2023 年 38 巻 2 号 論文ID: ME23019
2023 年 38 巻 2 号 論文ID: ME23019
Pseudomonas putida is a major species belonging to the genus Pseudomonas. Although several hundred strains of P. putida have been deposited in culture collections, they potentially differ from the genetically defined “true Pseudomonas putida” because many were classified as P. putida based on their phenotypic and metabolic characteristics. A phylogenetic analysis based on the concatenated sequences of the 16S rRNA and rpoD genes revealed that 46 strains of P. putida deposited in Japanese culture collections were classified into nine operational taxonomic units (OTUs) and eleven singletons. The OTU7 strain produces N-acylhomoserine lactone as a quorum-sensing signal. One of the OTU7 strains, JCM 20066, exhibited a ppuI-rsaL-ppuR quorum-sensing system that controls biofilm formation and motility. The P. putida type strain JCM 13063T and six other strains were classified as OTU4. Classification based on the calculation of whole-genome similarity revealed that three OTU4 strains, JCM 20005, 21368, and 13061, were regarded as the same species as JCM 13063T and defined as true P. putida. When orthologous genes in the whole-genome sequences of true P. putida strains were screened, PP4_28660 from P. putida NBRC 14164T (=JCM 13063T) was present in all true P. putida genome sequences. The internal region of PP4_28660 was successfully amplified from all true P. putida strains using the specific primers designed in this study.
Pseudomonas is a heterotrophic, motile, Gram-negative, rod-shaped bacterium found in natural environments, such as plants, soil, animals, and water (Palleroni, 1993; Diggle and Whiteley, 2020). The genus Pseudomonas was initially described by Migula according to the morphological characteristics of its members (Migula, 1894). The genus Pseudomonas was subsequently subdivided into five groups based on the findings of DNA-DNA and rRNA-DNA hybridization (Palleroni et al., 2005). Among these groups, group I is referred to as the genus Pseudomonas, which is called “true Pseudomonas” or “Pseudomonas sensu stricto”, whereas different designations have been proposed for the members of groups II-V (Scarpellini et al., 2004). Pseudomonas group I includes many fluorescent and non-fluorescent strains. The most common fluorescent species in Pseudomonas group I are Pseudomonas fluorescens and Pseudomonas putida, which are known as fluorescent Pseudomonas (Scarpellini et al., 2004).
P. putida is one of the major species of Pseudomonas group I, which performs many functions, such as plant growth promotion and bioremediation. For example, P. putida F1 is a soil isolate that is capable of chemotaxis for the degradation of numerous aromatic compounds (Hughes et al., 2017). P. putida KT2440 induces systemic resistance in plants in response to certain pathogens (Matilla et al., 2010). In addition, strain KT2440 metabolizes a wide range of aromatic compounds and has potential for the environmental bioremediation of industrial waste (Gong et al., 2017). In contrast, some strains of P. putida are opportunistic human pathogens responsible for bacteremia, sepsis in neonates, and neutropenia (Horii et al., 2005). Several hundred strains of P. putida have been deposited in culture collections worldwide. Many of the P. putida strains deposited in these culture collections have been classified as P. putida based on their phenotypic and metabolic characteristics, such as morphology, cultural characteristics, and biochemical testing, and not on genetic techniques. Therefore, the classification of these strains potentially differs from P. putida. In the present study, we defined the P. putida type strain and its genetically related strains as “true P. putida”. We reclassified P. putida strains deposited in Japanese culture collections based on genomic and phenotypic diversities and developed specific PCR primers to identify true P. putida among these bacterial strains.
The P. putida strains used in the present study are listed in Table 1. Forty-six strains of P. putida were obtained from the Japan Collection of Microorganisms (JCM) or NITE Biological Resource Center (NBRC). Escherichia coli was grown in Luria-Bertani (LB) medium at 37°C. P. putida strains were grown in trypticase soy broth (TSB; Becton, Dickinson and Co.) or King B medium (KB; Eiken) at 30°C. Chromobacterium violaceum CV026 (McClean et al., 1997) and VIR07 (Morohoshi et al., 2008) were grown in LB medium at 30°C. A solid bacterial medium was prepared by adding agar to obtain a final concentration of 1.5%. Antibiotics were added at final concentrations of 100 μg mL–1 (ampicillin and carbenicillin) and 50 μg mL–1 (kanamycin), as required. N-(3-oxodecanoyl)-l-homoserine lactone (3-oxo-C10-HSL) was synthesized, as previously described (Chhabra et al., 2003). 3-oxo-C10-HSL was dissolved in dimethyl sulfoxide (DMSO) to prepare a 10 mM stock solution and added to the medium at a final concentration of 1 μM for the biofilm and motility test described below.
| Strains | OTUa | AHL productionb | Motilityc | Fluorescence | Antifungal activity |
|
|---|---|---|---|---|---|---|
| CV026 | VIR07 | |||||
| JCM 9802 | 1 | – | – | ++ | + | – |
| JCM 9800 | 1 | – | – | + | + | – |
| JCM 9045 | 1 | – | – | – | + | – |
| JCM 31910 | 1 | – | – | – | + | – |
| JCM 20048 | 1 | – | – | + | + | – |
| JCM 20045 | 1 | – | – | – | + | – |
| JCM 20004 | 1 | – | – | + | + | – |
| NBRC 12668 | 1 | – | – | ++ | + | – |
| NBRC 109110 | 1 | – | – | – | + | – |
| NBRC 109109 | 1 | – | – | – | + | – |
| NBRC 101019 | 1 | – | – | + | + | – |
| NBRC 100988 | 1 | – | – | ++ | + | – |
| NBRC 100650 | 1 | – | – | – | + | – |
| JCM 6157 | 2 | – | – | – | + | – |
| JCM 20129 | 2 | – | – | + | + | – |
| JCM 20089 | 2 | – | – | ++ | + | – |
| JCM 20028 | 3 | – | – | + | + | – |
| NBRC 14671 | 3 | – | – | ++ | + | – |
| JCM 20188 | 4 | – | – | – | + | – |
| JCM 20187 | 4 | – | + | – | – | – |
| JCM 20005 | 4 | – | – | + | + | – |
| JCM 14351 | 4 | – | + | – | + | – |
| JCM 13063T | 4 | – | – | – | + | – |
| JCM 13061 | 4 | – | – | – | + | – |
| JCM 21368 | 4 | – | – | – | + | – |
| NBRC 15366 | 5 | – | – | ++ | + | – |
| NBRC 12996 | 5 | – | – | + | + | – |
| NBRC 109347 | 5 | – | – | ++ | + | + |
| JCM 20228 | 6 | – | – | ++ | + | – |
| JCM 20029 | 6 | – | – | – | + | – |
| JCM 20112 | 7 | – | ++ | – | – | – |
| JCM 20102 | 7 | – | ++ | ++ | – | – |
| JCM 20066 | 7 | – | ++ | ++ | + | – |
| JCM 20111 | 8 | – | – | + | + | – |
| NBRC 101020 | 9 | – | – | ++ | + | – |
| NBRC 3738 | ST | – | ++ | – | – | – |
| NBRC 14796 | ST | ++ | + | ++ | + | + |
| NBRC 13696 | ST | – | – | – | + | – |
| NBRC 12653 | ST | – | – | + | + | – |
| NBRC 110482 | ST | – | + | ++ | + | – |
| NBRC 110474 | ST | – | – | + | + | – |
| NBRC 102093 | ST | – | – | ++ | + | – |
| NBRC 102092 | ST | – | – | ++ | + | – |
| NBRC 102090 | ST | – | – | – | + | – |
| JCM 20120 | ST | – | – | – | + | – |
| JCM 13062 | ST | – | – | – | + | – |
aST, singleton; bAHL production means strong (++), weak (+), or no (–) induction of violacein production in AHL reporter strains; cDiameter of the spread colony after a 24-h incubation, ≥15 mm (++) >5 mm (+), or no motility (–).
The 16S rRNA and rpoD genes were amplified by PCR using Blend Taq Plus DNA polymerase (Toyobo) and the specific primer sets 16SF3/16SR3 (Sawada et al., 2011) and PsEG30F/PsEG790R (Mulet et al., 2010), respectively (Table S1). PCR for the 16S rRNA gene was performed using the following cycling parameters: 94°C for 30 s, 50°C for 30 s, and 72°C for 1.5 min for 30 cycles. PCR for the rpoD gene was conducted using the following cycling parameters: 98°C for 10 s, 55°C for 30 s, and 72°C for 1 min for 30 cycles. Sequencing was performed using the BigDye Terminator ver. 3.1 and an Applied Biosystems 3500 Series Genetic Analyzer (Applied Biosystems). A phylogenetic tree based on the concatenated sequences of the 16S rRNA and rpoD genes was constructed using the neighbor-joining method with the ClustalW program in MEGA 7 (Kumar et al., 2016). Clustering of the concatenated sequences of the 16S rRNA and rpoD genes for operational taxonomic unit (OTU) predictions was performed according to a previously described method (Morohoshi et al., 2018). OTUs were defined with ≥98% identity for clustering analyses.
Assay for fluorescence production, motility, and antifungal activityColonies of P. putida strains were formed on TSB agar plates. To assess fluorescence production, colonies of P. putida strains were inoculated onto the KB agar plate and incubated at 30°C for two days. Fluorescence was detected after irradiation with ultraviolet (UV) light (λ=365 nm). To evaluate motility, colonies of P. putida strains were inoculated onto KB soft agar (0.5 wt%) medium. After an incubation at 25°C for 24 h, motility was observed by measuring the spread of colonies on assay plates. To detect antifungal activity, a mycelial disc of Rhizoctonia solani MAFF 726551 was placed on top of a dNBYG agar plate (Takeuchi et al., 2014). P. putida strains were inoculated at the bottom of dNBYG agar plates. After an incubation at 25°C for three days, antifungal activity was estimated by inhibiting the development of the mycelia of R. solani.
Identification of N-acylhomoserine lactone (AHL) moleculesAHL production was tested by cross-streaking two AHL reporter strains, C. violaceum CV026 and VIR07 (Morohoshi et al., 2021). CV026 and VIR07 were streaked horizontally on LB agar plates, while P. putida strains were streaked vertically next to them. After an overnight incubation at 30°C, AHL produced by the strains under study diffused and induced the production of the purple pigment violacein by AHL reporter strains. AHL-producing activity was evaluated on three levels (strong, weak, and none) based on the intensity of the purple color. AHL molecules produced by P. putida were extracted from the culture supernatant using a previously described method (Morohoshi et al., 2019). AHL molecules were identified using Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS), as previously described (Morohoshi et al., 2019).
Whole-genome shotgun sequencingThe genomic DNA of P. putida strains was extracted using a standard protocol with sodium dodecyl sulfate (SDS) and proteinase K. Library construction and sequencing using the Illumina NovaSeq 6000 and HiSeq X Ten platforms were performed using the commercial services of Eurofins Genomics. Adapter-trimmed raw reads were assembled using SPAdes v3.13.0 (Bankevich et al., 2012). Assemblies were annotated using the DDBJ Fast Annotation and Submission Tool (DFAST) version 1.2.18, which is a bacterial genome annotation pipeline (Tanizawa et al., 2018). Briefly, coding sequences (CDSs) were predicted using MetaGeneAnnotator (Noguchi et al., 2008). Genes coding for tRNA and rRNA were identified using Aragorn 1.2.38 (Laslett and Canback, 2004) and Barrnap 0.8 (https://github.com/tseemann/barrnap), respectively.
Comparative genome analysisThe whole genome sequences of P. putida were retrieved from sequences deposited in the NCBI genome website (https://www.ncbi.nlm.nih.gov/genome) on Jan 20, 2023. Whole-genome similarity was analyzed by calculating average nucleotide identity (ANI) and digital DNA-DNA hybridization (dDDH) values. ANI values were calculated by the pairwise calculation method using the OrthoANIu online tool (https://www.ezbiocloud.net/tools/ani) (Yoon et al., 2017). dDDH values were calculated using formula two of the Genome-to-Genome Distance Calculator 3.0 (https://ggdc.dsmz.de/ggdc.php) (Meier-Kolthoff et al., 2022). Orthologous genes among P. putida strains were inferred using OrthoFinder 2.5.4 (Emms and Kelly, 2015). To detect the presence of one orthologous gene, PP4_28660, the internal region of PP4_28660 was amplified by direct colony PCR using the KOD One PCR Master Mix (Toyobo) and the specific primer sets, Ppu28660-F/Ppu28660-R (Table S1). PCR was performed using the following cycling parameters: at 98°C for 10 s, 60°C for 5 s, and 68°C for 30 s for 30 cycles.
Cloning and disruption of quorum-sensing genesThe ppuI and ppuR coding regions in the JCM 20066 genome were amplified using Blend Taq Plus DNA polymerase and specific primers (Table S1). PCR products were cloned into the pGEM-T Easy Cloning Vector (Promega). To remove the internal sequences of the target genes, sequences upstream and downstream of the target gene were amplified using pGEM-T-containing plasmids as the template and specific primers for gene deletion (Table S1). Amplified PCR fragments were excised via BamHI digestion and self-ligated. The gene-coding region with a deletion in the internal sequence was excised using EcoRI and inserted into the EcoRI site of the suicide vector pK18mobsacB (Schafer et al., 1994). Deletion mutants of quorum-sensing genes were generated by homologous recombination using a previously described method (Morohoshi et al., 2022).
Biofilm formation assayOvernight cultures of JCM 20066 and its mutants were diluted to an OD600 of 0.01 in TSB medium. One hundred microliters of this dilution was transferred to a 96-well polystyrene plate (AsOne). After an incubation at 30°C for 18 h, 25 μL of 1% crystal violet solution was added to each well. The plates were then incubated at room temperature for 15 min and rinsed twice with distilled water. Crystal violet was dissolved in 100 μL of 99.5% ethanol. Biofilm formation was quantified by measuring absorbance at 570 nm (A570) using an Infinite M200 microplate reader (Tecan Japan). The A570 values of the wells were averaged, and standard deviations were calculated. Means were separated using Tukey’s honest significant difference (HSD) test (P<0.01).
Nucleotide sequence accession numberThe whole genome shotgun sequences of P. putida were deposited in the DDBJ/ENA/GenBank databases under the accession numbers listed in Table 2. The raw sequencing reads used in this study have been deposited in the DDBJ Sequence Read Archive under the accession number DRA015616. The nucleotide sequences of 16S rRNA and rpoD reported in this study were deposited in the DDBJ/ENA/GenBank databases under the accession numbers listed in Table S2.
| Strains | OTUa | ANI (%)b | dDDH (%)b | Genome information | |||
|---|---|---|---|---|---|---|---|
| Total size (bp) | GC cont. (%) | CDSs | Accession no.c | ||||
| JCM 13063T (=NBRC 14164T) | 4 | 100 | 100 | 6,156,701 | 62.3 | 5,372 | AP013070* |
| JCM 20005 | 4 | 99.4 | 95.6 | 6,055,601 | 62.3 | 5,387 | BSKE01000000 |
| JCM 21368 | 4 | 99.2 | 94.8 | 6,321,123 | 62.3 | 5,770 | BSKH01000000 |
| JCM 13061 | 4 | 98.4 | 87.0 | 6,473,766 | 61.8 | 5,930 | BSKD01000000 |
| JCM 20187 (=NRRL B-252) | 4 | 94.9 | 60.3 | 6,087,214 | 62.5 | 5,364 | RWKF00000000* |
| JCM 14351 (=NRRL B-251) | 4 | 94.8 | 60.4 | 6,068,462 | 62.6 | 5,352 | RWKE00000000* |
| JCM 20188 | 4 | 93.6 | 54.0 | 6,201,866 | 62.1 | 5,567 | BSKG01000000 |
| NBRC 14671 | 3 | 90.7 | 43.6 | 5,793,231 | 61.9 | 5,189 | BSKJ01000000 |
| JCM 20028 | 3 | 90.7 | 43.6 | 5,784,640 | 61.9 | 5,188 | BSKF01000000 |
| NBRC 110474 | ST | 90.3 | 41.5 | 6,066,026 | 63.0 | 5,364 | BSKM01000000 |
| NBRC 109347 | 5 | 90.1 | 41.2 | 5,723,681 | 63.1 | 5,162 | BSKL01000000 |
| NBRC 12996 | 5 | 89.7 | 40.0 | 5,796,075 | 62.6 | 5,223 | BSKI01000000 |
| NBRC 15366 | 5 | 89.5 | 39.5 | 5,669,176 | 63.0 | 5,082 | BSKK01000000 |
| JCM 20066 | 7 | 86.9 | 32.8 | 6,317,210 | 62.2 | 5,528 | BSKN01000000 |
aST, singleton; bANI and dDDH values were calculated using P. putida NBRC 14164T as the reference genome; cAsterisks indicate sequences previously deposited in the NCBI database.
The 16S rRNA genes of 46 strains of P. putida and 14 type strains of other species of Pseudomonas were sequenced and used for an OTU clustering analysis. When the OTUs were strictly defined based on ≥99% identity, 39 strains of P. putida and type strains of P. mosselii, P. monteilii, and P. plecoglossicida were classified into the same OTU (Fig. S1). These results indicate that 16S rRNA sequences were not suitable for the classification of P. putida and other Pseudomonas species. Therefore, another housekeeping gene, rpoD, was sequenced and used for OTU clustering. The phylogenetic tree based on rpoD sequences showed more diverse branching than that based on 16S rRNA sequences (Fig. S2). Based on these results, concatenated sequences of the 16S rRNA and rpoD genes were used for OTU clustering for a more accurate classification of P. putida strains. A phylogenetic tree based on the concatenated sequences of the 16S rRNA and rpoD genes is shown in Fig. 1. Clustering of the concatenated sequences of the 16S rRNA and rpoD genes at 98% identity revealed that 46 strains of P. putida were classified into nine OTUs and eleven singletons. Strains classified as OTU2, OTU4, OTU8, and OTU9 showed high identity with type strains P. monteilii, P. putida, P. korrensis, and P. veronii, respectively. Since the other strains did not show a certain degree of identity with the type strains of Pseudomonas species, these strains may be classified as novel Pseudomonas species or subspecies. The P. putida type strain JCM 13063T was classified as OTU4, which includes only six strains: JCM 13061, JCM 14351, JCM 20005, JCM 20187, JCM 20188, and JCM 21368. Therefore, only these seven strains were classified as true P. putida.
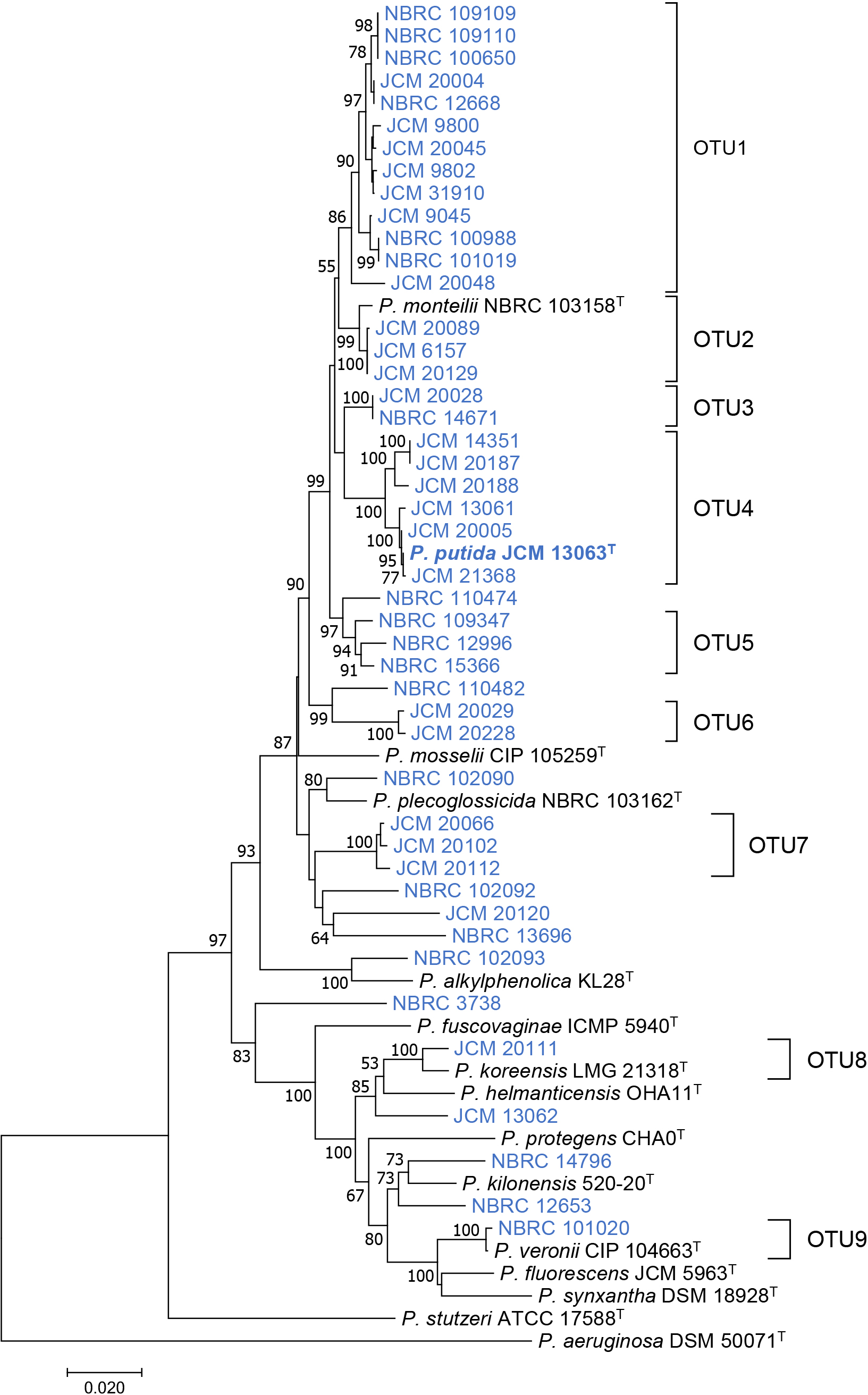
Phylogenetic tree based on concatenated sequences of 16S rRNA and rpoD genes from Pseudomonas putida strains (blue) and type strains of other Pseudomonas species (black). The P. putida type strain JCM 13063T is shown in bold. The phylogenetic tree was constructed by the neighbor-joining method using the ClustalW program of the MEGA 7 program. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are shown next to the branches. The scale bar represents 0.02 substitutions per nucleotide position.
Regarding classification based on physiological differences, various typical phenotypes were compared among P. putida strains. P. putida belongs to a wide group of fluorescent Pseudomonas species (Scarpellini et al., 2004). The fluorescence of P. putida strains was assayed using KB agar medium. Most strains produced fluorescence; however, a few non-fluorescent strains were scattered despite their OTU classification (Table 1). Motility was assayed using a KB plate containing 0.5 wt% agar. Although 26 strains of P. putida showed motility, the presence or absence of motility was not related to the OTU classification (Table 1). Some P. putida strains have been shown to inhibit the growth of plant pathogenic fungi (Bagnasco et al., 1998). Therefore, the antifungal activities of the 46 P. putida strains were assayed by cross-streaking them against the plant pathogen R. solani. However, only two strains, NBRC 109347 and NBRC 14796, exhibited antifungal activity (Table 1). One antifungal strain, NBRC 14796, was phylogenetically different from the other P. putida strains and was related to P. protegens, which is a well-known biocontrol agent (Takeuchi et al., 2014). Therefore, we hypothesized that P. putida did not essentially have effective antifungal activity.
Specific P. putida group strains harbor a quorum-sensing systemSome P. putida strains produce AHL as a quorum-sensing signaling compound, and various phenotypes are controlled by AHL-mediated quorum sensing (Steidle et al., 2002; Bertani and Venturi, 2004; Dubern et al., 2006). Therefore, AHL production by P. putida strains was assayed using the cross-streaking method with the AHL reporter strains CV026 and VIR07, which respond to short acyl-chain AHL (C ≤8) and long acyl-chain AHL (C ≥8), respectively (Table 1). The results of the AHL production assay revealed that three OTU7 strains, two OTU4 strains, and three singletons produced AHL. All three strains belonging to OTU7 produced AHL; therefore, the whole-genome shotgun sequencing of one of the OTU7 strains, JCM 20066, was performed using the HiSeq X Ten platform (Table 2). JCM 20066 comprises a quorum-sensing system consisting of the AHL synthesis gene ppuI (PPUJ20066_41380), transcriptional regulatory gene rsaL (PPUJ20066_41370), and AHL receptor gene ppuR (PPUJ20066_41360). The ppuI-rsaL-ppuR quorum-sensing system has been also detected in P. putida PCL1445 (Dubern et al., 2006), P. putida IsoF (Steidle et al., 2002), P. putida WCS358 (Bertani and Venturi, 2004), and Pseudomonas sp. StFLB209 (Kato et al., 2015). The LC-MS/MS analysis revealed that JCM 20066 produced 3-oxo-C10-HSL as a quorum-sensing signaling compound as well as the three Pseudomonas strains described above (Fig. S3). To elucidate the phenotype controlled by 3-oxo-C10-HSL-mediated quorum sensing, we constructed a deletion mutant of the ppuI and ppuR genes in JCM 20066. AHL production and motility completely disappeared, and biofilm formation clearly decreased in both mutants. The addition of 1 μM 3-oxo-C10-HSL restored the motility of and biofilm formation by the ppuI mutant, but not by the ppuR mutant (Fig. S4). Therefore, 3-oxo-C10-HSL produced by PpuI may bind to the PpuR receptor protein and the PpuR-3-oxo-C10-HSL complex may activate the transcription of genes involved in motility and biofilm formation.
Comparative genome analysis of P. putida strainsSince marked differences were not observed in the phenotypes of OTU4 strains that may be identified as true P. putida, a comparative genome analysis was performed to cluster the seven OTU4 strains using whole-genome similarity. The whole-genome shotgun sequencing of the seven OTU4 strains and related strains was performed using the NovaSeq 6000 platform (Table 2). Whole-genome similarity with P. putida type strain NBRC 14164T (=JCM 13063T) was calculated using ANI and dDDH values. ANI ≥95% and dDDH ≥70% are classified as the same species (Goris et al., 2007; Srivastava et al., 2020). Based on the results of calculations of the ANI and dDDH values of P. putida strains against the genome sequence of the P. putida type strain, three strains, JCM 20005, JCM 21368, and JCM 13061, were identified with the same species as that of the P. putida type strain (Table 2). The other three OTU4 strains, JCM20187, JCM 20188, and JCM 14351, did not meet this criterion (ANI <95% and dDDH ≤60%). Therefore, these strains may be characterized as different from true P. putida. Furthermore, since five strains belonged to OTU3 or OTU5, and singletons (NBRC 110474) showed low ANI (≤90%) and dDDH (≤45%) values, these strains belonged to phylogenetically different species from P. putida. Since the OTU4 strains were divided into two slightly different branches in the phylogenetic tree (Fig. 1), true P. putida may be classified using minor differences in the concatenated sequences of the 16S rRNA and rpoD genes.
Development of specific PCR primers for the identification of true P. putidaTo identify common genes among true P. putida strains, orthologous genes present in only four true P. putida strains were searched using the amino acid sequences of the CDSs identified from the whole-genome shotgun sequences listed in Table 2. Twenty-six orthologous genes were identified. The whole-genome sequences of 257 strains of P. putida have been deposited on the NCBI Genome website as of January 20, 2023. Twenty-nine genome sequences that showed high ANI values with the genome sequence of the P. putida type strain were selected (Table S3). The presence of 26 orthologous genes in the 29 genome sequences was assessed using the BLAST program (Altschul et al., 1990). Seven orthologous genes were highly conserved among the genome sequences that showed high ANI and dDDH values with those of P. putida JCM 13063T (Table S3). One of these, PP4_28660, derived from the complete genome sequence of P. putida JCM 13063T, was selected as the target ortholog. PP4_28660 comprised 639 bp, encoded a hypothetical protein, and was present in all genome sequences that showed high genome similarity (ANI ≥95% and dDDH ≥70) with that of the P. putida type strain. Based on the DNA sequences of PP4_28660 and its homolog in the P. putida genome, specific primers for the amplification of the internal region of the PP4_28660 homolog were designed (Table S1). To evaluate the potential use of these specific primers for the selection of true P. putida, we amplified the PP4_28660 partial region by direct colony PCR. The results obtained showed that a 622-bp PCR fragment of PP4_28660 was successfully amplified from the colony of only four strains, JCM 13063T, JCM 13061, JCM 20005, and JCM 21368 showing high genome similarity (ANI ≥95% and dDDH ≥70) with that of the P. putida type strain (Fig. 2). These results demonstrate that specific primers for the amplification of the PP4_28660 homolog are useful for selecting only true P. putida strains from miscellaneous bacterial isolates.
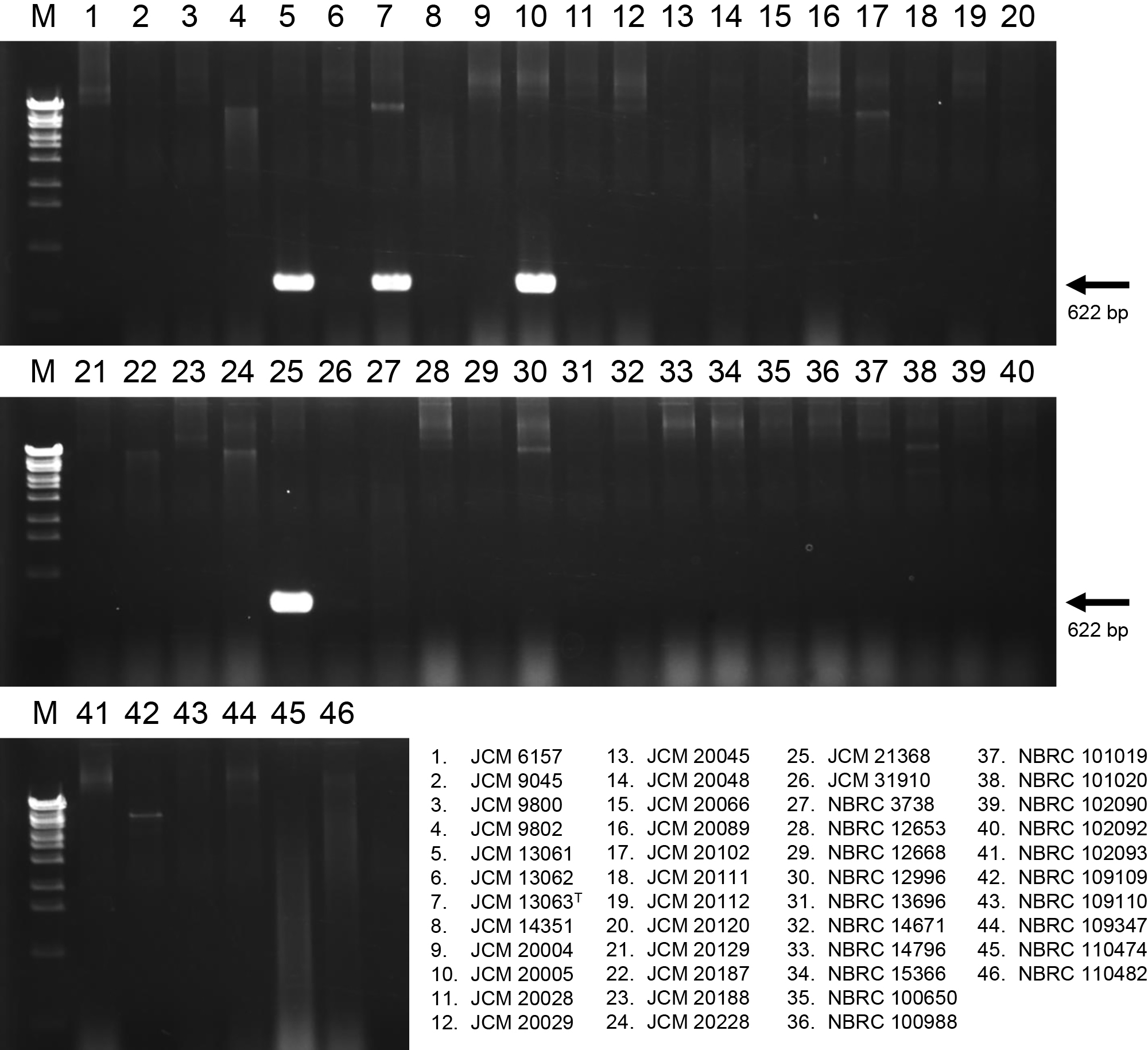
Specificity testing of primers designed from the sequence of PP4_28660 by agarose gel electrophoresis. PCR products were electrophoresed on 1.5% agarose gel. Lane M, One Step Marker 6 (Nippon Gene) used for the DNA size marker. Lane 1–46, Pseudomonas putida strains used for the PCR template described at the bottom of the picture.
We herein demonstrated that 46 strains of P. putida obtained from Japanese culture collections may be classified into multiple OTUs based on the concatenated sequences of the 16S rRNA and rpoD genes. Only four strains may be identified as true P. putida, while the other 42 strains may be reclassified as other species or subspecies of the genus Pseudomonas. Historically, numerous P. putida strains have been isolated, characterized, and utilized in various fields. For example, P. putida KT2440 is a well-known P. putida strain that was isolated in the 20th century (Regenhardt et al., 2002), and is expected to be used as a biocontrol and bioremediation agent (Matilla et al., 2010; Gong et al., 2017). However, the complete genome sequence of strain KT2440 (accession no. AE015451) showed lower ANI (89.85%) and dDDH (40.60%) values against those of P. putida JCM 13063T. Therefore, most of the bacterial strains classified as P. putida globally may be essentially different species from P. putida. While difficulties are associated with establishing whether a large number of P. putida strains may be classified as true P. putida using traditional classification methods, the newly developed primers in the present study render the identification of P. putida easier. However, P. putida isolates, which are potentially different from true P. putida, included specific bacterial groups with unique phenotypes, such as OTU7, which contains the ppuI-rsaL-ppuR quorum-sensing system. In the future, a comparative genome analysis of P. putida strains that have already been roughly classified may enable the identification of novel Pseudomonas species with unique phenotypes.
Morohoshi, T., Yaguchi, N., and Someya, N. (2023) Genomic Reclassification and Phenotypic Characterization of Pseudomonas putida Strains Deposited in Japanese Culture Collections. Microbes Environ 38: ME23019.
https://doi.org/10.1264/jsme2.ME23019
We thank Prof. Xiaonan Xie from Utsunomiya University for technical assistance with the LC-MS/MS analysis.