes of their high sensitivity to H2O2. A promoter search analysis by GENETYX-MAC v.21.0.1 software (GENETYX CORPORATION) revealed the presence of putative promoter and Shine-Dalgarno sequences at the upstream regions of each catalase gene in the OS-1 and OS-4 genomes. These native promoters scored 40 to 50 by the software, while the bacterial consensus promoter used in the present study scored 77. Although native promoter strength and regulation in wild-type OS-1 and OS-4 remain unknown, the consensus promoter may have markedly up-regulated catalase gene expression in the recombinant strains. The present results imply the presence of numerous similar environmental microbes that are highly sensitive to H2O2 in the agar plate, regardless of whether functional catalase genes were present in their genomes. These microbes may be able to tolerate the H2O2 pressure found in natural environments, but may be vulnerable to the amount of H2O2 in agar medium. H2O2 concentrations detected in a natural environment are always in the nanomolar order (Wilson et al., 2000; Takeda et al., 2018; Garcia et al., 2020), which may not be a threatening concentration as exogenous H2O2; therefore, for many microbes, the expression of catalase genes may not be required under natural conditions. The presence of environmental microbes possessing the enzyme that decomposes H2O2 concentrations in the micromolar order is of interest; however, the expression of these genes may not be effectively induced on agar plates.
To decrease H2O2 concentrations in agar media, previous studies applied 20–100 units mL–1 bovine liver catalase enzyme (Olson et al., 2000; Kawasaki and Kamagata, 2017; Adam et al., 2018), which successfully increased the colony yield of environmental microbes; however, the routine preparation of this medium is expensive. Moreover, the presence of other growth inhibitors in the agar plate has been reported (Kawasaki and Kamagata, 2017), and their degradation may require more than the simple supplementation of inexpensive enzymes. Under these conditions, the potential detoxifying abilities of the genomes of microbes may enhance their colony formation.
Although the catalase gene cloning technique used in the present study significantly improved the colony-forming abilities of the OS-1 and OS-4 strains, this technique is not always easily applied to a number of environmental microbes because host–vector systems and gene introduction methods strongly depend on and differ among strains. A novel pre-treatment or modified cultivation technique that effectively induces the expression of catalase genes (or other functional genes that degrade other growth inhibitors) in each microorganism is needed to improve colony-forming abilities, thereby increasing opportunities to isolate novel environmental microbes.
Watanabe, M., Igarashi, K., Kato, S., Kamagata, Y., and Kitagawa, W. (2023) Self-cloning of the Catalase Gene in Environmental Isolates Improves Their Colony-forming Abilities on Agar Media. Microbes Environ 38: ME23006.
https://doi.org/10.1264/jsme2.ME23006
This work was supported by JSPS KAKENHI grants (grant numbers 18H05295 and 20H05594), a JST SPRING grant (grant number JPMJSP2119), and the Institute for Fermentation, Osaka (IFO).