2020 年 45 巻 8 号 p. 503-513
2020 年 45 巻 8 号 p. 503-513
Creatine kinase (CK) and lactate dehydrogenase (LDH) serve as biomarkers for skeletal muscle injury in preclinical toxicity studies, but have a limitation regarding tissue specificity. Circulating miR-206 was recently reported to be a useful biomarker for skeletal muscle disorders in humans. Here, we sought to determine whether serum miR-206 can be used as a biomarker in preclinical toxicity studies to detect drug-induced skeletal muscle injury with higher sensitivity and specificity than the biomarkers CK, LDH, skeletal troponin I (sTnI), and myosin light chain 3 (Myl3). We established rat models of skeletal muscle injury through treatment with the muscle toxicant 2,3,5,6-tetramethyl-p-phenylenediamine (TMPD) as well as four in-house compounds. We found that serum miR-206 levels significantly increased after treatment with TMPD, and tended to be higher in rats treated with in-house compounds than in control rats. ROC analysis revealed that the specificity of serum miR-206 for detection of skeletal muscle injury was higher compared with those of other markers. Further, serum miR-206 levels were unchanged in rats with isoproterenol-induced cardiotoxicity. These findings demonstrate that serum miR-206 may serve as a highly specific biomarker for preclinical analysis of rats with drug-induced skeletal muscle injuries.
Skeletal muscle injury is occasionally induced by treatment with certain medications, such as statins (Di Stasi et al., 2010) and fibrates (Hodel, 2002; Okada et al., 2009). This adverse event can potentially lead to withdrawal of a drug from the market. For example, cerivastatin caused 100 rhabdomyolysis-related deaths (Mendes et al., 2014). To avoid developing drug candidates with such severe myotoxicity, preclinical toxicity studies are required to predict the risk of adverse events.
Plasma creatine kinase (CK) and lactate dehydrogenase (LDH), which are commonly used as conventional clinical biomarkers of muscular disorders, are measured in preclinical toxicity studies using rodents as well as other animal species. A disadvantage of these biomarkers is that, because CK and LDH are also expressed in the heart, brain, or liver, their levels are increased by injuries of tissues other than muscle. Further, CK and LDH levels can be increased by exercise (Sastre et al., 1992), age, muscle mass, pregnancy, and alcohol consumption (Matsuzaka et al., 2014).
Skeletal muscle troponin I (sTnI) and myosin light chain 3 (Myl3) were identified as useful biomarkers of skeletal muscle injury and other disorders in rats and humans (Matziolis et al., 2011; Simpson et al., 2005; Burch et al. 2016). The United States Food and Drug Administration and the European Medicines Agency have issued biomarker Letters of Support, which aim to encourage further studies of biomarkers such as sTnI and Myl3, in research, nonclinical, and early clinical drug-development programs to detect and monitor skeletal muscle injuries (European Medicines Agency, 2015; U.S. FDA, 2015). However, these biomarkers have several disadvantages, including their rapid clearance from the bloodstream and alteration by renal dysfunction (Tonomura et al., 2012). Therefore, identifying additional biomarkers with high specificity and sensitivity is critically important.
Circulating microRNAs (miRNAs) represent potential markers of diseases such as cancer (Mitchell et al., 2008; Xu et al., 2011), hepatic fibrosis (Roderburg et al., 2011), heart failure (Tijsen et al., 2010), and Alzheimer’s disease (Kumar et al., 2013). miRNAs are endogenous, short non-coding RNAs 19-25 nucleotides in length that negatively regulate gene expression via translational repression or mRNA degradation (Ambros, 2001). To date, more than 2,600, 1,900, and 700 miRNAs have been identified in humans, mice, and rats, respectively (miRBase version 22). Certain miRNAs are expressed in a tissue-specific manner (Liang et al., 2007), and are stable in plasma (Mitchell et al., 2008), serum (Mitchell et al., 2008), saliva (Michael et al., 2010; Park et al., 2009), and urine (Hanke et al., 2010). MyomiRs such as miR-206, miR-1, miR-208, miR-133a, and miR-133b are described as striated muscle-specific or muscle-enriched miRNAs, and are involved in myoblast proliferation/differentiation, muscle regeneration, or fibretype specification (Siracusa et al., 2016). Among them, miRNA expressed in skeletal muscle but not heart is only miR-206 (Minami et al., 2014). Additionally, serum miR-206 has been reported to be a useful biomarker of muscular disorders such as Duchenne muscular dystrophy (Cacchiarelli et al., 2011; Li et al., 2014). Moreover, plasma miR-206 levels are elevated in rats treated with notexin, a tiger snake venom which induces muscle injury (Siracusa et al., 2016).
Here, we sought to determine whether serum miR-206, which is a skeletal muscle-specific miRNA, can be a sensitive and specific biomarker for skeletal muscle injury caused by new chemical compounds in pre-clinical toxicity studies in rats.
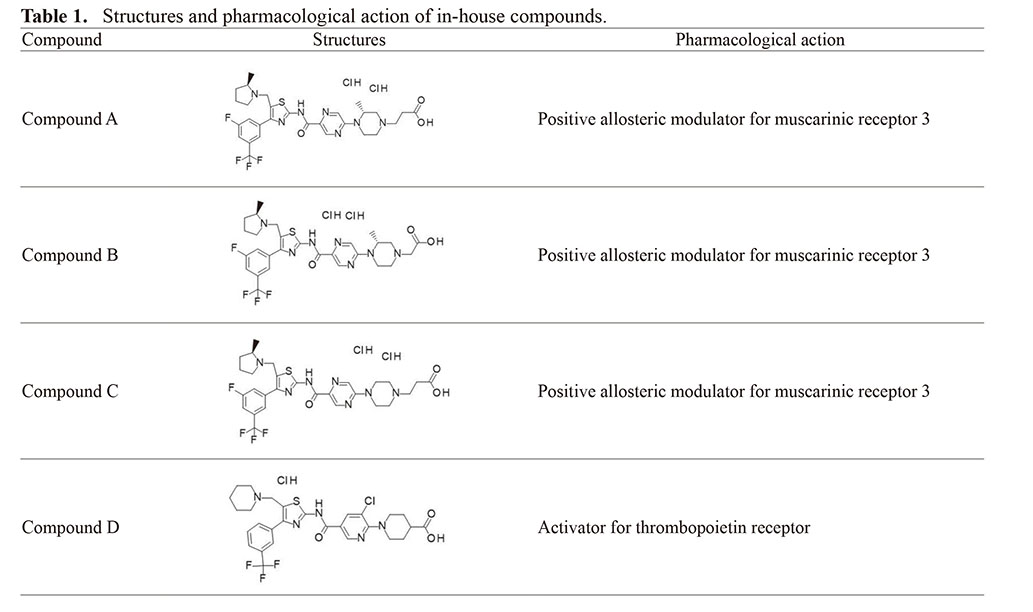
Chemicals and their sources were as follows: 2,3,5,6-tetramethyl-p-phenylenediamine (TMPD), Sigma-Aldrich (St. Louis, MO, USA); DL-isoproterenol hydrochloride, Nacalai Tesque Inc. (Kyoto, Japan); corn oil, MP Biomedicals (Santa Ana, CA, USA); methyl cellulose (MC), Shin-Etsu Chemical (Tokyo, Japan); and saline, Otsuka Pharmaceutical Factory (Tokushima, Japan). In-house compounds A-D were synthesized in-house and are characterized in Table 1. All other chemicals and solvents were of the highest grades commercially available.
 Animal models
Animal models
Animal experiments were approved by the Committee for Animal Experiments of Astellas Pharma, Kashima or Tsukuba Facilities, which have been fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Sprague-Dawley (SD) and F344 rats were purchased from Charles River (Shiga, Japan). Rats were housed in a controlled environment (temperature, 20°C to 26°C; humidity, 40%–70%; and 12 hr light/12 hr dark cycle) in the institution’s animal facility with free access to food and water. Rats were acclimatized before use in the experiments.
To determine whether serum miR-206 levels change in association with skeletal muscle injury, a rat model was established using TMPD, which is typically used to induce severe skeletal muscle-specific injury (Laterza et al., 2009). The TMPD group, 9-week old male SD rats (n = 4-5) were subcutaneously injected twice daily for 3 days with 4 mg/kg of TMPD dissolved in corn oil. These rats were sacrificed at 3 hr after the fifth administration or 16 hr after the last (sixth) administration.
To determine whether the miR-206 level changed in association with slight necrosis, samples obtained from past toxicity or pharmacological studies were used. In these studies, the four in-house compounds shown in Table 1 were used. In-house compound A, B, and C groups consisted of 8-week-old female SD rats (n = 1–2) which were orally administered compound A at 100 mg/kg or compound B or C at 100 mg/kg or 300 mg/kg dissolved in 0.5% MC once daily for 3 days. The rats were sacrificed at 24 hr after the final treatment. Compound D group consisted of 9-week-old male F344 rats (n = 3) which were orally administered a single dose of compound D at 300 mg/kg dissolved in 0.5% MC. These rats were sacrificed at 24 hr after administration.
At the time of being sacrificed, blood samples from the abdominal aorta and skeletal muscle tissues were collected. The femoral and cervical multifidus muscles of all rats were removed, and the crural muscles of the TMPD and compound D groups and the diaphragms of the TMPD group were removed. To prepare plasma, the anticoagulant EDTA was added to blood samples. After centrifugation, plasma or serum was collected and stored at −80°C.
To determine whether the elevation of serum miR-206 levels was specific for skeletal muscle injury, we prepared a model of cardiac muscle injury as follows: 6-week-old male SD rats (n = 10) were subcutaneously administered a single dose of isoproterenol at 0.1 mg/kg dissolved in saline. Sera and hearts were collected 24 hr after administration. Plasma and sera were stored at −80°C. Tissues were fixed in 10% phosphate-buffered formalin for histopathological examination.
To determine whether the stress of frequent blood sampling influenced the serum level of miR-206, 8-week-old male SD rats (n = 7) were orally administered a single dose of 0.5% MC, and blood samples (approximately 100 µL to 200 µL) were collected from the cervical vein 0.5, 1, 2, 4, and 8 hr after treatment and from the abdominal aorta 24 hr after treatment. Sera were prepared by centrifugation and stored at −80°C.
Biochemical assays and histopathological examinationThe activities of plasma CK and LDH were measured using an automated blood chemistry analyzer (Model 7170S; Hitachi, Tokyo, Japan). Serum levels of sTnI and Myl3 were measured using a SECTOR Imager 6000 (Meso Scale Diagnostics, Rockville, MD, USA) with a Muscle Injury Panel 1 (rat) Assay Kit (Meso Scale Diagnostics). Fixed skeletal muscles (femoral, crural, and cervical multifidus muscles and diaphragm) and heart were embedded in paraffin, sectioned, and then stained with hematoxylin and eosin. Necrosis grades were defined as follows: –, none; +, very slight; ++, slight; +++, moderate.
RNA isolation from serum and measurement of serum miR-206Total RNA was isolated from 50 µL to 200 µL of serum using QIAcube (QIAGEN, Hilden, Germany) and an miRNeasy Serum/Plasma Kit (QIAGEN) according to the manufacturer’s protocols. Synthetic cel-miR-39 (2.5 nmol) was added to serum as a spiked control. The cDNAs were synthesized from total RNA using a miScript II RT Kit (QIAGEN) according to the manufacturer’s protocols. Preamplification was performed using an miScript PreAMP PCR Kit (QIAGEN) according to the manufacturer’s protocols. The miR-206 and cel-miR-39 levels were measured using quantitative real-time PCR (40 cycles) with an miScript SYBR Green PCR Kit (QIAGEN) and miScript Primer Assay Rn_miR-206_1 (Qiagen, CA # MS00000623) and Ce-miR-39_1 (Qiagen, CA# MS00019789) using a QuantStudio 12K Flex Real-Time PCR System (Life Technologies, Carlsbad, CA, USA) in accordance with the manufacturer’s recommended cycling protocol. The PCR was run for 40 cycles. Standard curve for absolute quantification was generated using a commercially available synthetic RNA oligonucleotide (rno-miR-206, 5’-UGGAAUGUAAGGAAGUGUGUGG-3’) (Hokkaido System Science, Hokkaido, Japan). Fifteen serial dilutions ranging from 6.67 x 103 pmol/L to 1.39 pmol/L were prepared by 3-fold dilutions with synthetic miR-206 oligonucleotide and processed in parallel to the samples with the same protocol. The miR-206 concentrations were determined with the standard curve using the normalized Ct values. The normalized Ct values were calculated as follows: The median Ct of cel-miR-39 across all samples was calculated, and the Ct value of cel-miR-39 in each sample was divided by the median Ct stated above. The lower limit of quantification (LLOQ) for each miRNA was determined as follows: log miR-206 concentration (pmol/L) versus Ct was plotted for all serial dilutions in standard miRNA and linear regression analysis was performed. The lowest concentration was omitted until the R-squared (R2) value was found to be greater than 0.96. The lowest concentration at R2 > 0.96 was then determined to be the LLOQ for miR-206.
Statistical analysisStatistical analyses were performed using GraphPad Prism 8.0.2 (GraphPad Software Inc., La Jolla, CA, USA), except for the data for compounds A-C. Comparisons were performed using a Wilcoxon test (unequal variance) or the Student t test (variance), according to the results of the F test. For the receiver operator characteristic (ROC) analysis, 38 rats from studies of TMPD and compounds A-D were divided into two groups: 18 rats with skeletal muscle necrosis as a positive group, and 20 rats without necrosis as a negative group. ROC curves were then generated. Sensitivity and specificity were calculated using ROC analysis with optimal cut-off values, which were determined according to the maximum value of Youden’s index [J] ([J] = sensitivity + specificity – 1).
Rats administered TMPD exhibited very slight to moderate necrosis of the skeletal muscles (femoral, crural, and cervical multifidus muscles, or diaphragm) (Table 2). Necrosis was observed in the cervical multifidus muscle in one of two rats treated with compound A, in the cervical multifidus muscle of all rats treated with compound B, in the cervical multifidus muscle of three of four rats treated with compound C, and in the femoral, crural, and cervical multifidus muscles of all rats treated with compound D, although the grade of necrosis ranged from very slight to moderate.
| Compound | Dose (mg/kg) |
Number of animals | Femoral muscle |
Crural muscle |
Cervical multifidus muscle | Diaphragm |
|---|---|---|---|---|---|---|
| TMPD 3 hr | 4 | 4 | +++(1), ++(2), +(1) | ++(2), +(2) | ++(1), +(3) | ++(2), +(2) |
| TMPD 16 hr | 4 | 5 | +++(1), ++(3), +(1) | ++(2), +(3) | ++(1), +(4) | ++(1), +(4) |
| Compound A | 100 | 2 | - | NE | +(1) | NE |
| Compound B | 100 | 2 | - | NE | +(2) | NE |
| 300 | 1 | +(1) | NE | +(1) | NE | |
| Compound C | 100 | 2 | - | NE | +(2) | NE |
| 300 | 2 | - | NE | +(1) | NE | |
| Compound D | 100 | 3 | ++(2), +(1) | ++(2) | +++(1), +(1) | NE |
Histopathological necrosis grades: -, none; +, very slight; ++, slight; +++, moderate. NE: not examined. The number in parentheses describes number of rats.
The levels of plasma CK and LDH, as well as those of serum miR-206, sTnl and Myl3 3 hr after the fifth administration and 16 hr after the sixth (final) treatments with TMPD were measured (Fig. 1). Three hours after the fifth treatment, the levels of LDH and miR-206 in rats treated with TMPD were significantly high compared to those of control rats. Further, the levels of the other parameters in rats treated with TMPD tend to be higher than those of control rats. The levels of CK, LDH, and sTnI in TMPD-treated rats were similar to those of control rats 16 hr after the final treatment (Fig. 1A, B, D). In contrast, the levels of miR-206 and Myl3 remained high, although in just one rat with moderate necrosis. Thus, the level of miR-206 could detect skeletal muscle injury in the same way as conventional markers, even after a certain period of time from administration.
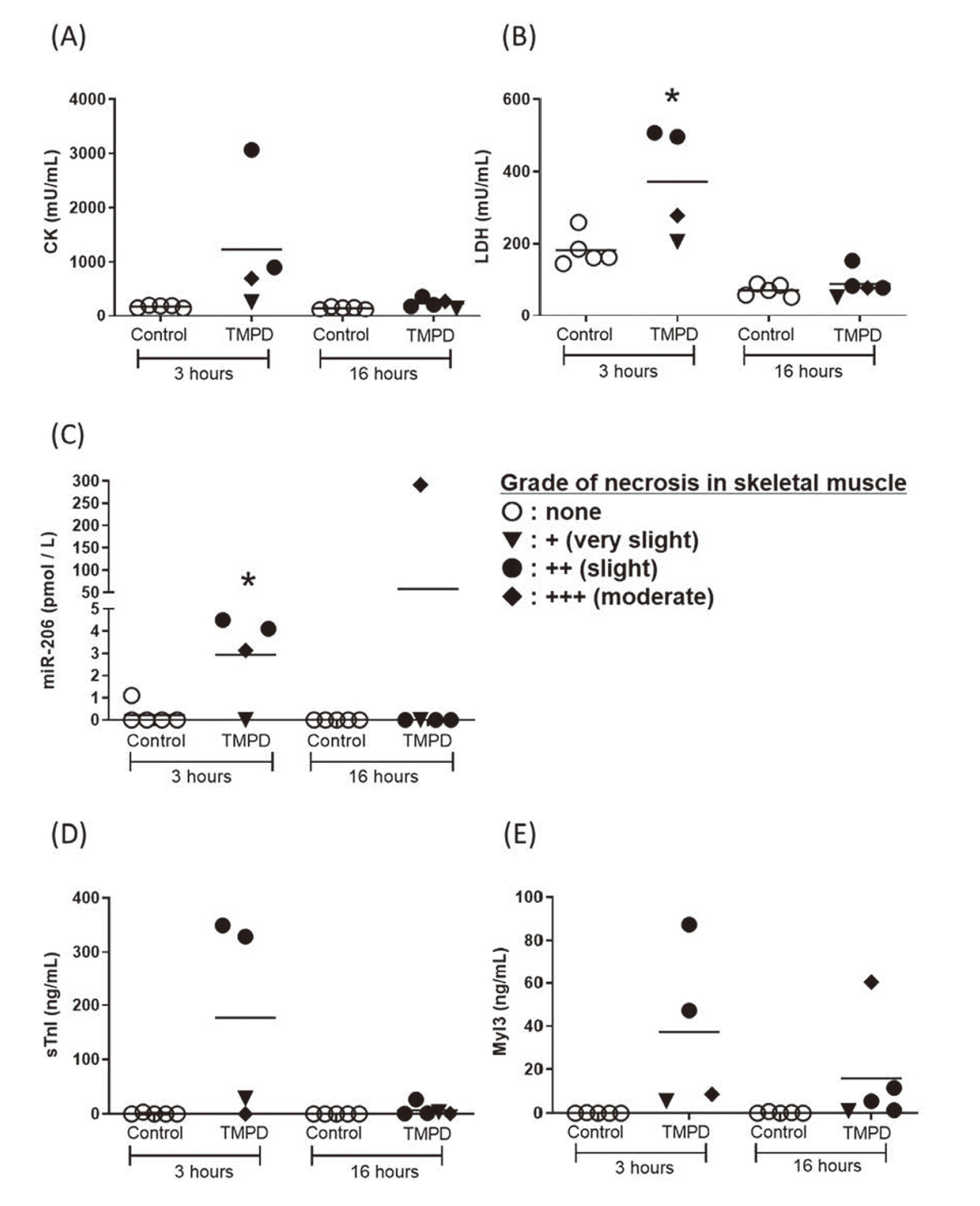
Changes in the levels of circulating CK (A), LDH (B), miR-206 (C), sTnI (D) and Myl3 (E) in TMPD-treated rats. Blood samples were taken 3 hr after the 5th treatment and 16 hr after the final (sixth) treatment. Skeletal muscle necrosis grades: ○, none; ▼, + (very slight); ●, ++ (slight); and ♦, +++ (moderate). *: p < 0.05 compared with the control group.
Next, we measured these five parameters in rats treated with in-house compounds A-D (A-C shown in Fig. 2., and D shown in Fig. 3.). In rats treated with compound A, the levels of test parameters, including miR-206, were unchanged in association with skeletal muscle necrosis. In rats treated with compound B, the levels of miR-206, sTnI and Myl3 were elevated in one rat only, whereas those of CK and LDH were unchanged. In rats treated with compound C, the level of miR-206 tended to be higher in most rats that exhibited skeletal muscle necrosis compared with those of control rats. In contrast, the levels of the other parameters were unchanged in most rats that exhibited skeletal muscle necrosis. The results of rats treated with compound D are shown in Fig. 3, because the strain and sex differed from those of the others. The levels of miR-206, sTnI and Myl3 were higher only in rats with skeletal muscle necrosis, whereas CK and LDH levels were higher in rats without skeletal muscle necrosis. These findings indicate that miR-206 may serve as a marker of skeletal muscle injury.
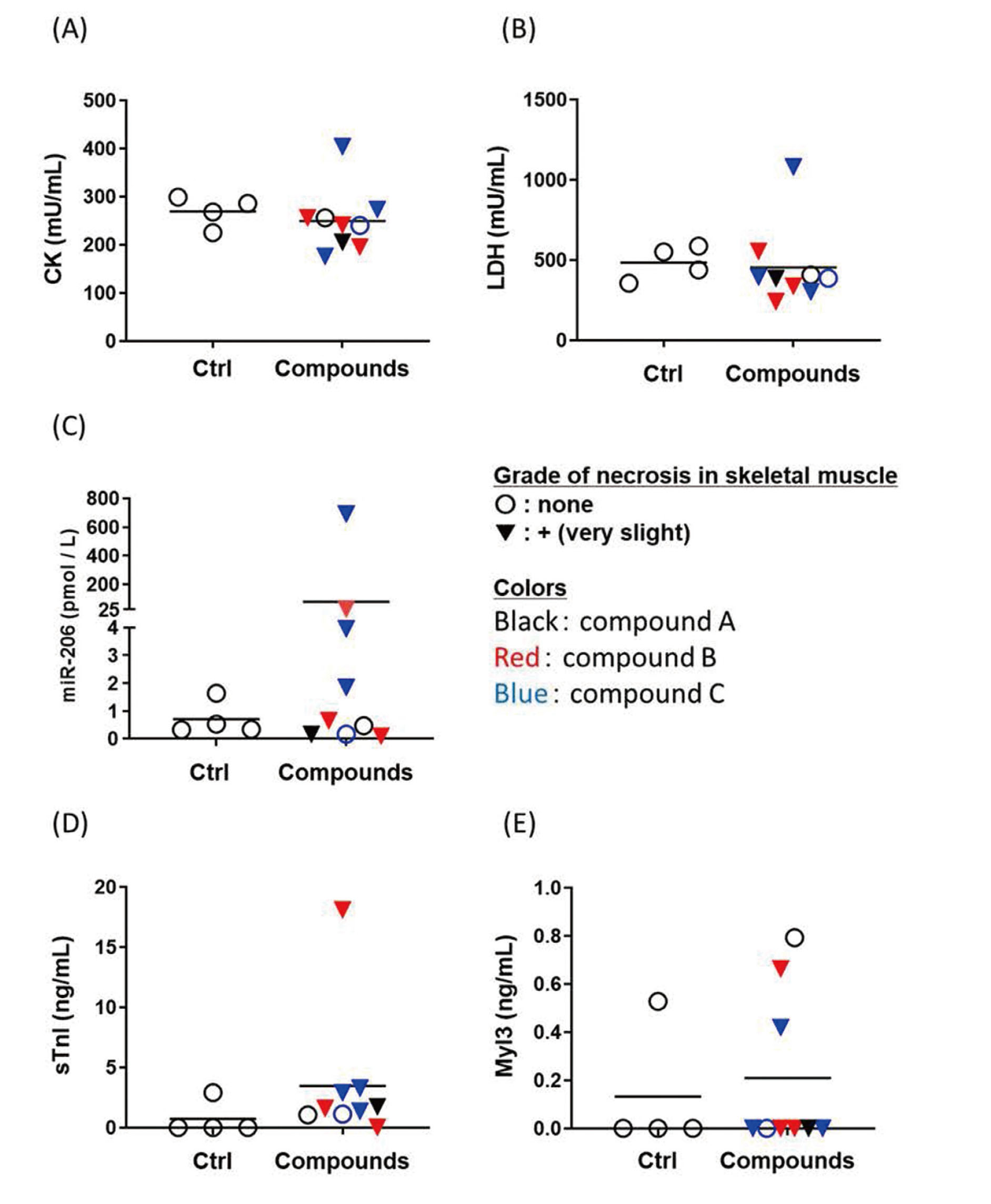
Changes in the levels of circulating CK (A), LDH (B), miR-206 (C), sTnI (D) and Myl3 (E) in rats treated with the in compounds A, B, or C. Blood samples were taken 24 hr after treatment. Skeletal muscle necrosis grades: ○, none; ▼, + (very slight). Black, red, and blue represent rats treated with compounds A, B, or C, respectively.
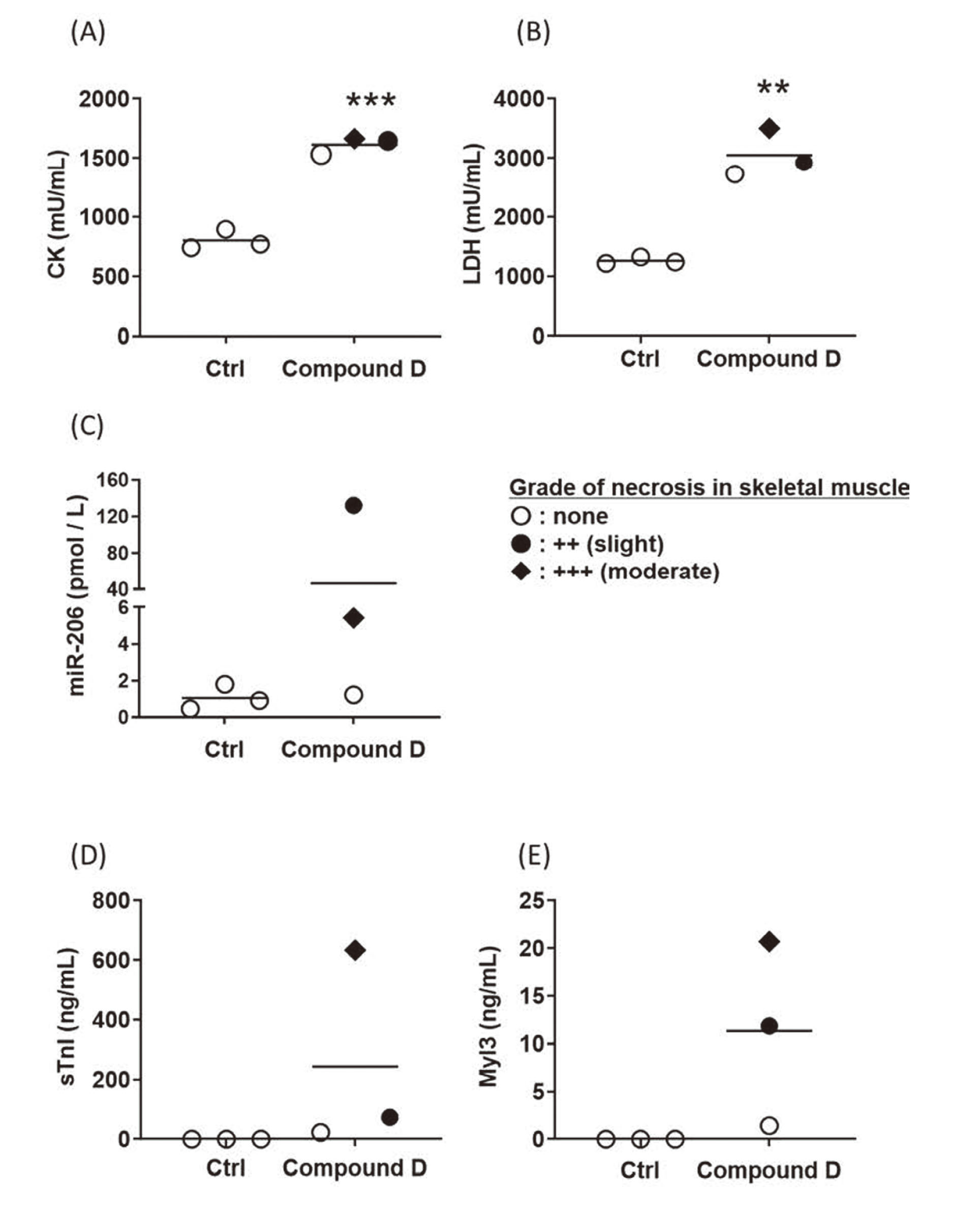
Changes in the levels of circulating CK (A), LDH (B), miR-206 (C), sTnI (D) and Myl3 (E) levels in compound D-treated rats. Skeletal muscle necrosis grades: ○, none; ●, 2+ (slight), +++ (moderate). Blood samples were taken 24 hr after the final treatment. **: p < 0.01, ***: p < 0.001 compared with the time-matched control group.
The sensitivities and specificities of each parameter were calculated using ROC analysis with optimal cut-off values (Table 3). The sensitivity and specificity of miR-206 were 56% and 100%, respectively. The sensitivities and specificities of the other parameters were as follows: CK, 83% and 45%; LDH, 72% and 45%; sTnI, 89% and 75%; Myl3, 78% and 85%. Thus, the specificity of miR-206 was highest, although the sensitivity was inferior to the others. sTnI had the highest area under the curve (AUC) value.
| Parameter | AUC | Cut-off | Sensitivity | Specificity |
|---|---|---|---|---|
| CK | 0.66 | 195 | 83 | 45 |
| LDH | 0.52 | 195 | 72 | 45 |
| miR-206 | 0.71 | 1.83 | 56 | 100 |
| sTnI | 0.85 | 0.41 | 89 | 75 |
| Myl3 | 0.84 | 0.36 | 78 | 85 |
Cut-off values were selected to maximize Youden’s index ([J] = sensitivity + specificity – 1).
We next determined if the serum miR-206 level distinguished skeletal muscle injury from cardiac injury. For this purpose, the levels of serum miR-206 and Myl3, which is also expressed in the heart, were measured in rats with isoproterenol-induced cardiotoxicity. Very slight or slight necrosis of the heart was observed in all rats treated with isoproterenol (n = 10) (Table 4). The levels of Myl3 were significantly high in rats treated with isoproterenol compared to those in control rats. On the other hand, the serum miR-206 level was not increased (Fig. 4), although an individual rat in the control group showed high serum miR-206 level, which was likely due to technical variation such as in blood sampling or restraint stress. In addition, we also measured the levels of serum cardiac troponin I, which is commonly used as a clinical biomarker of cardiac disorders, and serum miR-208, which is specifically expressed in heart. Results showed that these levels were elevated in isoproterenol-treated rats (data not shown). Thus, serum miR-206 level distinguished skeletal muscle injury from cardiac injury.
| Compound | Dose (mg/kg) |
Number of rats | Necrosis |
|---|---|---|---|
| Isoproterenol | 0.1 | 10 | ++(3), +(7) |
Histopathological grade of necrosis: very slight: +, slight: ++.
The numbers in parentheses indicate the number of rats.
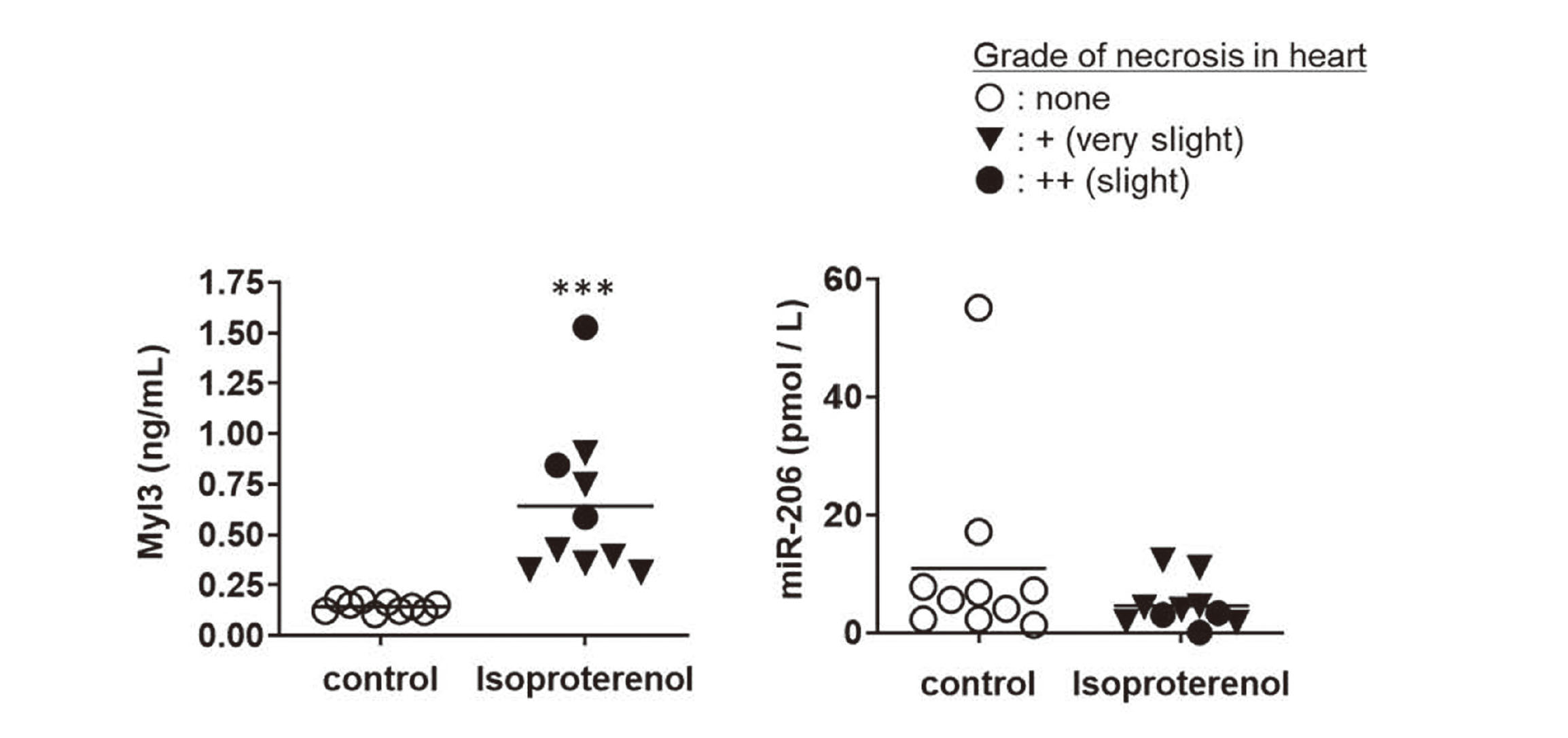
Changes in the levels of miR-206 and Myl3 in isoproterenol-treated rats. Cardiomyocyte necrosis grades: ○, none; ▼, 1+ (very slight); ●, 2+ (slight). ***: p < 0.001 compared with the time-matched control group.
The levels of CK and LDH, which are expressed in skeletal muscle, increased during frequent blood sampling in our previous studies (data not shown). When we investigated whether serum miR-206 levels were similarly increased, we found that the miR-206 level of each rat increased compared with that at the first sampling (Fig. 5). However, the extent was at most 5-fold, and relatively smaller than the increase associated with compound-induced skeletal muscle injury. Thus, serum miR-206 levels can be used as a useful biomarker of skeletal muscle injury.
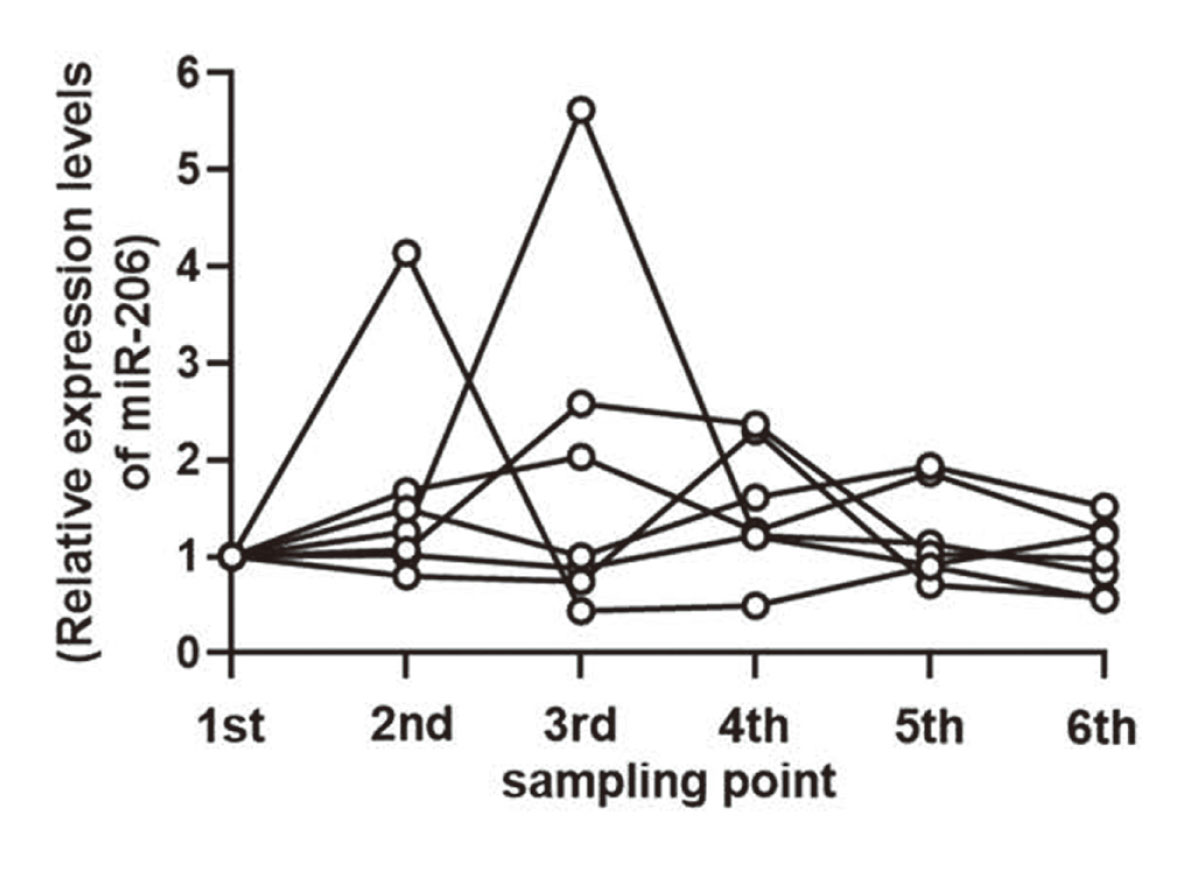
Effects of frequent blood sampling on serum miR-206 levels. Each circle represents the level of miR-206 in an individual rat. Values are relative to that of the first sampling (0.5 hr after vehicle treatment).
CK and LDH serve as biomarkers of myotoxicity of humans and animals. CK is abundant in the heart, intestine and brain as well, and its levels in plasma can be increased by injuries to tissues other than muscle. Similarly, LDH levels cannot be used to differentiate liver, hemocyte, heart, and skeletal muscle toxicities, because it is expressed in these tissues (Frank et al., 1978; Grindem et al., 2017). Evidence indicates the potential of sTnI and Myl3 as markers of muscle disease or injury (Tonomura et al., 2012; Burch et al., 2016). However, these proteins are rapidly cleared from the bloodstream (Tonomura et al., 2012), and Myl3 is abundantly expressed in the heart (Calvano et al., 2016). These reports indicate that these parameters do not detect skeletal muscle injury after a certain period of time from the administration of a chemical, and have the potential to detect non-muscular injuries. Therefore, a biomarker with improved specificity and sensitivity is required. miR-206, which is abundantly expressed in skeletal muscle, plays a critical role in the proliferation and differentiation of skeletal muscle cells (Chen et al., 2010; Dey et al., 2011; Ma et al., 2015). miR-206 has received much research interest as a potential biomarker for skeletal muscle disorders such as muscular dystrophy (Hu et al., 2014), skeletal muscle atrophy (Wang et al., 2017), and amyotrophic lateral sclerosis (Toivonen et al., 2014). Further, the plasma miR-206 level is elevated in rats treated with notexin, a tiger snake venom which induces muscle injury (Siracusa et al., 2016). However, it is uncertain whether circulating miR-206 is a useful biomarker for drug-induced skeletal muscle injury in preclinical toxicity studies of drugs under development compared with the standard markers CK, LDH, sTnI and Myl3. Therefore, we performed this study to address this question.
Here we show that the serum miR-206 levels in rats with TMPD-induced skeletal muscle injury were higher than those in control rats, and tended to be increased in rats with skeletal muscle injuries treated with in-house compounds B, C, or D, regardless of rat strain. Thus, the present study demonstrates that serum miR-206 levels were elevated not only in rats with severe necrosis of skeletal muscle but also in those with slight necrosis. Since miR-206 is abundant in skeletal muscle, the increase in serum may be explained by its release into the bloodstream from damaged skeletal muscle.
When we next compared the changes in miR-206 levels with those of CK, LDH, sTnI and Myl3, we found that the miR-206 and Myl3 levels remained high 16 hr after TMPD treatment, although only in one rat with moderate necrosis. Further, the CK, LDH, and sTnI levels in TMPD-treated rats were almost the same as those of control rats (Fig. 1), suggesting that miR-206 levels changed with skeletal muscle injury even after a certain period of time from the administration. Moreover, in rats treated with in-house compounds A–D, the levels of CK, LDH, sTnI and Myl3 were unchanged in most rats with skeletal muscle injury, despite the increasing tendency in miR-206 levels (Fig. 2). Therefore, miR-206 sensitively detected skeletal muscle injury. Moreover, miR-206, sTnI and Myl3 levels were higher only in rats with skeletal muscle necrosis, whereas CK and LDH levels were higher even in rats without skeletal muscle necrosis (Fig. 3).
ROC analysis indicated that the specificity of miR-206 was highest among the markers analyzed (Table 3) and that miR-206 levels did not change because of cardiotoxicity (Fig. 4). Thus, our data demonstrate that miR-206 detected skeletal muscle injury more specifically than the other markers. In contrast, sTnI achieved the highest accuracy (AUC = 0.85). As mentioned above, the level of sTnI did not detect skeletal muscle injury depending on when the sample was acquired, suggesting that measuring sTnI combined with miR-206 levels may help detect skeletal muscle injury, because sTnI was not a suitable biomarker of skeletal muscle injury.
The sequences of most miRNA are highly conserved across species (Ha et al., 2008). The sequence of miR-206 is the same in humans, dogs, rats and mice (miRBase version 22.1). Additionally, miR-206 is expressed in skeletal muscle in humans (McCarthy, 2008), dogs (Koenig et al., 2016) and mice (McCarthy, 2008). Therefore, serum miR-206 could detect drug-induced skeletal muscle injury across species. Further investigations will be needed to clarify these possibilities.
In preclinical toxicity studies, blood samples are collected by frequent sampling for toxicokinetics analysis, and are sometimes used for the measurement of biomarkers. In our experience, the levels of circulating biomarkers of skeletal muscle injury, such as CK and LDH, are increased by frequent blood sampling. Similarly, we found that miR-206 levels increased as much as 5-fold, which may be explained by its release into the bloodstream caused by repeated puncturing or the stress caused by restraining the rats. However, muscle toxicants caused at least an 8-fold increase under the same conditions. Thus, miR-206 may be used as a biomarker for drug-induced skeletal muscle injury, independent of the frequency of blood sampling.
While this paper was being prepared, Bailey et al. (2019) reported that several plasma miRNAs such as miR-1, miR-133a, miR-133b and miR-206 would serve as biomarkers for drug-induced skeletal muscle injury with improved specificity and sensitivity compared to the conventional markers. Additionally, it has been reported that serum miR-133a and miR-133b are sensitive markers of cardiac and skeletal muscle toxicities (Calvano et al., 2016). To distinguish between them, monitoring the serum level of miR-208, which is specifically and abundantly expressed in the heart, in combination with miR-133a and miR-133b, is desirable. Since the purpose of this study was to establish a biomarker for skeletal muscle injury, we evaluated miR-206 among the muscle-specific miRNAs such as miR-1, miR-133a and miR-133b. Our results demonstrated that the serum miR-206 level was elevated in association with skeletal muscle injury, but not with cardiac toxicity. Therefore, miR-206 may be more useful as a biomarker than miR-133a and miR-133b, considering the desirability of monitoring a single miRNA only.
In conclusion, we provide compelling evidence that in rats, miR-206 is a more specific biomarker of drug-induced skeletal muscle injury than the others tested here. miR-206 may therefore serve as a specific biomarker of drug-induced skeletal muscle injury, and hence be useful as an additional biomarker of skeletal muscle injury in preclinical toxicity studies using rats.
The authors thank Dr. Mihoko Ono for technical assistance and preparing the manuscript, and Dr. Keisuke Nagata and Atsushi Yamada for technical assistance.
Conflict of interestThe authors declare that there is no conflict of interest.