2020 年 45 巻 2 号 p. 77-86
2020 年 45 巻 2 号 p. 77-86
Sepsis-induced acute lung injury (ALI) has high morbidity and mortality rates, and there remains a need for therapeutic methods to improve the outcome of ALI patients. miR-483-5p is an important regulator for the development of various diseases such as sepsis. Nevertheless, it is not known whether miR-483-5p has an effect on sepsis-induced ALI. To explore this issue, this study used cecal ligation and puncture (CLP)-treated mice and lipopolysaccharide (LPS)-treated pulmonary microvascular endothelial cells (PMVECs) cells to simulate the models of sepsis-induced ALI in vivo and in vitro. Pathological and histological changes of lungs from sepsis-induced ALI mice were detected by Hematoxylin-eosin staining. The detection levels of caspase-3, interleukin (IL)-6 and IL-1β were used to reflect the effect of miR-483-5p on apoptosis and inflammation of sepsis-induced ALI. The detection level of lactate dehydrogenase (LDH) in PMVECs cells was used to reflect the severe extent of sepsis-induced injury. The expression of miR-483-5p in lung tissues of sepsis-induced ALI mice was determined by qRT-PCR. In addition, the interaction of miR-483-5p with PIAS1 was identified and validated by Targetscan website and luciferase reporter assay, respectively. The results showed that miR-483-5p was up-regulated in the lung tissues of sepsis-induced ALI mice. Knockdown of miR-483-5p effectively ameliorated lung injury in mice with sepsis-induced ALI and inhibited inflammation and apoptosis of LPS-treated PMVECs cells. Furthermore, in vitro experiment revealed that PIAS1 was a potential target of miR-483-5p. Moreover, miR-483-5p could suppress PIAS1 expression to aggravate inflammation and apoptosis of LPS-treated PMVECs cells. These findings suggest miR-483-5p is a potential therapeutic and diagnostic biomarker for sepsis-induced ALI and provide a new insight for understanding the molecular mechanism of sepsis-induced ALI.
Sepsis is known as a serious and life-threatening condition caused by a dysregulated host response to infection (Sinha et al., 2018). The extreme inflammatory response is a major pathophysiological response of sepsis, which leads to multiple organ failure or death (Perner et al., 2016; Van Wyngene et al., 2018). The respiratory failure is the first and most common complication in sepsis, and thus approximately 50% of severe sepsis patients can develop into acute lung injury (ALI) or acute respiratory distress syndrome (ARDS, ALI’s more severe form) (Qin et al., 2019; Sadowitz et al., 2011). ALI has been found to be associated with inflammatory responses, neutrophil infiltration, oxidative stress and the accumulation of protein-rich fluid in the lung (Kissoon et al., 2016). As previously reported, patients with sepsis-induced ALI have a poor outcome, with mortality rates of more than 40% (Mo et al., 2018). Currently available treatments for ALI, antibiotics and pulmonary supportive measures, can hardly improve the outcome of the patients (Aziz et al., 2018). Therefore, it is a considerable challenge to find effective therapeutic methods for sepsis-induced ALI.
MicroRNAs (miRNAs) are a group of small non-coding RNA molecules with 20-24 nucleotide length, and they bind to the complementary sequences in the 5’ or 3’ UTR (untranslated region) of the target mRNAs to regulate gene expression (Mohajeri et al., 2018). Many studies suggest that miRNAs are critical regulators for the development of various cancers (Leonetti et al., 2019). Important roles of miRNAs in sepsis have also been found (Benz et al., 2016). For instance, miR-150 levels in plasma samples of sepsis patients had a negative correlation with interleukin (IL)-18, IL-10 and TNF-α, and miR-150 could be considered as a marker of early sepsis (Vasilescu et al., 2009). miRNA-143 and -150 have been found to be able to detect T-cell immunoparalysis in sepsis (Möhnle et al., 2018). Recent evidence indicates that miR-483-5p plays key roles not only in diverse cancers but also in sepsis (Agosta et al., 2018; Tian et al., 2019; Wang et al., 2012). Serum miR-483-5p showed a lower level in surviving sepsis patients when compared with the non-surviving group, and miR-483-5p has the potential to be an effective marker for the prognosis of sepsis (Kingsley and Bhat, 2017; Wang et al., 2012). However, it is not known whether miR-483-5p has an effect on sepsis-induced ALI.
To explore the effect of miR-483-5p on sepsis-induced ALI, we firstly tested the level of miR-483-5p in the lung tissues of sepsis-induced ALI mice. Then, our study detected the injury of mice with ALI, the inflammation and apoptosis in lipopolysaccharide (LPS)-induced PMVECs cells after knocking down miR-483-5p. In addition, the interaction among miR-483-5p and its target PIAS1 in sepsis-induced ALI was further explored.
Twenty-four C57BL/6 mice (male, 21-28 g weight, 8- to 10-week-old) were purchased from the Shanghai Laboratory Animal Center (Shanghai, China). These mice were raised in a temperature-controlled room with a humidity of 50-60% and a 12 hr light/dark cycle at 24 ± 1°C, and they were allowed to free access to water and food throughout the study. We performed the animal experiments based on the guidelines from the Administration of Animal Experiments for Medical Research Purposes issued by the Ministry of Health of China.
Construction of sepsis animal modelIn this study, animal model of sepsis-induced ALI was constructed in mice using CLP (cecal ligation and puncture) method. Before CLP operation, 5 μg of miR-483-5p antagomir (GenePharma, Shanghai, China) or control antagomir (NC, GenePharma) was dissolved in 5 μL RNase-free water, which was mixed with 5 μL of in vivo transfection reagent (EntransterTM-In Vivo, Engreen Biosystem, Beijing, China). The mixture was injected into the mice via the tail vein on day 1 and day 3. After 4 days of injection, the mice were anesthetized with sodium pentobarbital (1%, 50 mg/kg), fixed on the operating table using adhesive tape, and shaved on their abdomen followed by disinfection with 10% povidone iodine. Next, the cecum was exposed after performing a 1-cm vertical incision at the median of the abdomen, then ligated using a 4-0 silk suture 1.5 cm to its distal end, and punctured twice with a 22-gauge needle to eject a small amount of feces. Next, the cecum was returned in the abdomen, and then the incision was closed using a 4-0 silk suture in two layers. Subsequently, mice were injected with 0.9% saline (1 mL). The operation in the sham group was the same, but did not ligate and puncture. Finally, the mice were replaced in their cages and allowed free access to water and food. We randomly divided the mice into four groups: sham group + miR-483-5p antagomir; sham group + negative control antagomir (NC); sepsis-induced ALI model group + miR-483-5p antagomir; sepsis-induced ALI model group + control antagomir (NC).
Cell culture, transfection, and simulation of a septic model in vitroMouse pulmonary microvascular endothelial cells (PMVECs cells) were obtained from Shanghai Qincheng Biotechnology Co, Ltd. (Shanghai, China). HEK-293 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were incubated with DMEM medium containing 100 U/mL penicillin, 100 μg/mL streptomycin and 10% FBS under 5% CO2 at 37°C in a humidified incubator.
miR-483-5p inhibitor and control inhibitor were synthesized by GenePharma, and were used to transfect into HEK-293 cells for 24 hr with Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
For simulation of a septic model in vitro, we used LPS (1 μg/mL) to stimulate the transfected cells for 4 hr.
Hematoxylin-eosin stainingThe right lung of the above treated mice was removed, fixed using 4% paraformaldehyde for 24 hr at 4°C, dehydrated by ethanol, embedded in paraffin, and sectioned at 5 μm thickness by a microtome (Leica RM2125RT, Leica, Nussloch, Germany), followed by staining with hematoxylin and eosin. The section was recorded by Olympus BX 51 light microscope (Olympus Corporation, Tokyo, Japan) at 200 × magnification. The severity of lung injury was histologically scored in a blinded manner and evaluated by four independent parameters: pulmonary interstitial thickening, alveolar edema, neutrophil infiltration, and hemorrhage in lung tissue.
Immunohistochemistry (IHC)The lung tissues were 4% paraformaldehyde, embedded in paraffin and then sectioned by a microtome (Leica RM2125RT; 5 μm thickness). After deparaffinization, rehydration and antigen retrieval, the section was incubated at 4°C with primary antibodies against caspase-3 (Cell Signaling Technology, Danvers, MA, USA, dilution 1:1000, cat. No 9662) overnight, followed by incubating at room temperature with secondary antibodies (HRP (horseradish peroxidase)-conjugated- goat anti-rabbit IgG antibody, Abcam, dilution 1:2000, Cat no. ab205718,) for 30 min. The section was then washed with PBS, incubated with diaminobenzidine and then counterstained with hematoxylin (Sigma-Aldrich, St. Louis, MO, USA). Finally, an Olympus BX 51 light microscope (Olympus Corporation) was used to record the tissue section.
Enzyme-linked immunosorbent assays (ELISA)Interleukin (IL)-6 and IL-1β levels in plasma of septic mice and LPS-treated PMVECs cells were measured by mouse ELISA kits (BD Biosciences, Franklin Lakes, NJ, USA) based on the manufacturer’s protocols.
Measurement of the injury markerBased on the manufacturer’s instructions, the leakage level of lactate dehydrogenase (LDH) from PMVECs cells was determined by a commercial assay kit (Pointe Scientific, Lincoln Park, MI, USA).
Caspase-3 enzyme activity assayA fluorimetric assay system kit (Sigma) was utilized to evaluate the caspase-3 enzyme activity in LPS-treated PMVECs cells. The proteins from the cells were extracted by RIPA lysis buffer (Beyotime, Shanghai, China), quantified using BCA method, and equally added into the assay buffer (pH 7.4, 20 mM Hepes, 0.1% CHAPS5, 2 mM EDTA, and mM DTT) with DEVD-AMC substrate molecule (10 μM). The fluorescence intensity of the sample was measured at 37°C in a Synergy H1 fluorometer (BioTek, Winooski, VT, USA; the excitation wavelength, 370 nm; emission wavelength, 450 nm).
Quantitative real-time PCR (qRT-PCR)Lung tissues and cells were utilized to extract total RNA with TRIzol reagent (Invitrogen; Carlsbad, CA, USA). After quantifying the concentration of total RNA, RNA was reverse transcribed into the cDNA (complementary DNA) using the PrimeScript™ RT reagent Kit (Takara, Kyoto, Japan). cDNA as a template was used for PCR amplification and quantified with TB Green® Premix Ex Taq™ RT-PCR Kit (Takara) on the ABI7500 Real-Time PCR machine (ABI Life Technologies, Waltham, MA, USA). Relative mRNA expression was evaluated using the 2-ΔΔCq method. β-actin and U6 were respectively utilized as internal control genes for normalization. Table 1 shows the sequences of the primers.
| Primer | Sequence (5’-3’) |
|---|---|
| miR-483-5p forward | ACACTCCAGCTGGGAAGACGGGAGGAAAGAA |
| miR-483-5p reverse | CTCAACTGGTGTCGTGGA |
| PIAS1 forward | GCGGACAGTGCGGAACTAAA |
| PIAS1 reverse | ATGCAGGGCTTTTGTAAGAAGT |
| β-actin forward | CGTGAAAAGATGACCCAGATCA |
| β-actin reverse | TGGTACGACCAGAGGCATACAG |
| U6 forward | CTCGCTTCGGCAGCACA |
| U6 reverse | AACGCTTCACGAATTTGCGT |
PMVECs cells were lysed using RIPA lysis buffer (Beyotime) to obtain total protein. After quantification with BCA method, equal protein (20 µg) was separated by 10% SDS-PAGE, followed by transferring to PVDF membranes. After 5% non-fat milk was used to block the membranes for 1 hr at room temperature, the membranes were incubated with primary antibodies against PIAS1 (Cell Signaling Technology; dilution 1:1,000; cat no. 3550) and β-catenin (Cell Signaling Technology, Danvers, MA, USA; dilution 1:1,000; cat no. 8480) overnight at 4°C. Next, the membranes were washed with 0.5% TBST buffer and cultured for 1 hr at room temperature with HRP-conjugated secondary antibodies (goat anti-rabbit, ProteinTech Group, Inc., dilution 1:2,000, Cat no. SA00001-2). Finally, the ECL Kit (Millipore, Burlington, MA, USA) was used to record the protein bands. The subsequent quantitative calculation was performed by ImageJ software. β-actin was utilized to be a loading control for normalization.
Luciferase reporter assayThe Targetscan website (http://www.targetscan.org/vert_72/) was used to identify the binding sites between PIAS1 and miR-483-5p. The 3’-UTR fragment of PIAS1 with the putative binding sites of miR-483-5p was amplified by PCR and then cloned into the pGL3 vector (Promega, Madison, WI, USA) to form a luciferase reporter vector, named PIAS1 WT. PIAS1 with the corresponding mutated sequences was also cloned into the pGL3 vector (Promega) to obtain the control luciferase reporter vector, named PIAS1 MUT. The above luciferase reporter vectors were respectively transfected into HEK-293 cells using Lipofectamine 3000 for 48 hr. Then a luminometer (Promega) was used to determine the luciferase activity under a dual-luciferase reporter assay system (Promega).
Statistical analysisThe data from three independent repeated experiments are presented as mean ± standard deviation (SD). SPSS 19.0 software was carried out to analyze these data. The statistical differences between groups were analyzed using one-way ANOVA or Student’s t test. A p-value less than 0.05 was considered statistically significant.
In order to study the expression of miR-483-5p in sepsis-induced ALI, we first constructed the animal model of sepsis-induced ALI, and then detected the miR-483-5p level in the animal model. The sepsis-induced ALI model was established in mice using the CLP method. Figure 1A revealed that the lung in the sham group had normal lung structures, while the lung in mice with sepsis-induced ALI showed obviously histopathological changes, including edema, alveolar collapse, increased the thickness of the alveolar wall, inflammatory cell infiltrations, and severe hyperemia and hemorrhage of pulmonary interstitial. Thus, the septic mice model had been successfully constructed. Applying this model, the analysis of qRT-PCR indicated the overexpression of miR-483-5p in the lung tissues of mice with sepsis-induced ALI in comparison to the sham group (Fig. 1B).

miR-483-5p is over-expressed in mice with sepsis-induced ALI. (A) Pathological and histological changes of lungs from sepsis-induced ALI mice (CLP group) or untreated mice (sham group) were detected by Hematoxylin-eosin staining. (B) The expression of miR-483-5p in the lung tissues of mice with sepsis-induced ALI (CLP-treated group, CLP) or untreated mice (sham group) was detected by qRT-PCR. **p < 0.01 vs. sham group. CLP, cecal ligation and puncture.
To study the effect of miR-483-5p on sepsis-induced ALI, in vivo experiments were performed. As shown in Fig. 2A, miR-483-5p antagomir significantly suppressed the expression of miR-483-5p in the lung tissues of mice from sham and CLP groups. In the sham group, there were no significant histological changes between the lung sections transfection with miR-483-5p antagomir and control antagomir (NC) (Fig. 2B). Nevertheless, knockdown of miR-483-5p considerably ameliorated lung injury in mice treated with CLP (Fig. 2B).

Knockdown of miR-483-5p ameliorates lung injury in mice with sepsis-induced ALI. After miR-483-5p antagomir and control antagomir were respectively transfected in mice, the mice were randomly divided into four groups: sham group + miR-483-5p antagomir; sham group + negative control antagomir (NC); sepsis-induced ALI model group (CLP-treated group, CLP) + miR-483-5p antagomir; CLP + NC. (A) The expression of miR-483-5p in the lung tissues from the above four different groups was detected by qRT-PCR. (B) Pathological and histological changes of lungs in four different groups were detected by Hematoxylin-eosin staining. (C) The expression of caspase-3 in the lung tissues of mice was measured by immunohistochemistry (IHC) assay. (D) The mRNA levels of interleukin (IL)-6 and IL-1β in the lung tissues of mice were detected by qRT-PCR. (E) The protein levels of IL-6 and IL-1β in plasma were examined by ELISA assay. **p < 0.01.
Furthermore, compared with the CLP-untreated normal mice (sham group), the key pro-apoptotic biomarker caspase-3 was found to be over-expressed in the lung tissues of mice with sepsis-induced ALI. Meanwhile, knockdown of miR-483-5p in sepsis-induced ALI mice obviously suppressed the expression of caspase-3 (Fig. 2C).
Additionally, the mRNA levels of pro-inflammatory cytokines (IL-6, IL-1β) were greatly up-regulated in the lung tissues of mice with sepsis-induced ALI as compared to sham group, whereas knockdown of miR-483-5p effectively down-regulated the levels of IL-6 and IL-1β in mice with sepsis-induced ALI (Fig. 2D). This result was confirmed by ELISA test, as the protein levels of IL-6 and IL-1β in plasma have the same change tendency caused by the introduction of miR-483-5p antagomir (Fig. 2E).
Knockdown of miR-483-5p inhibits inflammation and apoptosis of LPS-treated PMVECs cellsTo further study the effect of miR-483-5p on sepsis-induced ALI, in vitro experiments were also carried out. PMVECs cells were stimulated by LPS to simulate a septic model in vitro. The analysis of qRT-PCR revealed that miR-483-5p level was enhanced after PMVECs cells treated with LPS, and its level in LPS-treated cells was decreased by the transfection of miR-483-5p inhibitor (Fig. 3A). Figure 3B suggested LDH had a higher leakage level from LPS-treated cells when compared with LPS-untreated normal PMVECs cells, and knockdown of miR-483-5p obviously decreased the level of LDH. Fig. 3C showed that the activity of caspase-3 was greatly enhanced after PMVECs cells treated with LPS, and its activity was inhibited by miR-483-5p knockdown in LPS-treated cells. Furthermore, qRT-PCR and ELISA assays suggested that the levels of IL-6 and IL-1β were up-regulated by the treatment of LPS in PMVECs cells, and miR-483-5p knockdown reduced the levels of IL-6 and IL-1β (Fig. 3D and 3E).
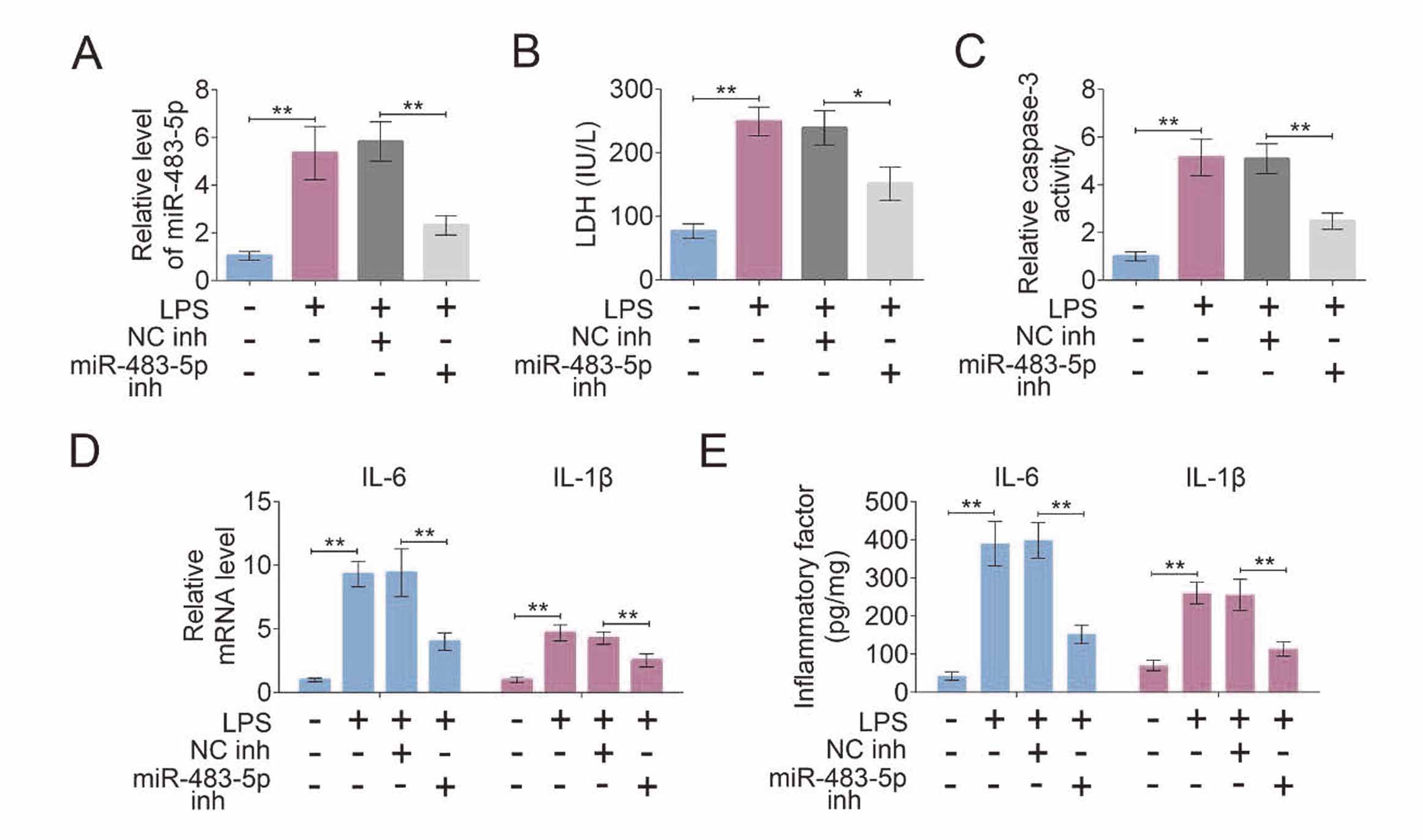
Knockdown of miR-483-5p inhibits inflammation and apoptosis of LPS-treated PMVECs cells. After miR-483-5p inhibitor and control inhibitor were respectively transfected into LPS-treated PMVECs cells, (A) the expression level of miR-483-5p was determined by qRT-PCR; (B) the level of lactate dehydrogenase (LDH) and (C) the activity of caspase-3 in PMVECs cells were measured using assay kits; (D) the mRNA and (E) protein levels of interleukin (IL)-6 and IL-1β were respectively detected by qRT-PCR and ELISA assays. **p < 0.01; *p < 0.05.
To explore the molecular mechanism of miR-483-5p, the Targetscan website was used to identify the potential targets of miR-483-5p. PIAS1 was selected as a candidate, and the putative binding sites between PIAS1 and miR-483-5p are listed in Fig. 4A. The high transfection efficiency of miR-483-5p mimics in HEK-293 cells was proven by qRT-PCR (Fig. 4B). Luciferase reporter assay was utilized to validate the putative relationship between PIAS1 and miR-483-5p (Fig. 4C). The luciferase activity remarkably decreased after the co-transfection of miR-483-5p mimics and PIAS1-WT into HEK-293 cells compared with NC mimic group, while there were no obvious changes in PIAS1-MUT luciferase activity between miR-483-5p mimics and NC mimic groups (Fig. 4C). Additionally, PIAS1 level was decreased by miR-483-5p mimics transfection as compared to the control group (Fig. 4D). Thus, PIAS1 could be negatively regulated by miR-483-5p.

PIAS1 is a possible target of miR-483-5p. (A) The putative binding sites between PIAS1 and miR-483-5p were identified using the Targetscan website. (B) The level of miR-483-5p was examined by qRT-PCR. (C) The putative relationship between PIAS1 and miR-483-5p was validated by luciferase reporter assay. (D) The protein level of PIAS1 was measured by western blot. **p < 0.01.
To further explore the roles of miR-483-5p with PIAS1, the co-transfection of miR-483-5p mimics with PIAS1 plasmid in LPS-treated PMVECs cells was measured. The mRNA and protein level of PIAS1 was considerably upregulated after LPS-treated PMVECs cells transfected with PIAS1 plasmid (Fig. 5A and 5B). Furthermore, the high release level of LDH induced by miR-483-5p mimics was reversed after the introduction of PIAS1 plasmid in LPS-treated cells (Fig. 5C). The activity of caspase-3 was enhanced by the transfection of miR-483-5p mimics, while its high activity was changed by the co-transfection of PIAS1 plasmid and miR-483-5p mimics in LPS-treated cells (Fig. 5D). The transfection of miR-483-5p mimics in LPS-treated cells considerably increased the levels of IL-6 and IL-1β, which was reversed after the introduction of PIAS1 plasmid (Fig. 5E and 5F). Hence, miR-483-5p could aggravate inflammation and apoptosis of LPS-treated PMVECs cells by negatively regulating PIAS1 expression.

miR-483-5p aggravates inflammation and apoptosis of LPS-treated PMVECs cells through regulating PIAS1 expression. The mRNA and protein expression of PIAS1 in LPS-treated cells was assessed by (A) qRT-PCR and (B) western blot, respectively. After miR-483-5p mimics + PIAS1 plasmid, miR-483-5p mimics + control vector, and vector + NC mimics were respectively transfected into LPS-treated cells, (C) the level of LDH and (D) the activity of caspase-3 were evaluated using assay kits; the (E) mRNA and (F) protein levels of interleukin (IL)-6 and IL-1β were respectively detected by qRT-PCR and ELISA assays. **p < 0.01.
Our study used CLP-treated mice and LPS-treated cells to simulate the models of sepsis-induced ALI in vivo and in vitro. MiR-483-5p levels were upregulated in the lung tissues of sepsis-induced ALI models as compared to the corresponding control groups. Knockdown of miR-483-5p decreased the level of caspase-3, IL-6 and IL-1β in lung tissues to ameliorate lung injury in mice with sepsis-induced ALI. Similarly, the level of LDH, IL-6 and IL-1β and the activity of caspase-3 were enhanced by LPS treatment in PMVECs cells, which was reversed by the transfection of miR-483-5p inhibitor. In addition, PIAS1 was found to be a possible target of miR-483-5p, and it could be negatively mediated by miR-483-5p. Furthermore, miR-483-5p could aggravate inflammation and apoptosis of LPS-treated PMVECs cells through targeting PIAS1 expression.
Currently, it is important to explore the molecular mechanism of sepsis-induced ALI because of the poor outcome of patients with sepsis-induced ALI and the lack of effective therapeutic methods (Aziz et al., 2018; Digiacomo et al., 2018). Plenty of reports suggest that miR-483-5p can be regarded as a tumor promoter or suppressor gene in various cancers, and closely correlate with the tumor progression (Agosta et al., 2018; Liu et al., 2019; Tian et al., 2019; Yang et al., 2017). Moreover, serum miR-483-5p has been proven to be a potential marker for the prognosis of sepsis (Wang et al., 2012). Nevertheless, the effect of miR-483-5p on sepsis-induced ALI has not been reported. To explore this issue, our study firstly used CLP-treated mice and LPS-treated cells to simulate sepsis-induced ALI models in vivo and in vitro. Then, miR-483-5p over-expression was observed in the animal and cell models compared with the corresponding control groups. Thus, the level of miR-483-5p might be correlated with sepsis-induced ALI.
LDH is known to catalyze the conversion of pyruvate into lactate reversibly, and its level is positively associated with the severe extent of sepsis-induced injury (Duman et al., 2016; Lu et al., 2018). In addition, apoptosis widely exists in sepsis, and it is accompanied by DNA fragmentation and caspase-3 activation (Girardot et al., 2017). Most importantly, the extreme inflammatory response is a major pathophysiological response of sepsis (Kalbitz et al., 2016). The pro-inflammatory cytokines, mainly including IL-1β and IL-6, are critical for mediating inflammation, and their high levels in sepsis have been proven (Kurt et al., 2007). The present study showed that the high level of caspase-3, IL-6 and IL-1β in mice with sepsis-induced ALI was down-regulated by miR-483-5p knockdown. Similarly, the high activity of caspase-3 and the high level of LDH, IL-6 and IL-1β in LPS-treated cells were reversed by miR-483-5p knockdown. Collectively, miR-483-5p knockdown effectively ameliorated lung injury in mice with sepsis-induced ALI and inhibited inflammation and apoptosis of LPS-treated cells.
It is known that miRNAs can regulate its target genes to participate in diverse biological processes mainly including cell invasion, apoptosis, and proliferation (Zhou et al., 2017). To further study the molecular mechanism of miR-483-5p in sepsis-induced ALI, we identified PIAS1 as a possible target of miR-483-5 through performing the Targetscan website and luciferase reporter assay. PIAS1 (protein inhibitor of activated STAT 1) is a member of the PIAS family and initially found to negatively regulate the STAT1 signaling (Ke et al., 2019). In addition, PIAS1 can be involved in the differentiation of hematopoietic stem cells and T cells (Zhang et al., 2017). Moreover, PIAS1 is critical for inhibiting inflammation in the lung and adipose (Liu et al., 2016; Liu et al., 2015). Liu et al. have indicated that PIAS1 could suppress the level of IL-4 and IL-1β in the NF-κB pathway to inhibit the inflammation in the lung (Liu et al., 2016). Consistent with previous reports, the activity of caspase-3 and the level of LDH, IL-6 and IL-1β were enhanced by miR-483-5p mimics, which could be effectively changed by the introduction of PIAS1 in LPS-treated cells. Accordingly, it can be speculated that miR-483-5p aggravated inflammation and apoptosis of LPS-treated PMVECs cells through inhibiting PIAS1 expression.
In conclusion, the high expression of miR-483-5p promoted inflammation and apoptosis of LPS-treated PMVECs cells through inhibiting PIAS1 expression. Therefore, miR-483-5p can function as a potential therapeutic and diagnostic biomarker for sepsis-induced ALI. The interaction of miR-483-5p with PIAS1 offers a novel insight for understanding the molecular mechanism of sepsis-induced ALI.
Conflict of interestThe authors declare that there is no conflict of interest.