2018 年 43 巻 2 号 p. 129-134
2018 年 43 巻 2 号 p. 129-134
The aim of the present study is to investigate the “chronotoxicity” of seven metal compounds (Hg, Pb, Ni, Cr, Cu, Zn, or Fe) by assessing how their toxicity varies with circadian periodicity. Male ICR mice were injected with each metal compound intraperitoneally at 6 different time points over the course of a day (zeitgeber time [ZT]: ZT2, ZT6, ZT10, ZT14, ZT18 and ZT22). Mortality was then monitored until 14 days after the injection. Our investigation demonstrated that mice were tolerant against Ni toxicity during dark phase, on the other hand, they were tolerant against Cr toxicity during light phase. The chronotoxicity of Hg and Pb seemed to be biphasic. Further, mice were susceptible to toxicities against Cu and Zn in the time zone during which light and dark were reversed. Interestingly, no significant differences were observed for Fe exposure at any time of the day. Our results propose that the chronotoxicology may provide valuable information regarding the importance of injection timing for not only toxicity evaluation tests but also the reproducibility of animal experiments. Furthermore, our data suggests that chronotoxicology may be an important consideration when evaluating the quality of risk assessments for night shift workers who may be exposed to toxic substances at various times of the day.
Many biochemical, physiological, and behavioral processes show circadian rhythms, which are generated by an internal time-keeping mechanism referred to as the biological clock. The circadian system regulates 24-hr biological rhythms, which mainly regulate events necessary for life support, such as sleeping and feeding (Aschoff, 1963). It is well known that biological defense factors show diurnal variation in their expression levels or activities (Zhang et al., 2009). This diurnal variation may result from alterations in drug metabolism activities, e.g., bioactivation or bioinactivation by cytochrome P450 enzymes (Košir et al., 2013). These facts suggest that the timing of administration may affect the toxicity of applied compounds.
Previously, we have focused on the relationship between injection timing and the severity of cadmium (Cd) toxicity, which we advocated as “chronotoxicology.” Our studies clearly indicated that mice showed remarkable daily fluctuations in the severity of the toxic response to Cd (Miura et al., 2013, 2012). Exposure to Cd during the dark phase was more tolerated than exposure during the light phase. This difference was not only observed through mortality rates (Miura et al., 2013, 2012), but also through other indicators of toxicity such as hepatotoxicity (Miura et al., 2013) by multidirectional analysis (Miura et al., 2017). In addition, we recently investigated the chronotoxicity of bromobenzene (BB), which is known to induce severe hepatic injury and mild renal injury (Yoshioka et al., 2017). The chronotoxicity of BB was shown to have specific patterns of diurnal variation that were similar to Cd. Moreover, other groups have reported that chloroform and carbon tetrachloride (CCl4) also demonstrate this same tendency (Chen et al., 2009; Skrzypińska-Gawrysiak et al., 1995). However, acetaminophen (APAP)-induced hepatotoxicity was reduced when it was injected at night (Matsunaga et al., 2004). Interestingly, although these chemicals are well known to induce hepatotoxicity in mice (Kakan et al., 2011; Yoshioka et al., 2016a, 2016b), their peak toxicities occur at opposite times in the circadian period. This presents the possibility that each chemical will have a unique circadian sensitivity. If this is true, it is important to understand the circadian influences on the tolerance of many chemicals. Therefore, we investigated whether or not diurnal variation was observed in the toxicity of seven kinds of metal compounds.
HgCl2 was purchased from Nacalai Tesque (Kyoto, Japan). Other metals were purchased from Wako Pure Chemical Industries (Osaka, Japan). (CH3COO)2Pb · 3H2O was dissolved in distilled water. Other metals were dissolved in saline.
Animal treatmentMale ICR mice were purchased from Japan SLC Inc. (Shizuoka, Japan) and maintained under standard conditions of 24 ± 1°C, 55 ± 5% humidity, and 12:12 hr light/dark cycles, with free access to water and food. Experimental treatments were performed on 7-week-old animals. At the end of the experiment (14 days after injection), surviving mice were euthanized using pentobarbital. All experiments were approved by the Institutional Animal Care and Experiment Committee of Kinjo Gakuin University (No. 130) and the Institutional Animal Care and Use Committee of the National Institute of Occupational Safety and Health, respectively.
Experimental protocolSeven-week-old male ICR mice were assigned to 6 groups of 5 animals each per compound tested and were administrated intraperitoneally (i.p.) with 12.8 mg (47.1 µmol Hg) /kg HgCl2, 277 mg (731 µmol Pb) /kg (CH3COO)2Pb · 3H2O, 42.75 mg (330 µmol Ni) /kg NiCl2, 61.5 mg (209 µmol Cr) /kg K2Cr2O7, 9.5 mg (70.3 µmol Cu) /kg CuCl2, 53.9 mg (392 µmol Zn) /kg ZnCl2, or 130 mg (650 µmol Fe) /kg FeCl2 · 4H2O at one of 6 different time points per group (clock time; 10:00, 14:00, 18:00, 22:00, 02:00, or 06:00), corresponding to zeitgeber time (ZT); ZT2, ZT6, ZT10, ZT14, ZT18, or ZT22. In the case of injections during the dark period (ZT14, ZT18, or ZT22), these were done under red light (Miura et al., 2013). Mortality was monitored until 14 days after the injection. Administration dose was decided according to safe data sheet in each compounds and other reported literature (Park et al., 2001). The mean survival time (MST) was estimated using Kaplan-Meier analysis.
In this study, we investigated the lethal chronotoxicity induced by seven metal compounds. These metals were classified generally as harmful metals (Hg and Pb), rare metals (Ni and Cr), and essential transition metals (Cu, Zn, and Fe). Survival over 14 days following injection is shown in Figure 1-1.
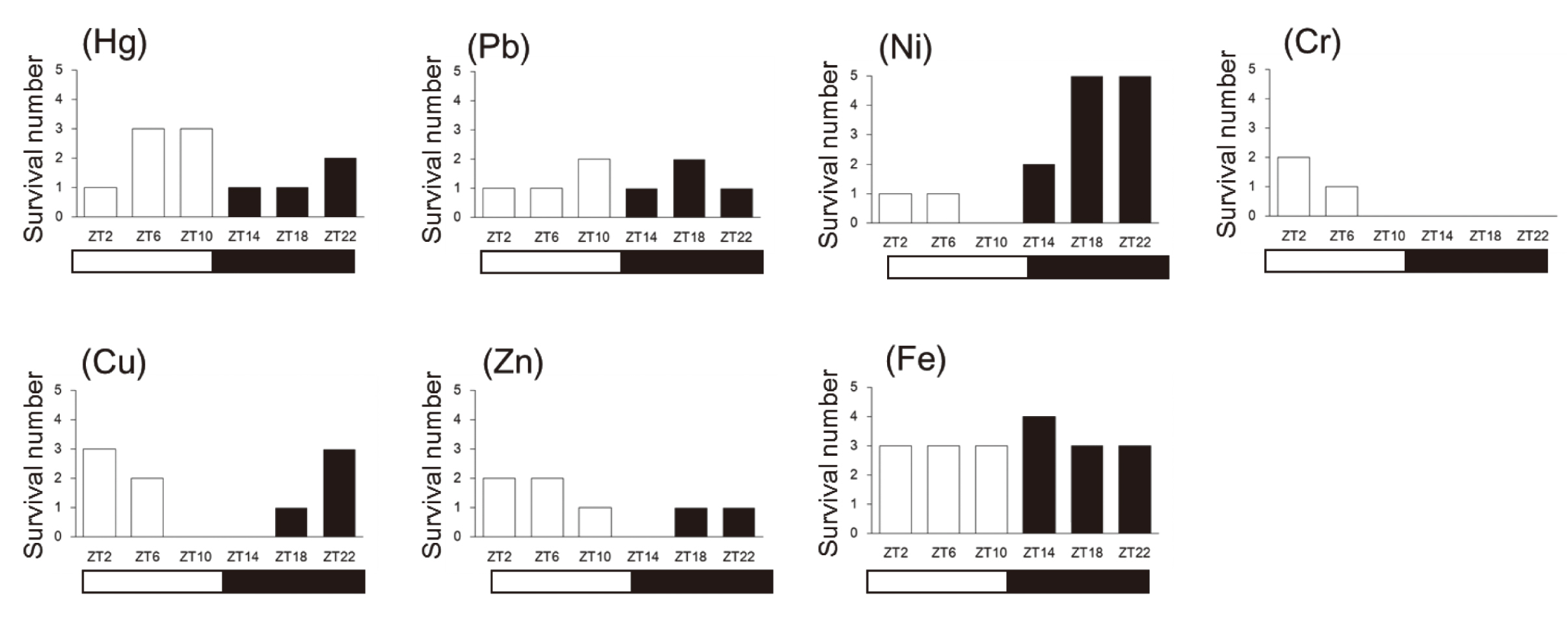
Survival numbers of mice after injection of metal compounds at different time points. Male ICR mice (n = 5) were injected i.p. with seven metal compounds (Hg, Pb, Ni, Cr, Cu, Zn, or Fe) at ZT2, ZT6, ZT10, ZT14, ZT18, or ZT22. The survival numbers were observed for 14 days to calculate survival number of mice in each groups.
In the Hg treatment group, mice were susceptible at ZT14, ZT18, even at ZT2, while being slightly insensitive at ZT6, ZT10, even at ZT22. This indicates that the chronotoxicity of Hg is biphasic, having two peaks. In the Pb treatment group, mice were susceptible at ZT2, ZT6, ZT14, and ZT22, and slightly insensitive at ZT10 and ZT18 suggesting that Pb toxicity also seemed to be biphasic.
In the Ni treatment group, all of the mice died within 6 days when treated at ZT10, while all of the mice survived when treated at ZT18 and ZT22. Therefore, mice were tolerant to Ni-induced lethal toxicity during dark phase. This pattern is similar to that of Cd treatment (Miura et al., 2013). In contrast, in the Cr treatment group, mice were susceptible from ZT10 to ZT22, while slightly resistant at ZT2. Therefore, mice seemed to be tolerant to Cr-induced lethal toxicity during light phase.
In the Cu treatment group, mice were susceptible at ZT10 and ZT14, while slightly resistant at ZT2, ZT6, and ZT22. In the Zn treatment group, mice were susceptible at ZT14, while slightly insensitive at ZT2 and ZT6. Therefore, it seemed that toxicities of Cu and Zn tended to occur in mice in the time zone during which light and dark were reversed. These results indicate that lethal chronotoxicity is also observed following treatment with essential trace elements. Interestingly, no diurnal variation in lethal toxicity was observed for the Fe treatment group.
The number of surviving mice in each treatment group was recorded every day during the experiment. Changes in survival numbers are shown in Figs. 1-2 (a-g). The MST was calculated using the Kaplan-Meier analysis. The patterns in MST (Figs. 1-2, h-n) were almost the same as those for the raw survival numbers (Fig. 1-1), indicating support for the observed lethality data.

Changes in survival numbers and mean survival times of metal-induced chronotoxicity. Mice were observed for 14 days to calculate surviving mice in each groups (a-g). The mean survival times (MST) were estimated by Kaplan-Meier analysis (h-n).
Our previous studies indicated that mice were tolerant to Cd toxicity when exposed during the night phase, as deduced from mortality and hepatotoxicity assessments (Miura et al., 2013, 2012). Following on from these studies, we sought to investigate whether there are diurnal variations in sensitivity to seven kinds of metal compounds, based on their injection time. As a result, the chronotoxicity was recognized with all of the metals except Fe. The time period during which susceptibility increased differed depending on the metal.
It is well known that Hg can induce metallothionein (MT) (Bracken and Klaassen, 1987) and that Hg-induced toxicity is protected by MT (Liu et al., 1991). However, the protective effect of MT against Hg is lower than that against Cd and Zn (Park et al., 2001). Further, glutathione (GSH)-depleted animals are markedly more sensitive to Hg toxicity (Naganuma et al., 1990), suggesting that GSH levels might be more important than MT in determining sensitivity to Hg toxicity. GSH is known to protect the liver against oxidative injury by reducing hydrogen peroxide and scavenging reactive oxygen and nitrogen radicals (Yuan and Kaplowitz, 2009). Our group and others have demonstrated that hepatic GSH levels vary with daily periodicity (Matsunaga et al., 2004; Miura et al., 2013). In addition, hepatic GSH levels are lowest during the light phase and highest during the dark phase. In further investigation, we need to determine whether or not renal GSH levels show similar diurnal variation, since the target organ of Hg toxicity is the kidney (Kanda et al., 2008).
Several reports have shown that Ni induces hepatotoxicity (Pari and Amudha, 2011; Sidhu et al., 2004). Our study showed that all Ni-treated mice died within 6 days of injection when given at ZT10, while all mice survived when injected at ZT18 and ZT22 injections. This result was similar to that of Cd (Miura et al., 2013, 2012). Moreover, similar to Cd, Ni also depletes GSH levels (Pari and Amudha, 2011; Sidhu et al., 2004). These observations indicate that Ni-induced toxicity was tolerated during the dark phase. Since Ni and Cd treatments show similar phenotypes, their patterns of diurnal variation in sensitivity may be similar.
The pattern of Cr-induced lethal toxicity appeared to be the same as Cu, Zn, and Pb. Cr is known to undergo redox cycling, resulting in the generation of reactive oxygen species (Sugiyama, 1992). These mechanisms were also observed for Cu. However, although Zn or Cu is known to induce MT, Cr inhibits MT induction, that is opposite effect of Zn or Cu (Stohs and Bagchi, 1995). Therefore, MT might not be an important factor for determining sensitivity to Cr-induced chronotoxicity. Both Cu and Zn induce MT and bind to MT, resulting in suppression of their toxicity. As mentioned above, however, Cr may suppress MT synthesis while Pb induces MT but does not bind (Nolan and Shaikh, 1992). These facts indicate that MT may be involved in the chronotoxicity exhibited by Cu and Zn while not participating as a determining factor for the chronotoxicity seen with Cr and Pb. We assume that the target organ provides important information about the method of chronotoxicity. For instance, the target organs of Cd-induced acute toxicity are liver and testis (Miura et al., 2012; Ohtani et al., 2013). However, while the liver is the target organ in Zn-induced acute toxicity, Onosaka reviewed that the pancreas is more sensitive than the liver (Onosaka et al., 2002). In addition, not only the liver but also the kidney is recognized as a target organ in Cu-induced acute toxicity (Gaetke and Chow, 2003). Therefore, there is a possibility that a difference in target organs may regulate chronotoxicity.
Very interestingly, notable diurnal variation was not confirmed for Fe treatment. This metal is classified as an indirect MT inducer since it only induces production in vivo and only in the liver (Bracken and Klaassen, 1987). Moreover, acute toxicity with Fe is more likely to result in renal failure than hepatic failure (Hamazaki et al., 1985), suggesting a difference of target organs might be involved. Although our present study observed only one fatal case in this treatment group, further investigation is needed to focus on the toxicity induced by lower concentrations of metal compounds, because it is possible for lower metal concentrations to demonstrate different chronotoxicity profiles than those described in this paper.
In conclusion, our present study demonstrated that many metal compounds showed chronotoxicity. We propose that chronotoxicology may provide valuable information for future toxicology studies through awareness of the importance of injection timing for both toxicity evaluation tests as well as the reproducibility of animal experiments. Furthermore, our data suggests that chronotoxicology may be an important consideration when evaluating the quality of risk assessments for night shift workers who may be exposed to toxic substances at various times of the day.
This work was supported by a grant-in-aid for fundamental research (N-F29-02) from JNIOSH and Kinjo Gakuin University Research Grant.
Conflict of interestThe authors declare that there is no conflict of interest.