Abstract
We analyzed electropharmacological characteristics of microminipigs under halothane-anesthesia using anti-influenza virus drug oseltamivir, which has been known to possess multi-channel blocking properties, including Na+, Ca2+ and K+ channels (n = 4). Oseltamivir in doses of 0.3, 3 and 30 mg/kg was intravenously infused over 10 min with an interval of 20 min, which provided peak plasma concentrations 1.4, 7.4 and 125.5 µg/mL, respectively. The low dose did not alter any of the cardiovascular variables. The middle dose decreased the heart rate at 30 min after the start of the infusion. The high dose transiently returned the heart rate toward the baseline for 10-15 min, but decreased it for 20-60 min; decreased the mean blood pressure for 5-60 min; prolonged the PR interval for 10-60 min, and the QRS width for 10-20 min; but shortened the QT interval for 10-30 min, and the QTc for 5-60 min. Thus, oseltamivir can suppress the sinus automaticity, and atrioventricular nodal and intraventricular conduction; and decrease the mean blood pressure, extents of which were greater in microminipigs than in beagle dogs in our previous observation in spite of similar plasma concentrations, reflecting higher sensitivity of microminipigs for Na+ and Ca2+ channel inhibition than that of beagle dogs. In contrast to beagle dogs, oseltamivir shortened the repolarization period in microminipigs, indicating that oseltamivir can more potently inhibit the inward currents than the outward ones in the hearts of microminipigs. This information may help improve utilizatione of microminipigs as a laboratory animal.
INTRODUCTION
Microminipigs are extraordinarily small-sized miniature pigs, weighing approximately 7 kg at 6 months of age when they mature, which were developed by Fuji Micra Inc. (Shizuoka, Japan) (Kaneko et al., 2011). We have pharmacologically characterized microminipig as an in vivo laboratory animal for safety pharmacology study under halothane-anesthesia using several typical cardiac ion channel and receptor modulators (Cao et al., 2017; Matsukura et al., 2017; Yokoyama et al., 2017). Namely, we assessed the effects of pilsicainide, verapamil and E-4031 on microminipigs, which preferentially suppress Na+, Ca2+ and K+ channels, respectively (Matsukura et al., 2017); those of dl-sotalol, which can block both ß adrenoceptor and K+ channel (Yokoyama et al., 2017); and those of moxifloxacin and terfenadine, which inhibit various cardiac ionic channels, leading to benign and malignant QT-interval prolongation, respectively (Cao et al., 2017). We have also compared those with the beagle dogs in our previous studies using the same experimental conditions, and shown that microminipigs may have smaller effective volume of drug distribution; greater basal sympathetic tone resulting in lessened hypotension-induced, reflex-mediated increase of sympathetic tone; and/or smaller repolarization reserve than beagle dogs (Cao et al., 2017; Matsukura et al., 2017; Yokoyama et al., 2017).
Oseltamivir (oseltamivir phosphate) is a prodrug of oseltamivir carboxylate, which is a neuraminidase inhibitor prescribed for patients with influenza virus A and B infections (Safrin, 2015). Importantly, in our recent studies using in vitro guinea-pig hearts and in vivo canine model, we have demonstrated that oseltamivir may have multi-channel blocking actions on Na+, Ca2+ and K+ channels (Kitahara et al., 2013; Nakamura et al., 2016; Takahara et al., 2013), although no significant electrophysiological action was confirmed for its major active metabolite oseltamivir carboxylate (Chugai Pharmaceutical Co., Ltd. and F. Hoffmann-La Roche Ltd., 2007). It should be noted that intravenous oseltamivir administration did not induce torsade de pointes in the chronic atrioventricular block dogs, one of the well-established proarrhythmia models (Nakamura et al., 2016; Sugiyama, 2008), which rather tended to shorten the short-term variability of QT interval, predicting its antiarrhythmic property. Indeed, the use of oseltamivir against influenza infection was shown to be associated with significant decrease in the risk of recurrent cardiovascular events in subjects with a history of cardiovascular disease (Casscells et al., 2009).
In this study, we tried to electropharmacologically characterize microminipigs as a laboratory animal by assessing pharmacokinetic and pharmacodynamic actions of oseltamivir in comparison with beagle dogs in our previous studies (Kitahara et al., 2013; Nakamura et al., 2016), since the dog is the most commonly used non-rodent animal for safety pharmacology study.
MATERIALS AND METHODS
Male microminipigs of 18 ± 2 months old, weighing approximately 10.2 ± 0.6 kg, were obtained from Fuji Micra Inc. All experiments were approved by the Toho University Animal Care and User Committee (No. 16-53-275) and performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Toho University.
Drugs
Oseltamivir for in vivo experiment was extracted from Tamiflu® capsule (Chugai Pharmaceutical Co., Ltd., Tokyo, Japan), which was dissolved with saline in concentrations of 0.3, 3 and 30 mg/mL. The dose of oseltamivir was calculated as its free base (FW = 312.4). Meanwhile, oseltamivir for plasma concentration assay (Sigma-Aldrich Japan K.K., Tokyo, Japan), ketamine (Ketalar®, Daiichi Sankyo Co., Ltd., Tokyo, Japan), xylazine (Celactal®, Bayer Yakuhin, Ltd., Osaka, Japan), propofol (Fresenius Kabi Japan K.K., Tokyo, Japan), halothane (Fluothane®, Takeda Pharmaceutical Co., Ltd., Osaka, Japan) and heparin calcium (Caprocin®, Sawai Pharmaceutical Co., Ltd., Osaka, Japan) were purchased.
Cardiovascular variables
Microminipigs (n = 4) were pre-anesthetized by an intramuscular injection of ketamine hydrochloride (16 mg/kg as ketamine) and xylazine hydrochloride (1.6 mg/kg as xylazine). A 24G cannula was introduced into a superficial auricular vein, and 1 mg/kg of propofol was intravenously injected to induce anesthesia. After intubation with a 6 mm cuffed endotracheal tube, 1% halothane vaporized with 100% oxygen was inhaled with a volume-limited ventilator (SN-480-3; Shinano Manufacturing Co., Ltd., Tokyo, Japan). Tidal volume and respiratory rate were set at 10 mL/kg and 15 breaths/min, respectively. Heparin calcium in a dose of 100 IU/kg was intravenously administered to prevent blood clotting via the auricular vein. A clinically available 4F-size catheter-sheath set (XEMEX ISA0740BBA, Zeon Medical Inc., Tokyo, Japan) was placed in the right femoral artery to measure the aortic pressure and to sample the blood.
Experimental protocol
The electrocardiogram and aortic pressure were monitored by using a polygraph system (RM-6000, Nihon Kohden, Co., Ltd., Tokyo, Japan), and analyzed with a real-time fully automatic data analysis system (WinVAS3 for Windows ver. 1.1R24v; Physio-Tech Co., Ltd., Tokyo, Japan). Three recordings of consecutive complexes were used to calculate the mean for the cardiovascular variables. The electrocardiogram and aortic pressure were recorded under sinus rhythm.
After the basal assessment, oseltamivir in a low dose of 0.3 mg/kg was intravenously infused over 10 min, and each variable was assessed at 5, 10, 15, 20 and 30 min after the start of infusion. Then, oseltamivir in a middle dose of 3 mg/kg was infused over 10 min, and each variable was assessed in the same manner as the low dose. Finally, oseltamivir in a high dose of 30 mg/kg was infused over 10 min, and each variable was assessed at 5, 10, 15, 20, 30, 45 and 60 min after the start of infusion. The doses of oseltamivir were determined according to the previous reports (Kitahara et al., 2013).
Plasma drug concentration
A volume of 2.5 mL of blood was withdrawn from the right femoral artery. The blood samples were centrifuged at 1,500 × g for 15 min at 4°C. The plasma was stored at –80°C until the drug concentration was measured. A volume of 100 μL of plasma was treated with 20 μL of 60% (v/v) perchloric acid and 10 μL of H2O or standard solution. After vortex mixing, the samples were centrifuged at 20,000 × g for 5 min at 4°C. An aliquot of the supernatant (30 μL) was injected to an analytical C18 reversed-phase column (250 × 4.6 mm, 5 µm, MightysilRP-18; Kanto Chemical, Tokyo, Japan) maintained at 40°C. The mobile phase in the HPLC system was 21% (v/v) of acetonitrile in 0.01 mol/L sodium phosphate buffer (pH 3.0) at a flow rate of 1.6 mL/min. The elution profile of oseltamivir was monitored with a UV detector at wavelength of 216 nm.
Statistical analysis
Data are presented as mean ± S.E.M. The statistical significances within a parameter were evaluated with one-way, repeated measures analysis of variance (ANOVA) followed by Contrasts as a post-hoc test for mean values comparison. A p-value < 0.05 was considered to be statistically significant.
RESULTS
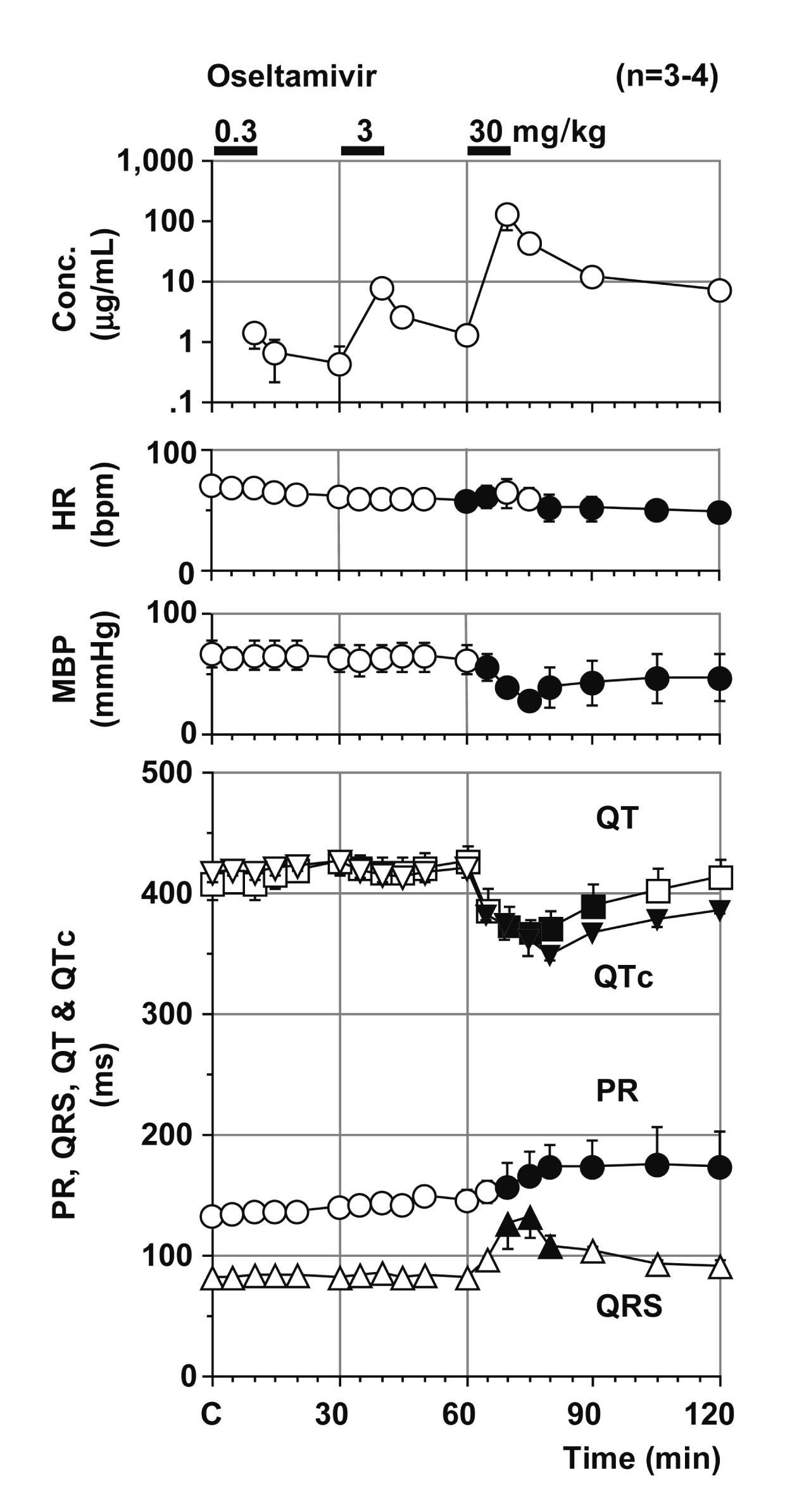
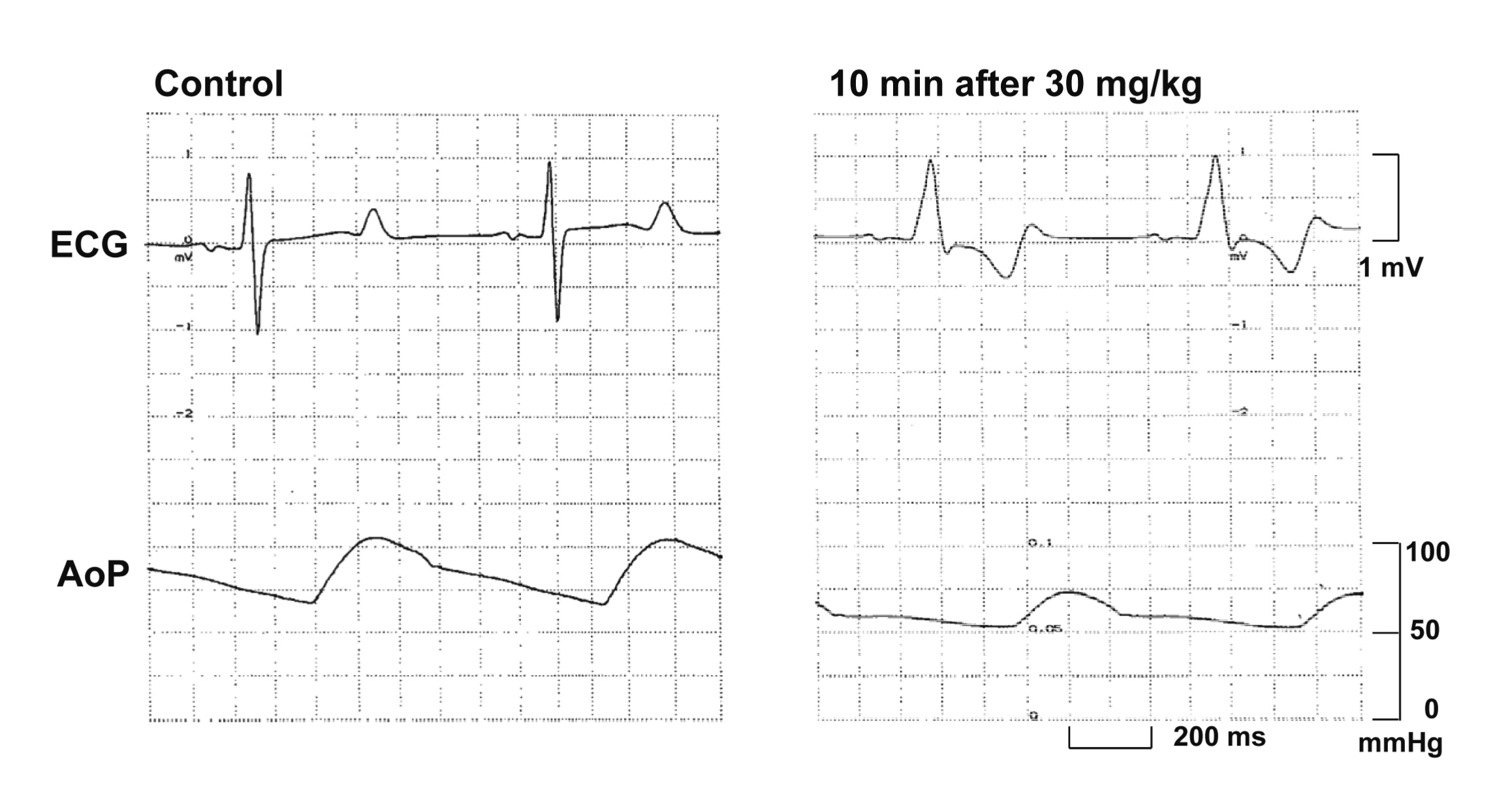
Typical tracings showing the effects of oseltamivir on the electrocardiogram and aortic pressure are depicted in Fig. 1. Time courses of the changes in the plasma concentration of oseltamivir, heart rate, mean blood pressure, PR interval, QRS width, QT interval and QTc are summarized in Fig. 2. The decrease in the plasma concentration of oseltamivir showed a pattern that could be predicted by the two-compartment theory of pharmacokinetics. The peak plasma concentrations of oseltamivir after 0.3, 3 and 30 mg/kg infusions were 1.4 ± 0.6 (4.5 ± 1.9), 7.4 ± 0.7 (23.7 ± 2.2) and 125.5 ± 51.7 µg/mL (401.7 ± 165.5 µmol/L), respectively. The pre-drug control values (C) of the heart rate, mean blood pressure, PR interval, QRS width, QT interval and QTc were 70 ± 5 beats/min, 66 ± 11 mmHg, 132 ± 5 ms, 81 ± 1 ms, 407 ± 13 ms and 417 ± 8, respectively. The low dose did not alter any of the variables. The middle dose decreased the heart rate at 30 min after the start of the infusion, whereas no significant change was detected in the other variables. The high dose transiently returned the heart rate toward the baseline for 10-15 min, but decreased it for 20-60 min; decreased the mean blood pressure for 5-60 min; prolonged the PR interval for 10-60 min and the QRS for 10-20 min; but shortened the QT interval for 10-30 min, and the QTc for 5-60 min after the start of the infusion. Severe hypotension occurred in 1 animal after the start of the high dose infusion leading to cardiohemodynamic collapse. Thus, data of this animal for 20-60 min after the high dose were not included for the statistical analysis.
DISCUSSION
In this study, we characterized halothane-anesthetized microminipigs using oseltamivir in comparison with beagle dogs (Kitahara et al., 2013), and found that oseltamivir decreased the heart rate and mean blood pressure, and delayed the atrioventricular nodal and intraventricular conduction in microminipigs like in beagle dogs, but that it shortened the repolarization period unlike in beagle dogs.
Rationale of drug doses
Oseltamivir of 75 mg/body is clinically prescribed twice a day. According to the interview form from the manufacturer (31th edition, revised in November 2017; Chugai Pharmaceutical Co., Ltd.), the administration of single p.o. dose of 150 mg of oseltamivir in healthy subjects attained its peak plasma concentrations of 115 ng/mL. The peak plasma concentrations of oseltamivir after 0.3, 3 and 30 mg/kg infusion over 10 min were 1.4, 7.4 and 125.5 µg/mL, respectively. Hence, the doses of oseltamivir used in this study could provide much higher plasma concentrations of oseltamivir than clinically relevant ones. On the other hand, in our previous experiment using the halothane-anesthetized canine model, the infusion of 0.3, 3 and 30 mg/kg of oseltamivir over 10 min provided the peak plasma concentrations of 1.2, 10.6 and 117.5 µg/mL (Kitahara et al., 2013) which were 1.2 times lower, 1.4 times higher and 1.1 times lower than in microminipigs, respectively. Thus, the peak plasma concentrations of oseltamivir were similar between microminipigs and beagle dogs. Since smaller effective volume of distribution of a drug and less whole-body fat content have been reported in miniature pigs than in dogs (Mitchell et al., 1996; Morton et al., 1993), one can speculate that oseltamivir might be converted to oseltamivir carboxylate by esterases greater in microminipigs than in beagle dogs.
Cardiohemodynamic effects
The low or middle dose of oseltamivir did not alter the cardiohemodynamic variables in microminipigs except that the heart rate decreased at 30 min after the middle dose. The high dose decreased the heart rate and mean blood pressure, of which maximum changes were –22 beats/min (–31%) at 60 min and –38 mmHg (–58%) at 15 min, respectively. It should be noted that severe hypotension occurred in 1 animal out of 4 after the start of the high dose infusion, leading to cardiohemodynamic collapse and ventricular tachycardia/fibrillation. In our previous experiment using halothane anesthetized dogs with the same experimental protocol, qualitatively the same responses were observed, but the maximum changes were smaller, which were –9 beats/min (–9%) at 10 min and –7 mmHg (–7%) at 10 min after the start of infusion of 30 mg/kg over 10 min, respectively (Kitahara et al., 2013). In spite of similar plasma drug concentrations, the extents of changes in the heart rate and mean blood pressure were greater in microminipigs than in beagle dogs, indicating higher drug sensitivity of microminipigs. Also, cardiohemidynamic collapse occurred in 1 microminipig, which was not observed in beagle dogs (Kitahara et al., 2013), supporting previously proposed characteristics of this new animal that microminipigs exert less great reflex-mediated increase of sympathetic tone on hypotension (Yokoyama et al., 2017).
Electrophysiological effects
The low or middle dose of oseltamivir did not alter any of the electrocardiographic variables. The high dose of oseltamivir prolonged the PR interval and QRS width, of which maximum changes were +42 ms (+32%) at 45 min and +50 ms (+61%) at 15 min after the infusion, respectively. In our previous study in beagle dogs (Kitahara et al., 2013), qualitatively the same responses were observed under the same experimental protocol, but their extents were +18 ms (+18%) at 15 min and +8 ms (+13%) at 10 min after the start of infusion of 30 mg/kg over 10 min, respectively. The prolongation of the PR interval and QRS width suggest that oseltamivir could effectively inhibit the Ca2+ and Na+ channels in the hearts of microminipigs, respectively like those of beagle dogs (Kitahara et al., 2013), whereas larger extent of changes in microminipigs than in beagle dogs may also indicate higher drug sensitivity of microminipigs.
On the other hand, oseltamivir shortened the QT interval and QTc, of which maximum changes were –41 ms (–10%) at 15 min and –68 (–16%) at 20 min after the high dose, respectively, which was in sharp contrast with those in beagle dogs, since the QT interval and QTc were prolonged by +24 ms (+9%) and +20 (+7%) at 10 min after the start of infusion of 30 mg/kg over 10 min, respectively in beagle dogs (Kitahara et al., 2013). Meanwhile, qualitatively similar results to those of microminipigs were observed in our previous in vitro study with the guinea-pig right ventricular preparation, which showed that oseltamivir in concentration of 9.4 to 94 µg/mL (30 to 300 µmol/L) shortened the action potential duration (Nakamura et al., 2016). Thus, inward ionic currents of the ventricle compared with outward ones could be more easily suppressed in microminipigs and guinea pigs than in dogs.
In conclusion, oseltamivir can suppress the sinus automaticity, and atrioventricular nodal and intraventricular conduction; and decrease the mean blood pressure, extents of which were greater in microminipigs than those in beagle dogs in spite of similar plasma concentrations, reflecting their higher drug sensitivity. Also, oseltamivir shortened the repolarization period in microminipigs unlike in beagle dogs, indicating that inward ionic currents of the ventricle compared with outward ones could be more easily suppressed in microminipigs than in dogs. This information may help improve utilizatione of microminipigs as a laboratory animal.
ACKNOWLEDGMENTS
This study was supported in part by AMED Grant (#AS2116907E) and JSPS KAKENHI Grant (#JP16K08559). The authors thank Ms. Misako Nakatani, Dr. Kiyotaka Hoshiai and Mrs. Yuri Ichikawa for their assistance during preparation of the manuscript.
Conflict of interest
The authors declare that there is no conflict of interest.
REFERENCES
- Cao, X., Wada, T., Nakamura, Y., Matsukura, S., Izumi-Nakaseko, H., Ando, K., Naito, A.T. and Sugiyama, A. (2017): Sensitivity and reliability of halothane-anesthetized microminipigs to assess risk for drug-induced long QT syndrome. Basic Clin. Pharmacol. Toxicol., 121, 465-470.
- Casscells, S.W., Granger, E., Kress, A.M., Linton, A., Madjid, M. and Cottrell, L. (2009): Use of oseltamivir after influenza infection is associated with reduced incidence of recurrent adverse cardiovascular outcomes among military health system beneficiaries with prior cardiovascular diseases. Circ. Cardiovasc. Qual. Outcomes, 2, 108-115.
- Chugai Pharmaceutical Co. Ltd. and F. Hoffmann-La Roche Ltd. (2007): Non-clinical studies conducted by order of the basic working group and results of voluntary examination and analysis (No. 2). pp. 47-57, [The 4th working group for basic research and examination of oseltamivir phosphate] December 10. (in Japanese). http://www.mhlw.go.jp/shingi/2007/12/s1210-6.html
- Kaneko, N., Itoh, K., Sugiyama, A. and Izumi, Y. (2011): Microminipig, a non-rodent experimental animal optimized for life science research: preface. J. Pharmacol. Sci., 115, 112-114.
- Kitahara, K., Nakamura, Y., Tsuneoka, Y., Adachi-Akahane, S., Tanaka, H., Yamazaki, H., Takahara, A., Yamazaki, J., Ikeda, T. and Sugiyama, A. (2013): Cardiohemodynamic and electrophysiological effects of anti-influenza drug oseltamivir in vivo and in vitro. Cardiovasc. Toxicol., 13, 234-243.
- Matsukura, S., Nakamura, Y., Cao, X., Wada, T., Izumi-Nakaseko, H., Ando, K., Yamazaki, H. and Sugiyama, A. (2017): Characterization of microminipigs as an in vivo experimental model for cardiac safety pharmacology. J. Pharmacol. Sci., 133, 103-109.
- Mitchell, A.D., Conway, J.M. and Potts, W.J. (1996): Body composition analysis of pigs by dual-energy x-ray absorptiometry. J. Anim. Sci., 74, 2663-2671.
- Morton, D.B., Abbot, D., Barclay, R., Close, B.S., Ewbank, R., Gask, D., Health, M., Mattic, S., Poole, T., Seamer, J., Southee, J., Thompson, A., Trussell, B., West, C. and Jennings, M. (1993): Removal of blood from laboratory mammals and birds. First report of the BVA/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab. Anim., 27, 1-22.
- Nakamura, Y., Sasaki, R., Cao, X., Wada, T., Hamaguchi, S., Izumi-Nakaseko, H., Ando, K., Tanaka, H., Takahara, A. and Sugiyama, A. (2016): Intravenous anti-influenza drug oseltamivir will not induce torsade de pointes: evidences from proarrhythmia model and action-potential assay. J. Pharmacol. Sci., 131, 72-75.
- Safrin, S. (2015): Antiviral agents. In: Basic & Clinical Pharmacology 13th edition (Katzung, B.G. and Trevor, A.J. ed.), pp. 835-864, McGraw Hill Education, New York.
- Sugiyama, A. (2008): Sensitive and reliable proarrhythmia in vivo animal models for predicting drug-induced torsades de pointes in patients with remodelled hearts. Br. J. Pharmacol., 154, 1528-1537.
- Takahara, A., Suzuki, S., Hagiwara, M., Nozaki, S. and Sugiyama, A. (2013): Electrophysiological effects of an anti-influenza drug oseltamivir on the guinea-pig atrium: comparison with those of pilsicainide. Biol. Pharm. Bull., 36, 1650-1652.
- Yokoyama, H., Nakamura, Y., Saito, H., Nagayama, Y., Hoshiai, K., Wada, T., Izumi-Nakaseko, H., Ando, K., Akie, Y. and Sugiyama, A. (2017): Pharmacological characterization of microminipig as a model to assess the drug-induced cardiovascular responses for non-clinical toxicity and/or safety pharmacology studies. J. Toxicol. Sci., 42, 93-101.