2022 年 47 巻 10 号 p. 409-420
2022 年 47 巻 10 号 p. 409-420
Certain polyphenols exhibit low permeability; precise prediction of their intestinal absorption is important for understanding internal exposure in humans. Intestinal availability, which represents the fraction of administered compounds that reach the portal blood (FaFg), is calculated by dividing bioavailability (F) by hepatic availability (Fh), and F is obtained from pharmacokinetic data from both intravenous (i.v.) and oral (p.o.) administration. However, human FaFg of polyphenols is hardly reported, as human i.v. data are extremely scarce. In this study, we developed an estimation method for FaFg of polyphenols in humans based on the extrapolation of rat clearance using allometric scaling (allometric scaling-based FaFg calculation method, AS- FaFgCM). First, for quercetin, for which human i.v. data have been reported, we compared the FaFg obtained by AS-FaFgCM with the traditional approach using human i.v. and p.o. data. Less than two-fold difference in FaFg values was observed between the two approaches. Next, we obtained FaFg of structurally diverse polyphenols (genistein, baicalein, resveratrol, and epicatechin) using AS-FaFgCM, demonstrating that all of them were poorly absorbable. Furthermore, to utilize the pharmacokinetic data of the total concentration, including aglycones and metabolites, we modified the AS-FaFgCM to focus on their excretion. The FaFg value of naringenin was obtained using modified AS-FaFgCM and was nearly equal to that of baicalein, a structural isomer of naringenin. This study provides quantitative information on the intestinal absorption of polyphenols using comprehensive estimation methods.
Polyphenols are naturally occurring organic compounds present abundantly in plants, including fruits, vegetables, and herbs. They comprise a wide family of molecules containing one or more phenolic rings. Accumulating evidence has highlighted the beneficial effects of polyphenols on human health, including their antioxidant, anti-obesity, anti-inflammatory, and anti-carcinogenic properties (Joseph et al., 2016; Singh et al., 2020; Bonfili et al., 2008). Recently, growing awareness regarding the importance of health and wellness among individuals has compelled the interest in applications of polyphenols in functional foods and dietary supplements (de Araújo et al., 2021). Despite their beneficial effects, certain polyphenols at excessive dosage have been found to cause side effects, including hepatotoxicity (Scalbert et al., 2005; Mennen et al., 2005; Seeff et al., 2015). Therefore, extensive research is required to examine their internal exposure to determine the optimal dosage for effective and safe use.
The concentration and duration of bioactive compounds in the circulation and tissues depend on their absorption, distribution, metabolism, and excretion (ADME) (Selick et al., 2002). Generally, phase II metabolic enzymes and transporters are considered crucial for the ADME of polyphenols (Bohn, 2014; Yang et al., 2008). Numerous pharmacokinetic studies have revealed low plasma concentrations of polyphenol aglycones following oral ingestion in humans and animals, although their metabolites have been well observed in the plasma (Manach et al., 2005; Scalbert et al., 2002). Recent studies have made great progress in improving our understanding of polyphenol behaviors related to ADME. One of the major factors contributing to their poor oral bioavailability is their low intestinal absorption due to low solubility, low permeability, efflux transport, and extensive gut metabolism (Truzzi et al., 2021). Therefore, the intestinal absorption of certain polyphenols could be a key rate-limiting process in polyphenol kinetics. However, the quantitative evaluation of polyphenol absorption in humans remains to be carried out because of the analytical difficulties in the human gastrointestinal lumen and portal vein blood, as well as ethical issues.
To quantitatively determine the contribution of intestinal absorption to absolute bioavailability (F), fractions of the administered compounds that reached the portal blood (FaFg) were evaluated. In general, FaFg is calculated by dividing F by hepatic availability (Fh), and F is obtained from pharmacokinetic data of both intravenous (i.v.) and oral (p.o.) administration (Kato, 2008; Shibata et al., 2003). Human i.v. drug data are well obtained in the process of drug application and are based on the balance of risks and benefits. However, human i.v. studies of polyphenols are rarely implemented if they are not for medical use (Hu et al., 2017), creating a situation in which the FaFg values of polyphenols are currently scarcely available.
To predict intestinal absorption, pharmacokinetic models like Advanced Compartmental Absorption and Transit model (Agoram et al., 2001), QGut model (Gertz et al., 2010), and translocation model (Ando et al., 2015) were used. These sophisticated models have the potential to precisely predict the intestinal absorption of drugs; however, multiple scaling factors such as kinetic parameters of metabolic enzymes or transporters (Ando et al., 2015) are required to address the gaps between in vitro and in vivo data. Considering that the absorption mechanism of polyphenols is not well elucidated as that of drugs, it is practically difficult to apply these pharmacokinetic models for estimating the FaFg of polyphenols.
Over the years, interspecies allometric scaling methods have been developed to predict pharmacokinetic parameters (Dedrick, 1973). These equations relate body weight to specific pharmacokinetic parameters, which were originally derived from empirical observations relating to anatomical, physiological, and biochemical similarities across species (Choi et al., 2019). A simple allometric equation can predict the human total clearance (CLtot) of drugs from data obtained in rats (Chiou et al., 1998). This study exhibited good correlation coefficient between humans and rats using 54 extensively metabolized drugs (Chiou et al., 1998), whose pharmacokinetic features were similar to those of polyphenols (Di Lorenzo et al., 2021). One of the pharmacokinetic parameters used to calculate F is CLtot, which is generally calculated from i.v. data. Together, it was expected that human FaFg of polyphenols could be predicted without human i.v. data using the F value calculated from human CLtot estimated by allometric scaling.
In this study, we propose a practical method to estimate the FaFg of polyphenols by extrapolating rat i.v. data to human pharmacokinetic parameters (allometric scaling-based FaFg calculation methods, AS-FaFgCM). Using quercetin, for which human i.v. data are reported, we confirmed the validity of AS-FaFgCM by comparing the FaFg values obtained by AS-FaFgCM with the traditional approach using human i.v. data. Moreover, to utilize a broader range of existing data on polyphenols, we next focused on the pharmacokinetic data of the total concentration, including both aglycons and their metabolites. We further modified AS-FaFgCM by focusing on its excretion (allometric scaling-based FaFg calculation method focusing on excretion, AS-FaFgCME). Collectively, this study provides promising information on the intestinal absorption of polyphenols using comprehensive calculation methods that can be implemented without human i.v. data.
AS-FaFgCM and AS-FaFgCME were used to estimate human FaFg using rat i.v. and human p.o. data, respectively (Fig. 1). AS-FaFgCM focused on the metabolism and excretion kinetics of polyphenol aglycones (Fig. 2A). In contrast, AS-FaFgCME focused on the excretion kinetics of polyphenol aglycone and its metabolites, which are mainly applied to the total blood concentration data, including both aglycone and its metabolites (Fig. 2B). AS-FaFgCME assumed that all metabolites produced in the enterocytes were excreted into the lumen and were not absorbed into the portal vein. This assumption could be applied to baicalein and its structural analogues, based on the following findings:

Procedure for human FaFg estimation using AS-FaFgCM and AS-FaFgCME. A diagram describing the procedure of AS-FaFgCM for estimating human FaFg. (1) Rat i.v. data were collected, and total rat clearance from the i.v. study (rCLtot,iv) was estimated using Eqs. 1 and 7 for AS-FaFgCM and AS-FaFgCME, respectively. (2) The human total clearance (hCLtot,iv) was extrapolated using the allometric approach in Eqs. 2 and 8 for AS-FaFgCM and AS-FaFgCME, respectively. (3) Human p.o. data were collected, and human F was estimated by Eqs. 3 for AS-FaFgCM and human F' was Eq. 9 for AS-FaFgCME. (4) Human FaFg was estimated from F and Fh using Eqs. 4 and 5 for AS-FaFgCM and from F' and Fh' using Eqs. 10 and 11 for AS-FaFgCME.

Schematic diagram of the compartment models for AS-FaFgCM and AS-FaFgCME. The compartment models used for AS-FaFgCM (A) and AS-FaFgCME (B) are shown. (A) AS-FaFgCM focuses on the absorption, distribution, metabolism, and excretion (ADME) of aglycone. CLh, CLr, and clearance of aglycone (CLg) reflect hepatic metabolism of aglycone, renal excretion of aglycone, and intestinal efflux transport and metabolism of aglycone, respectively. CLh and CLr were extrapolated from the rat data using an allometric approach. FaFg and Fh are the fractions of the administered compounds that reach the portal blood and the hepatic bioavailability of aglycone, respectively. (B) AS-FaFgCME focuses on the ADME of both aglycone and its metabolites; a single quotation (') of CLh', CLr', and CLg' indicates the clearances of both aglycone and its metabolites. CLh' and CLr' were extrapolated from rat data using an allometric approach. CLg' is based on the assumption that all intestinal metabolites are excreted into the gut lumen by efflux transporters. Fh' refers to hepatic bioavailability, focusing on the bile excretion of aglycone and its metabolites.
i) Almost all the glucuronated baicalein was excreted into the intestinal lumen by the intestinal mucosal cells through multidrug resistance associated protein 2 (MRP2) (Akao et al., 2004).
ii) Baicalein metabolites appeared only in the apical side, when baicalein was applied to the Caco-2 cell monolayer model (Zhang et al., 2007).
In this study, AS-FaFgCME was applied to naringenin, one of the structural analogues of baicalein.
Collection of the rat intravenous dataThe pharmacokinetic data in which rats received i.v. polyphenol were collected by searching PubMed and Google Scholar for relevant publications.
Using data from previous studies, in which aglycone was administered and the blood concentration of the unchanged form was analyzed, were used for AS-FaFgCM. The data were excluded if the reliability of their plasma concentration-time profile was insufficient (e.g., blood sampling was performed for less than 30 min, stabilization of the sample was not performed).
The data from this study, in which aglycone was administered and the total blood concentration of aglycone and its metabolites were analyzed, were collected for AS-FaFgCME as the following examples:
i. The blood samples were deconjugated and the aglycone concentration was quantified.
ii. Blood concentrations of aglycone and its metabolites were quantified using reference standards.
iii. The total blood concentration was examined using radio isotope-labelled compound.
Collection of the human oral dataPharmacokinetic data in which polyphenols were orally administered to humans were collected by searching PubMed and Google Scholar for relevant publications.
The human p.o. reference data used in this study were obtained from studies in which aglycone was administered and the blood concentration of the unchanged form were analyzed. For FaFg calculation, the data were excluded from these studies if any of the following criteria were met: (i) repeated dose study, (ii) controlled absorption using technologies such as drug delivery systems, (iii) the chemical structure was changed before absorption (e.g., gut microbial metabolism and natural degradation), and (iv) the reliability of their plasma concentration-time profile was not sufficient (e.g., blood sampling was performed for less than 30 min, stabilization of the sample was not performed).
The procedure of AS-FaFgCMAS-FaFgCM is the method to obtain human FaFg using the following procedure.
1. Calculation of rat total clearance (rCLtot,iv)
2. Estimation of human total clearance (hCLtot,iv) by allometric scaling
3. Estimation of human F
4. Estimation of human FaFg
The detailed procedure is described below.
1. Calculation of rCLtot,ivThe RCLtot,iv of polyphenols was estimated using the following equation:
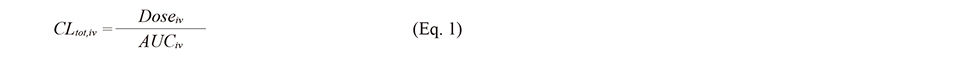
where CLtot,iv, Doseiv, AUCiv, are total clearance obtained using i.v. data, dose of an i.v. study, AUC of blood concentration versus time after dosage in an i.v. study, respectively. CLtot,iv of polyphenols was calculated based on the “blood concentration”. The blood-to-plasma ratios (Rb) of quercetin and resveratrol were 0.68 and 0.78, respectively (Furukawa et al., 2012); the plasma concentration was converted to the blood concentration by multiplying Rb value. For the other compounds with unknown Rb, Rb was assumed to be 1. In cases where i.v. data (e.g., dose, area under the curve (AUC), and body weight) of a specific polyphenol were available in several reports, CLtot,iv was estimated from each report, and the average value was used for the estimation of human clearance. In cases where several doses were examined in a report, the data of the minimum dose were adopted, considering the solubility, saturation of enzyme, transporter kinetics, and low intestinal absorption of polyphenols.
2. Estimation of hCLtot,iv, by allometric scalingExtrapolation of rat clearance to humans was performed by the extrapolation of clearance based on the relationship between pharmacokinetics and body weight of the animal.
The extrapolation of rCLtot,iv to hCLtot,iv was performed using the following allometric equation (Chiou et al., 1998):
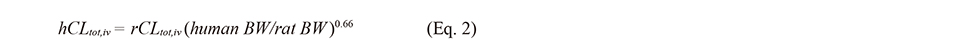
where 0.66 is the allometric exponent and BW is body weight.
3. Estimation of human FThe human F value was obtained from estimated hCLtot,iv, and human p.o. data. F was estimated from the following equation:

where F, hCLtot,iv, AUCpo and Dosepo are absolute bioavailability, human total clearance obtained from i.v. data, AUC of blood concentration versus time after dosage in a p.o. study, and dose of a p.o. study, respectively. In cases where p.o. data (e.g., dose, AUC, and body weight) in several reports were available, human F was estimated from each report, and the average value was used. If several doses were examined in a previous study (Barnett et al., 2015; Boocock et al., 2007; Li et al., 2014), the data of the minimum dose were adopted, considering the solubility and saturation of enzyme and transporter kinetics. In studies where only the dose per body weight was described, the dose was calculated using the average body weight of the participants. In the instances where body weight data were not available in the study, the average body weight in the area where the study was performed (Walpole et al., 2012) was used for calculation.
4. Estimation of human FaFgThe FaFg value was estimated using the following equations (Kato, 2008):

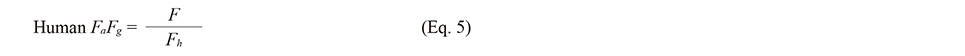
where Fh, FaFg, hCLh, and hQh are hepatic bioavailability, fraction of the administered compounds that reach the portal blood, human hepatic clearance, and human hepatic blood flow rate, respectively.
The value of hQh (96.6 L/h) was reported earlier (Kato et al., 2008). hCLh was calculated as follows:
i. The contribution of human renal clearance (hCLr) is less than 5% of hCLtot,iv: hCLh = ℎCLtot,iv
ii. hCLr is more than 5% of hCLtot,iv: hCLh = hCLtot, iv − hCLr
In cases where the value of hCLr was available (resveratrol (Boocock et al., 2007) and quercetin (Moon et al., 2008), the values were used for the calculation. In contrast, in cases where only the urinary excretion data in a human p.o. study were available without hCLr values (baicalein (Li et al., 2014)), hCLr was calculated using the below equation:
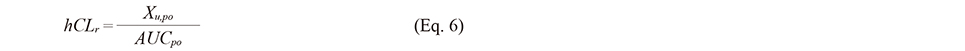
where Xu,po is the total amount of excretion in urine. In cases where only renal clearance data in rats (rCLr) were available (epicatechin (Yu, 2000)), the hCLr value was estimated using the allometric equation (Eq. 2). In cases where both renal clearance data in rats and humans (genistein) were not available, hCLtot, iv was regarded as ℎCLh.
Traditional approach to estimate FaFg using human intravenous data for quercetinThe F value was estimated using Eq. 3, using the reported value of hCLtot,iv in human i.v. studies. Human Fh and FaFg were estimated using Eqs. 4 and 5, considering the contribution of the renal clearance.
The procedure of AS-FaFgCMEAS-FaFgCME is the method to obtain human FaFg using the following procedure.
1. Estimation of rat CLtot,iv'
2. Estimation of human CLtot,iv' by allometric scaling
3. Estimation of human F'
4. Estimation of human FaFg
The detailed procedure is described below.
1. Estimation of rat CLtot,iv'The rCLtot,iv' of polyphenols was estimated using the following equation:

where the single quotation (') represents the value focusing on the excretion of both aglycone and its metabolites, and AUCiv, agri+metab is AUC of total blood concentration of aglycone and its metabolites versus time after dosage in an i.v. study.
2. Estimation of hCLtot,iv' by allometric scalingThe extrapolation of rCLtot,iv' to hCLtot,iv' was performed using the following allometric equation:

The human absolute bioavailability, focusing on the excretion of aglycone and its metabolites (F'), was obtained by the equation below:

The FaFg value was estimated using the following equations:


where a single quotation represents the values focusing on the excretion of both aglycone and its metabolites. The value of Qh (96.6 L/hr) was used as previously reported (Kato et al., 2008). hCLh' was calculated using the equation below:

hCLr' was estimated by the equation below:

where the single quotation represents the value focusing on the excretion of both aglycone and its metabolites, and Xu,po,agri+metab is the total amount of urinary excretion of aglycone and its metabolites.
Calculation of F, Fh, and FaFg in ratsIf the data for oral administration in rats was available, F, Fh, and FaFg in rats were calculated, combined with the rat i.v. data. Collection of the rat oral data was performed in the same way described in Collection of the human oral data.
Rat F was calculated using the below equation:

where F, rCLtot,iv, AUCpo, and Dosepo are absolute bioavailability, rat total clearance obtained from i.v. data, AUC of blood concentration versus time after dosage in a p.o. study, and dose of a p.o. study, respectively.
If several doses were examined in a previous study (Zhou et al., 2008), the data of the minimum dose were adopted, considering the solubility and saturation of enzyme and transporter kinetics.
Rat Fh and FaFg were calculated using the below equations:


where Fℎ, FaFg, rCLh, and rQh are hepatic bioavailability, fraction of the administered compounds that reach the portal blood, rat hepatic clearance and rat hepatic blood flow rate, respectively.
The value of rQh (0.853 L/hr) was described previously (Kato et al., 2008). rCLh was estimated as follows:
i. The contribution of rCLr is less than 5% of rCLtot,iv: rCLh = rCLtot, iv
ii. The rCLr is more than 5% of rCLtot,iv: rCLℎ = rCLtot, iv − rCLr
In cases where the value of rCLr was available (epicatechin (Yu, 2000)), the reported values were used for the calculation. In cases where the renal clearance data in rats (genistein) was not available, rCLtot,iv was regarded as rCLh.
To check the validity of AS-FaFgCM, the estimated values of FaFg for quercetin were compared between AS- FaFgCM and the traditional approaches using human i.v. data. The values of hCLtot,iv, human F, Fh, and FaFg estimated by AS-FaFgCM or the traditional approach are shown in Table 1. The values of hCLtot,iv, human F, and Fh in AS-FaFgCM were within 2-fold of those of the traditional approach. Also, The FaFg value estimated by AS-FaFgCM was 1.2- and 2.0-fold lower than that estimated by the traditional approach with two reference datasets, respectively. These results suggest that AS-FaFgCM could estimate pharmacokinetic parameters, such as FaFg, comparable to the traditional approach.
| AS-FaFgCM | Traditional approach | ||
|---|---|---|---|
| rCLtot,iv (L/hr) | 1.57 | - | - |
| Rat BW (kg) | 0.263 | - | - |
| hCLtot,iv (L/hr) | 59.12*1 | 34.3*2 | 46.7*3 |
| Human dosepo (mg) | 500*4 | ||
| Human AUCpo (mg*hr/L)† | 0.0541*4 | ||
| Human BW (kg) | 64.1*4 | - | - |
| Human F | 0.0062 | 0.00545 | 0.00744 |
| hCLr (L/hr) | 1.68*5 | ||
| hCLh (L/hr)†† | 59.12 | 34.3 | 46.7 |
| hQh (L/hr) | 96.6*6 | ||
| Human Fh | 0.388 | 0.645 | 0.516 |
| Human FaFg | 0.0165 | 0.00845 | 0.0144 |
The rCLtot,iv was estimated using the rat doseiv, AUCiv, and BW from three rat i.v. studies (Chang et al., 2015; Khaled et al., 2003; Yang et al., 2005). Rat BW was the mean value of three rat i.v. studies. *1: Estimated from “average rCLtot,iv” with the average human BW in Europe (Walpole et al., 2012); *2*3: values from human i.v. studies (*2: (Ferry et al., 1996); *3: (Gugler et al., 1975)); *4: (Riva et al., 2019); *5: (Moon et al., 2008); *6: (Kato et al., 2008); †: The plasma concentration was converted to the blood concentration by multiplying Rb value of 0.68 (Furukawa et al., 2012); ††: The value of hCLtot,iv was directly applied to the hCLh because hCLr was less than 5% of hCLtot, iv.
Next, we estimated the FaFg values of genistein, epicatechin, baicalein, and resveratrol using AS-FaFgCM. The values used for AS-FaFgCM and the estimated values for the four polyphenols are shown in Table 2. The estimated F and FaFg values of those polyphenols were less than 0.05 and 0.15, respectively. The FaFg value of epicatechin was lower than those of the other three polyphenols. These results suggest that these four polyphenols had low bioavailability and intestinal absorption, and epicatechin had the most incomplete intestinal absorption.
| Genistein | Epicatechin | Baicalein | Resveratrol | |
|---|---|---|---|---|
| rCLtot,iv (L/hr)† | 0.552 | 0.485 | 2.54 | 2.27 |
| hCLtot,iv (L/hr) | 21.9 | 25.9 | 79.2 | 78.5 |
| Human dosepo (mg) | 30.0*1 | 50.0*2 | 100*4 | 500*5 |
| 400*6 | ||||
| 500*7 | ||||
| Human AUCpo (mg∙hr/L)† | 0.0397*1 | 0.0199*2 | 0.0250*4 | 0.182*5 |
| 0.092*6 | ||||
| 0.121*7 | ||||
| Human BW (kg) | 62.7*1 | 80.7*2,3 | 62.2*4 | 75.8*3,5 |
| 70.8*3,6 | ||||
| 69.1*7 | ||||
| Human F | 0.029 | 0.0103 | 0.0198 | 0.0220 |
| hCLr (L/hr) | N.A. | 3.6*8 | 0.01*4 | 0.86*5 |
| hCLh (L/hr)†† | 21.9 | 22.3 | 79.2 | 78.5 |
| hQh (L/hr) | 96.6*9 | |||
| Human Fh | 0.773 | 0.769 | 0.180 | 0.19 |
| Human FaFg | 0.0375 | 0.0134 | 0.110 | 0.116 |
The rCLtot,iv was estimated using the rat doseiv, AUCiv, and BW from the following rat i.v. studies: genistein (Zhou et al., 2008; Coldham et al., 2002), epicatechin (Yu, 2000), baicalein (Lai et al., 2003), and resveratrol (Kapetanovic et al., 2011; Peñalva et al., 2018; Marier et al., 2002). *1: (Metzner et al., 2009); *2: (Barnett et al., 2015); *3: (Walpole et al., 2012); *4: (Li et al., 2014); *5: (Boocock et al., 2007); *6: (Vaz-da-Silva et al., 2008); *7: (Sergides et al., 2016). The average BW in Europe was used in (Vaz-da-Silva et al., 2008); that in North America was used in (Barnett et al., 2015); the average value of the average European BW and the average North America BW was used in (Boocock et al., 2007); and the average BW in each region was obtained from (Walpole et al., 2012). *8: the values was estimated from rat CLr reported in (Yu, 2000). *9: (Kato et al., 2008). †: In resveratrol, the plasma concentration was converted to the blood concentration by multiplying Rb value of 0.78 (Furukawa et al., 2012) ††: The value of hCLtot,iv was applied to the hCLh because hCLr was unavailable (baicalein) or less than 5% of hCLtot, iv (baicalein and resveratrol). N.A.: Not available
The FaFg value of naringenin was estimated by AS-FaFgCME using rat i.v. and human p.o. data of deconjugated samples by β-glucuronidase and sulfatase. The data used for the estimation and estimated values of naringenin are shown in Table 3. The estimated values of F', Fh', and FaFg were 0.134, 0.989, and 0.136, respectively. The FaFg value of naringenin was comparable to that of baicalein (0.11, Table 2), a structural isomer of naringenin. These results demonstrate the low intestinal bioavailability of naringenin.
| rCLtot,iv' (L/hr) | 0.0559 |
|---|---|
| Human BW (kg)*1 | 69.2 |
| Human dose (mg)*1 | 135 |
| Human AUCagri+metab (mg·hr/L)*1 | 9.42 |
| Human REagri+metab (%)*1 | 5.81 |
| hCLtot,iv' (L/hr) | 1.92 |
| Human F' | 0.134 |
| hCLr' (L/hr) | 0.832 |
| hCLh' (L/hr) | 1.09 |
| Human Fh' | 0.989 |
| Human FaFg | 0.136 |
The estimated values (rCLtot,iv', hCLtot,iv', hCLr', F', Fh', and FaFg) and the values from the human p.o. study (human dosepo, human AUCpo', human BW, and human renal excretion of aglycon and its metabolites (REagri+metab)) are shown. rCLtot,iv' was estimated using the rat doseiv, AUCagri+metab, and BW from the rat i.v. study (Wang et al., 2006). *1: (Kanaze et al., 2007).
The rat F, Fh, and FaFg values were compared with human values estimated for genistein and epicatechin, which have reliable rat p.o. data. The data used for the calculation and the calculated values are shown in Table 4. The rat F values were 0.20 and 0.102 for genistein, and 0.07 for epicatechin (Table 4), which were over 3-fold higher than that in humans (0.029 for genistein and 0.0103 for epicatechin, Table 2). In addition, rat FaFg values for genistein (0.57 and 0.29) and epicatechin (0.13) in Table 4 were approximately 10-fold higher than those in humans (0.0375 for genistein and 0.0134 for epicatechin in Table 2). However, rat Fh values for genistein (0.35) and epicatechin (0.51) in Table 4 were comparable to those in humans (0.773 for genistein and 0.769 for catechin in Table 2). These results suggested large species differences in intestinal availability.
| Genistein | Epicatechin | ||
|---|---|---|---|
| rCLtot,iv (L/hr) | 0.552 | 0.485 | |
| Rat dosepo (mg) | 1.25*1 | 1*2 | 13.7*3 |
| Rat AUCpo (mg∙hr/L) | 0.452*1 | 0.205*2 | 1.88*3 |
| Rat F | 0.20 | 0.102 | 0.07 |
| rCLr,iv (L/hr) | N.A. | 0.0709*4 | |
| rCLh (L/hr) | 0.552*5 | 0.414 | |
| rQh (L/hr) | 0.853*4 | ||
| Rat Fh | 0.35 | 0.51 | |
| Rat FaFg | 0.57 | 0.29 | 0.13 |
The rCLtot,iv was calculated using the rat doseiv, AUCiv, and BW from the following rat i.v. studies: genistein (Zhou et al., 2008; Coldham et al., 2002), epicatechin (Yu, 2000) *1: (Zhou et al., 2008); *2: (Coldham et al., 2002); *3: (Baba et al., 2001); *4: (Yu, 2000); *5: The value of rCLtot,iv was applied to the rCLh because rCLr was unavailable. N.A.: Not available
In this study, the validity of AS-FaFgCM was examined of quercetin, for which human i.v. data were reported in two studies. The results showed that FaFg of AS-FaFgCM (0.0165) was approximately 2.0 or 1.2 times of FaFg obtained by the traditional approach (0.00845 and 0.0144, respectively) (Table 1). Previously, it has been mentioned that inter-individual variability of bioavailability would occur up to four times, especially when the mean bioavailability is low (e.g., 10%) (Toutain and Bousquet-Mélou, 2004). As shown in Table 1, quercetin had a low bioavailability (approximately 1%), suggesting that the difference in FaFg between AS-FaFgCM and the traditional approach could be within the range of variation. Indeed, there was a 1.7-fold difference in the FaFg values between the two i.v. studies obtained using the traditional approach (Table 1). Thus, AS-FaFgCM can be considered to estimate values that are practically comparable to those of the traditional approach.
Both methods showed extremely low intestinal absorption of quercetin (FaFg = 0.00845 to 0.0165 in Table 1). Fh values of quercetin (0.388 to 0.645) were more than 10-times higher than FaFg values, suggesting that the contribution of hepatic metabolism on bioavailability of quercetin was not dominant. When rat intestine was perfused in situ with quercetin, 90% of the formed quercetin conjugates existed in the intestinal eluent and only 10% of the quercetin conjugates were recorded in the bile (Crespy et al., 2003). In addition, in vitro studies using human intestinal Caco-2 cells showed rapid intestinal conjugation and MRP2 mediated-efflux transport of quercetin (Nait Chabane et al., 2009; Ikeno et al., 1999). These data suggested that quercetin was extensively metabolized and excreted in the luminal side of small intestine, which is consistent with the results obtained with AS-FaFgCM. Therefore, AS-FaFgCM is applicable to the FaFg estimation of polyphenols.
We also determined the F and FaFg values for genistein, epicatechin, baicalein, and resveratrol using AS-FaFgCM (Table 2). The results showed that the F values were less than 0.05. It has been reported that the plasma AUC ratios of aglycone to its metabolites are approximately 1.3, 4,3, 3.1, and 1.0% for epicatechin, baicalein, resveratrol, and genistein, respectively (Barnett et al., 2015; Li et al., 2014; Boocock et al., 2007; Metzner et al., 2009). These data imply that the F values of these polyphenols were very low, which is consistent with the results of AS-FaFgCM, suggesting that AS-FaFgCM can accurately obtain human F from rat i.v. data.
The FaFg values of these four polyphenols were less than 0.15 (Table 2), suggesting low intestinal absorption; however, the FaFg of epicatechin was extremely lower compared to the others. Extensive intestinal metabolism of these polyphenols has been suggested in human and rat intestinal perfusion studies (Andlauer et al., 2000; Actis-Goretta et al., 2013; Zhang et al., 2005). In contrast, Caco-2 studies have shown that the permeability of epicatechin is in the range of poor absorption (Vaidyanathan and Walle, 2001; Yee, 1997), while genistein, baicalein, and resveratrol are classified as well-absorbed compounds (Chen et al., 2014; Willenberg et al., 2015; Kitaguchi et al., 2022). Therefore, lower FaFg of epicatechin could be derived from a lower fraction of dose absorbed (Fa), but not the fraction of dose passing through the gut wall without metabolism (Fg).
AS-FaFgCM is applicable to the pharmacokinetic data of aglycone; however, only the total concentration data of aglycone and its metabolites have been reported for several polyphenols owing to their rapid metabolism. Thus, AS-FaFgCM was further modified for application to the total concentration data of both aglycones and metabolites, namely AS-FaFgCME (Fig. 2B). Naringenin is a structural isomer of baicalein; naringenin glucuronides were excreted through MRP2 (Xu et al., 2009), suggesting that naringenin meets the assumption for applying AS-FaFgCME described in Materials and Methods. Table 2 shows the FaFg value of naringenin obtained by AS-FaFgCME using the pharmacokinetic data of deconjugated samples, demonstrating its low intestinal bioavailability. Naringenin is rapidly metabolized into glucuronide and sulfate conjugates, and the main plasma metabolite is naringenin-o-β-D-glucuronide, which constitutes 98% of the total metabolites detected (Joshi et al., 2018; El Mohsen et al., 2004). A mouse perfusion study revealed that naringenin was absorbed well in the small intestine and colon, but was extensively metabolized by phase II enzymes. These observations show the poor intestinal bioavailability of naringenin, which is consistent with the FaFg value estimated by AS-FaFgCME.
The kinetic parameters of intestinal metabolism and permeability of naringenin are similar to that of baicalein. The human intrinsic intestinal clearance values of baicalein and naringenin in human intestinal microsomes are 125 µL/min/mg protein (Li et al., 2012) and 166.6 µL/min/mg protein (Isobe et al., 2018), respectively. The apparent permeability values (Papp) for baicalein and naringenin in Caco-2 cells are 1.7 × 10−5 cm/s (Chen et al., 2014) and 1.7 × 10−5 cm/s (Nait Chabane et al., 2009), respectively. These results suggested that the FaFg values of naringenin and baicalein were similar. Indeed, the FaFg of naringenin estimated by AS-FaFgCME (0.136) was close to that of baicalein estimated by AS-FaFgCM (0.110) (Table 2 and 3). Therefore, AS-FaFgCME would be applicable to the estimation of human FaFg of polyphenols.
FaFg values for genistein and epicatechin in rats were approximately10-fold higher than that in humans (Table 2 and 4), suggesting that there are large species differences in intestinal availability between rats and humans. These discrepancies could be due to the difference in the expression of key drug metabolizing enzymes. UDP-glucuronosyltransferase (UGT) 1A10 is involved in the metabolism of genistein in the human gut (Doerge et al., 2000). Rat intestine lacks UGT1A10 (Jeong et al., 2005); therefore, the large difference in FaFg of genistein between rats and humans could be attributed to the difference in the expression of UGT1A10. Similar species differences are observed for the UGT1A10-metabolized drug; raloxifene, whose bioavailability in humans (2%) is much lower than that in rats (39%) (Jeong et al., 2005). Epicatechin was metabolized by sulfotransferase (SULT) 1A1 and SULT1A3 in the human small intestinal microsome (Vaidyanathan and Walle, 2002). However, SULT1A3 has no known animal orthologue (Peters et al., 2016); therefore, the lack of SUT1A3 in rat intestine influenced the higher FaFg value in rats compared to that in humans. The method proposed in this study could more precisely estimate the human FaFg of polyphenols than the traditional i.v. approach using a rat model.
AS-FaFgCM and AS-FaFgCME were based on previously reported allometric scaling methods (Chiou et al., 1998). Chiou et al. discussed that the allometric approach may inaccurately predict clearance if the liver is not the main metabolic tissue in humans, or if a drug is mainly excreted unchanged in humans but is mainly metabolized in the rat liver. Various polyphenols absorbed in the intestine undergo rapid hepatic phase II metabolism in both humans and rats, because they contain one or more phenolic rings with hydroxy groups; thus, the allometric approach is considered applicable to polyphenols. However, the linearity or nonlinearity of kinetic parameters, such as gastrointestinal absorption, first-pass metabolism, and apparent volume of distribution may also need to be considered. Although we adopted the data of the lowest dose in the reference studies for FaFg estimation to avoid saturation in enzyme and transporter kinetics, the FaFg values varied depending on the dose used for estimation. Finally, the validity of the AS-FaFgCME could not be confirmed; however, the applicability of AS-FaFgCM was demonstrated in comparison to the traditional i.v. approach. This is a limitation of this study; further research is warranted to ensure the validity of AS-FaFgCME.
In conclusion, this study determined the intestinal bioavailability of structurally diverse polyphenols and quantitatively demonstrated their poor intestinal absorption. In this study, we developed AS-FaFgCM and AS-FaFgCME, which are comprehensive approaches that can be used to obtain FaFg of polyphenols without using human i.v. data. Scarce i.v. data of polyphenols in humans have prevented us from understanding their intestinal absorption; however, this study provides a solution to this insufficient knowledge and potentially contributes to the development of novel alternative methods that can quantitatively predict the internal exposure of polyphenols.
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interestThe authors declare that there is no conflict of interest.