2023 年 38 巻 4 号 論文ID: ME23056
2023 年 38 巻 4 号 論文ID: ME23056
Rhizobia are soil bacteria that induce the formation of nodules in the roots of leguminous plants for mutualistic establishment. Although the symbiotic mechanism between Lotus japonicus and its major symbiotic rhizobia, Mesorhizobium loti, has been extensively characterized, our understanding of symbiotic mechanisms, such as host specificity and host ranges, remains limited. In the present study, we isolated a novel Rhizobium strain capable of forming nodules on L. burttii from agricultural soil at Iwate prefecture in Japan. We conducted genomic and host range analyses of various Lotus species. The results obtained revealed that the novel isolated Rhizobium sp. Chiba-1 was closely related to R. leguminosarum and had a wide host range that induced nodule development, including L. burttii and several L. japonicus wild-type accessions. However, L. japonicus Gifu exhibited an incompatible nodule phenotype. We also identified the formation of an epidermal infection threads that was dependent on the Lotus species and independent of nodule organ development. In conclusion, this newly isolated Rhizobium strain displays a distinct nodulation phenotype from Lotus species, and the results obtained herein provide novel insights into the functional mechanisms underlying host specificity and host ranges.
The mutually beneficial connection between legume plants and rhizobia is known as symbiotic nodulation. Rhizobia enter and colonize root nodules, new organs generated by the host legume plant, and convert atmospheric nitrogen into ammonium, which is then used by the host legume plant. In exchange, the host legume plant delivers carbon energy generated by photosynthesis to rhizobia (Udvardi and Poole, 2013). The capacity of these nodules to directly connect with each other through chemical signals and perception is critical to the development of this nodule symbiosis.
Legume roots produce phenolic chemicals known as flavonoids, which are recognized by rhizobia via the NodD protein in first contact for the formation of nodule symbiosis (Perret et al., 2000). In rhizobia, the NodD-flavonoid complex triggers the expression of nodulation (nod) genes, resulting in the production and secretion of lipochitooligosaccaride molecules known as Nod factors. Once the legume plant perceives and recognizes its Nod factors through NFR1/LYK3 and NFR5/NFP LysM-RLKs, it commences nodulation processes (Amor et al., 2003; Limpens et al., 2003; Madsen et al., 2003; Radutoiu et al., 2003). Symbiotic rhizobia then invade nodules through intracellular or intercellular mechanisms that are controlled in epidermal infection thread-dependent or -independent manners (Madsen et al., 2010; Montiel et al., 2021; Quilbé et al., 2022). These interactions depend on the ability of rhizobia and the host legume plant to perceive and respond to specific molecular signals from their counterparts.
These interactions also serve as the foundation for the host range and host specificity in symbiotic nodulation (Walker et al., 2020). Legume plants synthesize and exude different types of flavonoids, and only a particular Rhizobium has the ability to recognize these flavonoids using the NodD protein (Liu and Murray, 2016). A previous study reported that when the nodD gene of the broad-host range Rhizobium sp. NGR234 was introduced into the limited-host range R. leguminosarum biovar trifolii ANU843, this strain formed symbiotic nodules on the different host plants Trifolium sp. and non-legume Parasponia (McIver et al., 1989). Nod factor synthesis genes are also important for host specificity. When nodABC genes in S. meliloti GMI51 were transferred to R. tropici CNF299, the hybrid R. tropici strain more strongly induced nodules on alfalfa than R. tropici CNF299 (Roche et al., 1996). Furthermore, the introduction of R. meliloti RCR2011 nodH, nodEF, and nodQ genes into R. leguminosarum bv. trifolii ANU843 and R. leguminosarum bv. viciae 248 changed the host plant from T. repens and Vicia sativa to Medicago sativa (Debellé et al., 1988; Faucher et al., 1989). In contrast to the wild-type strain, transconjugant R. leguminosarum bv. viciae RBL5560 containing the nodZ gene, given the fucosyl residue on Nod factors from Bradyrhizobium diazoefficiens USDA110, exhibited the ability to induce nodule formation in M. atropurpureum, G. soja, V. unguiculata, and L. leucocephala (López-Lara et al., 1996). Therefore, Nod factors, which are critical for early interactions, dictate each Rhizobium’s host range and host specificity based on its core structure and side chains.
The rhizobial type III or IV secretion system, which injects these effector proteins directly into host plant cells, also controls host ranges and host specificity. Previous studies demonstrated that the NopP and BEL2-5 effectors of B. diazoefficiens USDA122 or B. elkanii USDA61 induced incompatibility with G. max carrying the Rj2 or Rj4 alleles (Faruque et al., 2015; Sugawara et al., 2018; Ratu et al., 2021); these specific interactions suppressed nodule development via effector-triggered immunity (Grundy et al., 2023). The msi059 effector injected into host cells through the type IV secretion system of Mesorhizobium loti R7A was found to inhibit nodule formation in L. corniculatus and Leucaena leucocephala (Hubber et al., 2004).
Lipopolysaccharides (LPS) and exopolysaccharides (EPS) are rhizobial polysaccharides that control the host range. Several mutants of S. meliloti Rm1021 with changes in LPS structure showed impaired symbiosis in M. truncatula, but not in M. sativa (Campbell et al., 2003). Exo genes from S. meliloti Rm1021, which are involved in the production of succinoglycans, were transferred to S. meliloti Rm41 forming non-nodule in M. truncatula A17. This led to the synthesis of similar succinoglycans and restored symbiotic nodules with them (Simsek et al., 2007). The exoU mutant of M. loti R7A synthesized truncated EPS and caused symbiosis incompatibility in L. japonicus Gifu by recognizing the EPR3 receptor, but did not in L. japonicus MG20 (Kelly et al., 2013; Kawaharada et al., 2015, 2017, 2021; Liu et al., 2018).
Several Rhizobium genera, including Mesorhizobium, Ensifer (Sinorhizobium), Bradyrhizobium, Rhizobium, and Aminobacter, have been isolated from Lotus nodules growing in natural areas (Andrews and Andrews, 2017; Lorite et al., 2018). These findings suggest the ability of Lotus species to form nodules with many types of rhizobia as well as their wide host range; however, their host specificity remains unknown. Recent studies showed that B. elkanii USDA61 formed nodules on L. burttii and L. japonicus MG20, but not on L. japonicus Gifu, whereas the same genus as Bradyrhizobium sp. SUTN9-2 formed nodules on L. burttii and L. japonicus Gifu, but not on L. japonicus MG20 depending on the type III secretion system (Hashimoto et al., 2020; Kusakabe et al., 2020). Another genus, S. fredii HH103, also exhibits a different nodule phenotype among Lotus species. L. burttii inoculated with S. fredii HH103 formed mature nodules through the intercellular infection process, whereas L. japonicus Gifu did not. These phenotypes were found to depend on the NopC effector injected by the type III secretion system (Acosta-Jurado et al., 2016, 2019, 2020; Jiménez-Guerrero et al., 2020). R. leguminosarum Norway isolated from L. corniculatus nodules has diverse nodule phenotypes in Lotus species (Gossmann et al., 2012). R. leguminosarum Norway induced nodule formation on L. burttii and invaded it by an intercellular infection mechanism, but did not induce nodules on L. japonicus Gifu (Liang et al., 2019). However, the host range and mode of invasion of the same Rhizobium genus into Lotus species remain unknown. In the present study, we identified the new Rhizobium sp. Chiba-1, which induced nodule formation on L. burttii and several L. japonicus MG accession lines, but not on L. japonicus Gifu or MG20. We discovered two types of epidermis-invading systems in Rhizobium sp. Chiba-1: epidermal infection threads and other systems that differ according to the host Lotus species.
Rhizobium and Mesorhizobium strains were cultured at 28°C in Tryptone Yeast medium (Beringer, 1974), while Escherichia coli was cultured at 37°C in Luria Bertani medium (Kawaharada et al., 2007). Antibiotics at the indicated concentrations were added to culture media when necessary: fosfomycin (100 μg mL–1), tetracycline (2–10 μg mL–1), streptomycin (250 μg mL–1), gentamycin (5 or 50 μg mL–1), and chloramphenicol (20 μg mL–1), for both Rhizobium and Escherichia strains.
Nodulation assaySeeds of Lotus species were sterilized using 2.4% sodium hypochlorite for 10 min, followed by seed coat scratching with sandpaper. After 3 days, seedlings were transferred onto filter paper (No. 4A; Advantech) on modified Long Ashton medium (Kawaharada et al., 2021) containing 1.4% agar slanted in 11×11 cm Petri dishes. Each plate was inoculated 2 days later with 1 mL of the rhizobia culture at OD600=0.04. Plants were grown at 23°C under a 16-h light/8-h dark cycle. Regarding T. repens (KANEKO SEEDS), 3-day-old seedlings from sterilized seeds were grown on 16-fold diluted modified Long Ashton medium.
Nodule complementation assayCo-inoculation experiments were conducted to assess the potential complementation of the nod factor-deficient phenotype in the M. loti ∆nodAC mutant. A 1-mL mixture of cultures consisting of the M. loti ∆nodAC mutant (ML101) (Takeda et al., 2005) carrying pFAJGFP (Kelly et al., 2013) and Rhizobium sp. Chiba-1 carrying pFAJDsRed (Kelly et al., 2013) was prepared at a ratio of 1:1, with each culture having an OD600=0.02. The mixture was then inoculated onto the roots of L. japonicus Gifu plants. Nodules were observed 3–6 weeks post-inoculation under a Leica M165 FC microscope.
Microscopic observations of infection thread formationLotus sp. roots were sonicated for 30 s for 7 to 10 days after the inoculation with Rhizobium sp. Chiba-1 harboring pFAJDsRed. Roots were examined under Olympus BX53 and Leica M165 FC microscopes. The CellSens Standard program (Olympus) was used to merge images of bright and DsRed fluorescence.
GUS stainingRoots from L. japonicus Nin promoter::GUS and Cbp1 promoter::GUS (T90) transgenic lines were analyzed in the present study. After the inoculation with M. loti MAFF303099 or Rhizobium sp. Chiba-1, roots were stained for ß-glucuronidase activity using GUS staining buffer. Staining buffer contained 0.5 mg mL–1 X-Gluc, 100 mM phosphate buffer (pH 7.0), 10 mM EDTA, 1 mM K4(Fe[CN]6), 1 mM K3(Fe[CN]6), and 0.1% Triton X-100. Stained root samples were observed under the Leica M165 FC microscope. The staining process was performed at room temperature overnight.
Phylogenetic analysisPhylogenetic trees for the 16S rRNA and nodC genes were constructed using the maximum likelihood method with MEGAX software (Tamura et al., 2021). The robustness of tree branchings was estimated using 1,000 bootstrap replicates. Phylogenetic trees were drawn using OIPH-PhyNE (https://www.iph.osaka.jp/s007/1000/20211129/20211129191934.html). Sequences used in phylogenetic analyses were obtained from NCBI (NR_118339.1, NR_044112.1, AY509899.1, CP000133.1, JX855169.1, EF035074.2, AB680662.1, FR870231.1, NR_041396.1, NZ_CP025012.1, AB680660.1, BA000012.4, AB231916.1, AY260145.1, AF271637.1, NR_113675.1, NZ_DF820425.1, CP001191.1, NC_020059.1, JQ795195.1, FJ596038.1, KC608575.1, EF209422.1, NZ_WISX01000115.1, BA000012.4, AF217268.1, HM441255.1, DQ060002.2, NC_022536.1, NZ_CP025015.1, NC_020061.1, NC_011368.1, NZ_DF820426.1, and AP013103.1). Homology and identity searches for genes and proteins were performed using local blasts.
Rhizobium sp. Chiba-1 genome sequenceGenomic DNA was extracted using phenol-chloroform. Regarding SMRTbell library preparation, genomic DNA was fragmented at 20 kb using Megaruptor 2 (Diagenode), and the library was constructed using SMRTbell express template prep kit 2.0 according to the manufacturer’s protocol (Pacific Biosciences). Barcodes were attached to each fragmented genome, and samples were pooled and cut off at 15 kb using the BluePippin size selection system (Sage Science). The genomic library was sequenced on a single PacBio sequel II system 2.0 cell. Reads were assembled using HGAP4 through SMRTlink (v 8.0.0), and the circularity of each contig was calculated with Circlator v 1.5.5 (Hunt et al., 2015). The DDBJ Fast Annotation and Submission Tool was used to annotate genes (Tanizawa et al., 2018). Sequence data were stored in AP028250 to AP028255.
To identify new rhizobia noduled with Lotus species, we collected various soil samples from agricultural and fruit cultivation areas at Iwate University in Japan (39°42′54.2″ N, 141°08′01.0″ E). When L. japonicus Gifu and L. burttii plants were grown in these mixed soils, one nodule formed in L. burttii after 5 weeks. The Rhizobium isolated from this nodule was named Chiba-1 strain and was reinoculated into both L. japonicus Gifu and L. burttii. Pink nodules were observed on L. burttii, but not on L. japonicus Gifu 3 weeks after the inoculation of Rhizobium sp. Chiba-1 strain (Fig. 1).

Lotus nodulation phenotypes inoculated with Rhizobium sp. Chiba-1.
(A) Images of nodulation phenotypes in L. burttii (left) and L. japonicus Gifu (right) 21 days after the inoculation with Rhizobium sp. Chiba-1. The scale bar represents 0.5 cm. Red arrows indicate mature-like nodules. (B) Average numbers of each nodule type formed over time (days after the inoculation) with Rhizobium sp. Chiba-1 in L. japonicus Gifu or L. burttii. n=17 for L. japonicus and 13 for L. burttii. Error bars represent standard errors (SE). (C) Nodule senescence in L. burttii. The upper panel shows a nodule 14 days after the inoculation, and the bottom panel shows the same nodule 22 days after the inoculation.
Whole genome sequencing by PacBio Sequel II revealed that Rhizobium sp. Chiba-1 consisted of one chromosome and five plasmids with 7,819 annotated genes (Fig. 2A). Based on a 16S rRNA gene sequence analysis, Rhizobium sp. Chiba-1 was the most closely related to R. leguminosarum bv. viciae strain USDA2370T and R. leguminosarum bv. trifolii WSM2304T with 100% identity. R. leguminosarum Norway, which had 99.9% identity to the 16S rRNA gene of Rhizobium sp. Chiba-1, was previously shown to induce root nodules in L. burttii, but not in L. japonicus Gifu with similar phenotypes to Rhizobium sp. Chiba-1 (Fig. 3A and Supplemental Table S1) (Gossmann et al., 2012). We then compared the genes in the two strains. atpD, recA, dnaK, and rpoB, four housekeeping genes from Rhizobium sp. Chiba-1 and R. leguminosarum Norway, exhibited strong homology of 93 to 99%. However, symbiotic-related genes involved in the transcription factor nodD, Nod factor backbone synthesis nodABC, adding side chains to Nod factor backbone nodEFIJLMN and nitrogenase nifABDEHKN in these strains, showed low homology of 69 to 82%, while nitrogen fixation regulator and cytochrome oxidase genes fixABCGHINOPQSX showed broad homology of 69 to 98% (Supplemental Table S1). Genes involved in EPS synthesis and the type IV secretion system (but not the type III secretion system), which are important for symbiotic interactions, were also identified in the Rhizobium sp. Chiba-1 genome (Supplemental Table S1). Low homology was noted between each symbiotic gene among rhizobia that caused nodule development in Lotus species when housekeeping genes and symbiotic genes were compared. Additionally, 16S rRNA and nodC gene comparisons, which included a number of rhizobia-forming nodules in Lotus species, failed to link these genes to symbiotic relationships (Fig. 3). Based on these results, the traits of nodule phenotypes did not appear to correlate with crucial genes for the development of symbiosis.

Genome structure of Rhizobium sp. Chiba-1.
(A) The chromosome and five plasmids of Rhizobium sp. Chiba-1. Plasmids were drawn at four times the scale of the chromosome. Contig lengths, positive and negative strand coding sequences, tRNA, rRNA, the GC content, and GC skew were displayed from outside of the circular genome. These circular genomes were generated using Genovi (Cumsille et al., 2023). (B) Comparison of symbiotic nodulation gene clusters between Rhizobium sp. Chiba-1 and R. leguminosarum Norway. Gray arrows indicate symbiotic genes, while blue arrows indicate symbiotic genes in the absence of R. leguminosarum Norway. Numbers between gene clusters indicate percent homology between each gene.
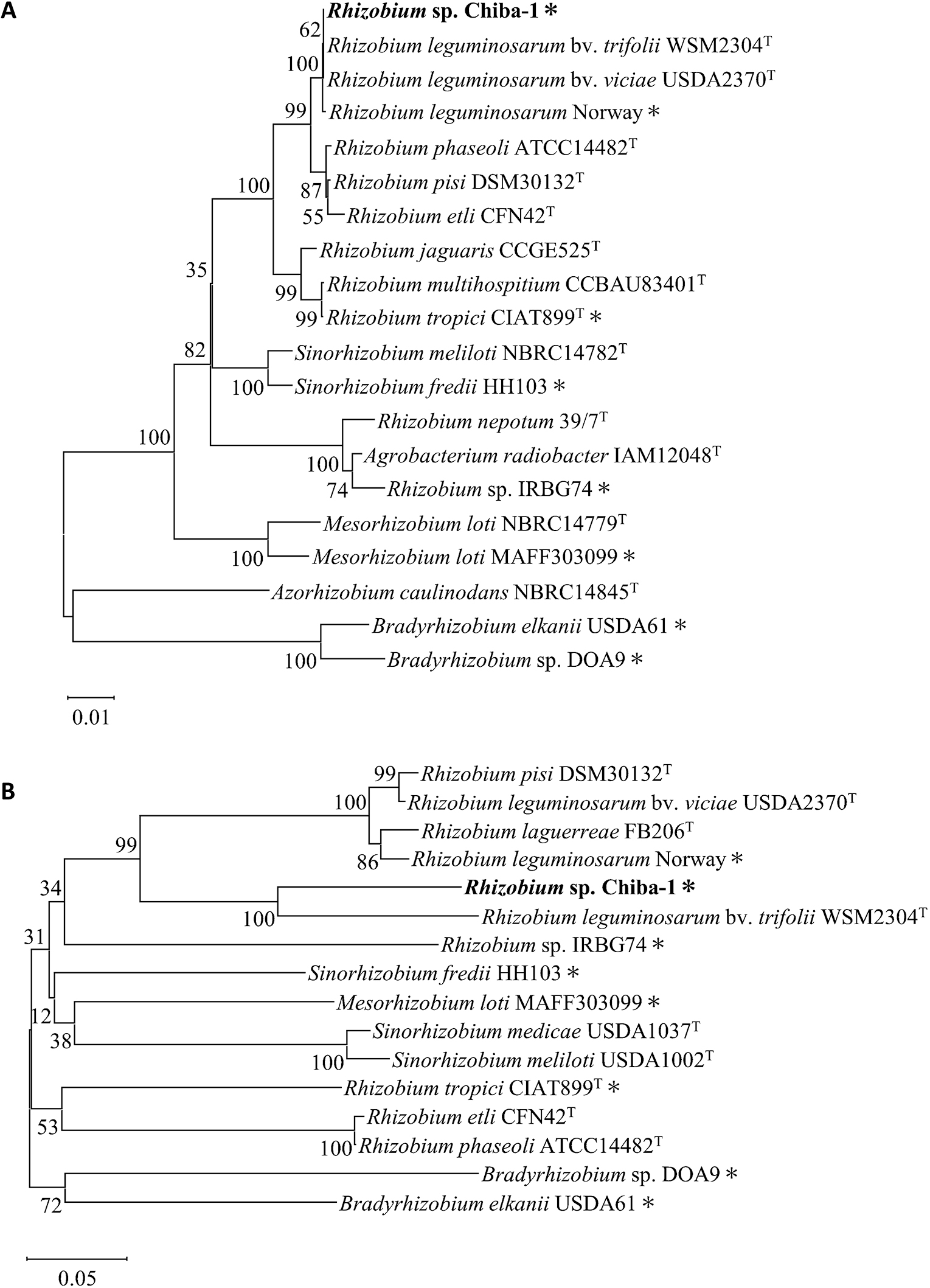
Phylogenetic trees of 16S rRNA and nodC genes.
The 16S rRNA and nodC genes were obtained from isolated strains and their related strains that formed symbiotic nodules on legume plants. (A) A neighbor-joining phylogenetic tree of the 16S rRNA gene was constructed using 1,362-bp partial nucleotide sequences. (B) A neighbor-joining phylogenetic tree of the nodC gene was constructed using 825-bp partial nucleotide sequences. Numbers at the nodes represent bootstrap values based on 1,000 replicates. The substitution rate was estimated at 0.01 and 0.05 substitutions per nucleotide position. Asterisks indicate strains that were identified to form nodules on Lotus species.
Some rhizobia species of the genera Rhizobium and Bradyrhizobium are known to exhibit nodule formation phenotypes with several Lotus wild-type accessions (Schumpp et al., 2009; Gossmann et al., 2012; del Cerro et al., 2015; Acosta-Jurado et al., 2016; Hashimoto et al., 2020; Kusakabe et al., 2020; Montiel et al., 2021). Therefore, we investigated the mechanisms by which Rhizobium sp. Chiba-1 induced root nodule formation in Lotus species. Rhizobium sp. Chiba-1 was inoculated onto L. burttii in our experimental system, and mature-like pink nodules were observed after 7 days, while approximately 2 mature-like nodules per plant had formed after 21 days (Fig. 1B). However, the growth promotion of plants that formed mature-like nodules was not confirmed. At the same time point, senescence nodules, which had greened from mature-like nodules, were also noted, and approximately 4 senescent nodules per plant had formed by 35 days after the inoculation (Fig. 1C). In contrast, nodule formation was not detected on L. japonicus Gifu, except for one white nodule in one plant 21 days after the inoculation. Similarly, neither L. filicaulis nor L. krylovii collected from China or Kazakhstan formed nodules (Table 1 and Supplemental Fig. S1). Therefore, we selected a number of L. japonicus wild-type accessions procured from various locations around Japan to assess the extent of nodule development among L. japonicus ecotype lines (Hashiguchi et al., 2011). Mature-like pink nodules including senescence green nodules were observed in less than 10% of plants in MG20, MG022, MG064, MG073, MG098, MG115, and MG122 35 days after the inoculation. No nodules were observed in any plants in MG20, MG064, or MG098. On the other hand, mature-like pink nodules were noted in the majority of plants in MG051, MG063, MG080, and MG119. Other MG accession plants showed an intermediate nodule phenotype (Table 1 and Supplemental Fig. S1). The present results revealed that the host range of Rhizobium sp. Chiba-1 included both L. japonicus intra-species and Lotus inter-species hosts. To investigate whether Rhizobium sp. Chiba-1 retained its nitrogen fixation ability in nodules, we inoculated Rhizobium sp. Chiba-1 onto T. repens, the main symbiotic partner of R. leguminosarum. Effective nodules were observed 10 days after the inoculation, one plant formed approximately 13 nodules, and nitrogen starvation in plants was eliminated 35 days after the inoculation (Supplemental Fig. S2).
Nodule phenotypes in Lotus species inoculated with Rhizobium sp. Chiba-1.
| n | No. of plants forming mature-like nodules, including green nodules |
% plants forming mature-like nodules, including green nodules |
Ave. no. of mature-like nodules, including green nodules, per plant |
Infection thread formation (11 dpi) |
||
|---|---|---|---|---|---|---|
| L. japonicus | Gifu | 17 | 0 | 0 | 0±0 | – |
| MG20 | 18 | 0 | 0 | 0±0 | nt | |
| MG008 | 20 | 5 | 25 | 0.4±0.2 | – | |
| MG018 | 30 | 3 | 10 | 0.1±0.1 | – | |
| MG022 | 18 | 1 | 6 | 0.1±0.1 | – | |
| MG048 | 24 | 5 | 21 | 0.2±0.1 | – | |
| MG051 | 21 | 20 | 95 | 3.6±0.4 | – | |
| MG056 | 21 | 12 | 57 | 2.3±0.6 | – | |
| MG063 | 30 | 26 | 87 | 5.8±0.7 | – | |
| MG064 | 27 | 0 | 0 | 0±0 | – | |
| MG073 | 20 | 1 | 5 | 0.1±0.1 | – | |
| MG080 | 20 | 18 | 90 | 5.7±0.7 | – | |
| MG098 | 8 | 0 | 0 | 0±0 | – | |
| MG100 | 21 | 2 | 10 | 0.1±0.1 | – | |
| MG111 | 30 | 4 | 13 | 0.3±0.2 | – | |
| MG113 | 30 | 16 | 53 | 1.0±0.3 | – | |
| MG115 | 30 | 1 | 3 | 0.1±0.1 | – | |
| MG119 | 30 | 23 | 77 | 1.6±0.3 | – | |
| MG122 | 29 | 2 | 7 | 0.1±0.1 | – | |
| MG132 | 30 | 5 | 17 | 0.2±0.1 | – | |
| L. burttii | 13 | 13 | 100 | 4.6±0.6 | + | |
| L. filicaulis | 6 | 0 | 0 | 0±0 | nt | |
| L. krylovii cv China | 10 | 0 | 0 | 0±0 | nt | |
| L. krylovii cv. Kazakhstan | 10 | 0 | 0 | 0±0 | nt | |
The nodulation phenotype 35 days after the inoculation
“nt” is not observed
Rhizobium sp. Chiba-1 did not exhibit the ability to induce nodules in L. japonicus Gifu. To investigate the initial response of L. japonicus Gifu, transgenic lines carrying the ß-glucuronidase (Gus) gene driven by Nin or Cbp1 promoters were examined (Webb et al., 2000; Radutoiu et al., 2003). M. loti MAFF3030399 strongly induced the expression of both Cbp1 and Nin 3 days after the inoculation, whereas Rhizobium sp. Chiba-1 only weakly induced that of Cbp1 in the epidermis (Fig. 4). However, more than 10 days after the inoculation, Rhizobium sp. Chiba-1 induced Nin expression in the epidermis. Interestingly, 28 days after the inoculation of Rhizobium sp. Chiba-1, Cbp1 and Nin both showed high expression in a spotted pattern in cortical cells (Fig. 4).

β-Glucuronidase expression induced by Cbp1 or Nin promoters.
Cbp1 promoters:: Gus and Nin promoters:: Gus transgenic lines in Lotus japonicus Gifu were inoculated with M. loti MAFF303099, Rhizobium sp. Chiba-1, or H2O. After 3 to 28 days, roots were stained with 5-bromo-4-chloro-3-indolyl-β-D-glucuronide (X-gluc). Scale bars indicate 1 mm.
Although Rhizobium sp. Chiba-1 is capable of inducing the expression of the early symbiotic genes necessary for nodule development, we conducted co-inoculation experiments to establish whether it complemented the phenotype of the M. loti ∆nodAC mutant (ML101) through the Nod factors produced by Rhizobium sp. Chiba-1. While the M. loti ∆nodAC mutant alone did not induce nodule formation, a co-inoculation with both Rhizobium sp. Chiba-1 and the M. loti ∆nodAC mutant (ML101) resulted in the same nodule phenotypes as the single inoculation of Rhizobium sp. Chiba-1. However, no mature nodules were observed on 20 plants 35 days after the inoculation.
Epidermal infection thread formation on L. burttii, but not L. japonicusNodule organ development on L. burttii was induced by R. leguminosarum Norway, which entered nodules via epidermal gaps between host cells (a process known as intercellular infection) in the absence of root hair infection threads (Liang et al., 2019). In contrast, M. loti MAFF303099 infiltrated nodules through the development of intracellular root hair infection threads. The invasion process of Rhizobium sp. Chiba-1 into Lotus species was examined under a fluorescent microscope using a fluorescent strain harboring pFAJDsRed. Epidermal infection threads harboring Rhizobium sp. Chiba-1 carrying pFAJDsRed were observed in root hairs 7 to 10 days after the inoculation into L. burttii (Fig. 5B). We classified two types of infection threads: long thin threads and short thick threads (Fig. 5C, D, E, and F). Many of the long infection threads formed from the middle of the root hair without curling of the root hair tip, while all of the short infection threads formed through root hair curling (Fig. 5C, D, E, and F). In addition, we noted a number of irregularly broken, halfway infected threads in root hairs (Fig. 5C and F). The total number of infection threads of L. burttii included approximately 129 long infection threads and 50 short infection threads 10 days after the inoculation with Rhizobium sp. Chiba-1 (Fig. 5G). Rhizobium sp. Chiba-1 was attached to the root surface of L. japonicus Gifu, and infection threads were not observed (Fig. 5A). Additionally, no infection threads were produced by L. japonicus MG accessions that did or did not develop mature-like nodules (Table 1). These results show that in the symbiotic relationship between Rhizobium sp. Chiba-1 and Lotus species, infection thread development and nodule formation were separate events.

Epidermal infection thread formation.
Lotus japonicus Gifu (A) or L. burttii (B, C, D, E, and F) 10 days after the inoculation with Rhizobium sp. Chiba-1 carrying pFAJDsRed. C and D show long infection threads, and E and F show short infection threads in L. burttii. Scale bars indicate 50 μm. (G) Average number of each epidermal infection thread 10 days after the inoculation with Rhizobium sp. Chiba-1 carrying pFAJDsRed. n=10.
Legume-rhizobia symbiosis exhibits the complex and varied characteristics of host specificity and host ranges, and the underlying molecular pathways have yet to be examined in detail. In the present study, we isolated a novel Rhizobium sp. Chiba-1 from nodules in L. burttii, identified the host range in Lotus species, and elucidated the mechanisms of invasion into nodules.
Rhizobium sp. Chiba-1, which has a high degree of similarity with R. leguminosarum Norway on the 16S rRNA gene, was discovered and exhibited host selectivity that was more similar to Lotus species than to R. leguminosarum Norway (Fig. 1 and Supplemental Table S1) (Gossmann et al., 2012). In addition, compared with symbiotic genes related to Nod factor synthesis and nitrogen fixation in each pRLN3 (557,386 bp) of R. leguminosarum Norway and pRC4 (353,970 bp) of Rhizobium sp. Chiba-1, these were widely different from one another (Liang et al., 2018) (Fig. 2B and Supplemental Table S1), suggesting that the genetic factors affecting the host range did not depend on the acquired symbiotic plasmid or main molecular cues for symbiosis. The diversity of results from phylogenetic analysis of 16S rRNA and nodC genes (Fig. 3) made it difficult to determine the host range of Lotus species.
M. loti, a major symbiotic partner of L. japonicus and L. burttii, forms elongated infection threads in root hairs and invades nodules (Acosta-Jurado et al., 2016). In contrast, B. elkanii USDA61, Rhizobium sp. IRBG74, R. leguminosarum Norway, S. fredii HH103, Rhizobium sp. NGR234, and R. tropici CIAT899, which exhibit minor nodulation with Lotus species, employ two distinct epidermal infection strategies. B. elkanii USDA61, Rhizobium sp. IRBG74, and R. leguminosarum Norway are unable to form infection threads in root hairs (Liang et al., 2019; Kusakabe et al., 2020; Montiel et al., 2021), while Rhizobium sp. NGR234 induces infection threads similar to M. loti (Schumpp et al., 2009) and R. tropici CIAT899 induces infection threads in a manner that is dependent on the host Lotus species (Ayala-García et al., 2022). In the present study, Rhizobium sp. Chiba-1 formed infection threads on L. burttii, while no infection thread was observed on L. japonicus MG051, MG063, or MG080, which formed mature like-nodules (Fig. 1 and 5, Table 1). These results showed that the invasion tactics by Rhizobium sp. Chiba-1 varied depending on the host plant. S. fredii HH103 is known to employ the NopC effector as a switch between intercellular invasion and infection thread formation in L. burttii (Jiménez-Guerrero et al., 2020). However, neither a type III secretion system nor NopC effector genes were present in the genome of Rhizobium sp. Chiba-1. These results showed that the ability of Rhizobium sp. Chiba-1 to switch between intracellular and extracellular invasion pathways is dependent on as-yet-unidentified activities; therefore, the interactions between Rhizobium sp. Chiba-1 and Lotus that contribute to this host specificity warrant further study. Furthermore, Rhizobium sp. strain Chiba-1 formed two different infection threads in L. burttii. Long thin infection threads were more likely to be induced in the absence of typical root hair curling, while short thick infected threads showed root hair curling (Fig. 5). These differences in infection thread formation are expected to occur in association with unusual intracellular infections or general infection threads reaching into the nodules, and, thus, further studies are needed to identify the type of infected thread that reaches into nodules.
Based on previous findings suggesting that differences in the attachment behaviors of Rhizobium sp. IRBG74 and M. loti R7A to the root surface of L. japonicus Gifu affect the infection process (Montiel et al., 2021; Quilbé et al., 2022), we examined the attachment behavior of Rhizobium sp. Chiba-1 on both L. japonicus Gifu and L. burttii (Fig. 5A and B). The differences observed in rhizobial attachment behavior may contribute to the inability of Rhizobium sp. Chiba-1 to complement the symbiosis of the M. loti ΔnodAC mutant in co-inoculation experiments.
Cbp1 expression was previously shown to be induced by Nod factors through a transcription factor such as CYCLOPS (Webb et al., 2000; Gong et al., 2022). In the present study, Rhizobium sp. Chiba-1 induced Cbp1 expression in L. japonicus Gifu 3 days after the inoculation, similar to M. loti (Fig. 4), suggesting that Nod factors produced by Rhizobium sp. Chiba-1 were recognized by L. japonicus Gifu. In contrast, Nin expression was induced and fine-tuned by the complex involvement of several proteins and phytohormones, such as NSP1, NSP2, CYCLOPS, IPN2, LAN, GA, and CK (Singh et al., 2014; Liu et al., 2019; Suzaki et al., 2019; Xiao et al., 2020; Akamatsu et al., 2021, 2022). Rhizobium sp. Chiba-1, unlike M. loti, was unable to induce Nin expression 3 days after the inoculation (Fig. 4), indicating that it may not be entirely capable of triggering the activation of these proteins. This variation in how the genes needed for symbiosis are expressed when Rhizobium sp. Chiba-1 is present may be directly connected to host specificity.
Rhizobium sp. Chiba-1, a unique Rhizobium that may symbiotically coexist with Lotus species, was successfully isolated in the present study. It differs from other known rhizobia in the host range and different infection processes between Lotus species. However, the molecular mechanisms underlying host specificity and the host range between Rhizobium sp. Chiba-1 and Lotus species remain unclear. Molecular genetic tools and bioresources from Rhizobium sp. Chiba-1 and Lotus species need to be used in the future to clarify signal molecular cues and perceptions, which are two functions connected to host specificity.
We obtained the new Rhizobium sp. Chiba-1 strain that developed mature-like nodules on L. burttii and several L. japonicus wild-type accessions, but not on L. japonicus Gifu, the model accession, which provides novel insights into host specialization and the host range in symbiotic processes. Furthermore, we discovered that the epidermal infection process of Rhizobium sp. Chiba-1 was characterized by the production of infection threads in root hairs in a manner that was dependent on the host plant.
Chiba, Y., Sasaki, M., Masuda, S., Shibata, A., Shirasu, K., and Kawaharada, Y. (2023) A Novel Rhizobium sp. Chiba-1 Strain Exhibits a Host Range for Nodule Symbiosis in Lotus Species. Microbes Environ 38: ME23056.
https://doi.org/10.1264/jsme2.ME23056
We would like to thank LegumeBase and Prof. Shusei Sato for providing MG accessions. We thank Prof. Jens Stougaard for providing several Lotus species seeds and T90 and Nin::GUS transgenic lines seeds. We thank Dr. Hisayuki Mitsui for providing M. loti ∆nodAC mutant (ML101) strains. This work was supported by New Energy and Industrial Technology Development Organization (JPNP18016) and JSPS Leading Initiative for Excellent Young Researchers to Y.K.