2022 年 47 巻 10 号 p. 429-437
2022 年 47 巻 10 号 p. 429-437
Antibiotic-associated encephalopathy (AAE) is a central nervous system disorder caused by antibiotics administration and classified into three types based on clinical symptoms. Type 1 AAE causes seizures and myoclonus, type 2 causes psychiatric symptoms, and type 3 is characterized by cerebellar ataxia. In this study, we investigated whether the electrical activity of in vitro human iPSC-derived neurons to antibiotics could be classified based on the 3 types of AAEs classified by clinical symptoms. Glutamatergic, GABAergic neurons and astrocytes differentiated from human iPS cells were seeded on micro-electrode array (MEA). The cumulative administration of 13 different antimicrobials detected changes in neural activity that differed according to AAE type. Next, we classified the antimicrobials by principal component analysis (PCA) and confirmed the AAE type of each agent. We found that Types 1–3 AAE agents were distributed separately. The classification of antibiotics depending on electrophysiological response characteristics was consistent with the clinical practice classification of AAEs. In conclusion, the combination of electrophysiological responses of human iPS cell-derived neural networks measured by MEA plus multivariate analysis methods will effectively detect and classify antibiotics developmental risks.
Antibiotic-associated encephalopathy (AAE) is a central nervous system (CNS) disorder caused by antibiotics administration. Delirium and encephalopathy occur in up to 80% of cases in the ICU (Inouye et al., 2014), with drug-related causes accounting for 12%–39% (Alagiakrishnan and Wiens, 2004). Because of other causes and severe symptoms, AAE is usually difficult to distinguish from the underlying disease, such as septic encephalopathy or encephalopathy. Therefore, there is a need to explain the causes of AAE and its prevention. Also, in the development of antibiotics, it is a vital issue to understand the AAE risk of the developed product and the symptoms of AAE caused.
A systematic review summarizing the clinical characteristics, electroencephalography (EEG) changes, and MRI images of each antibiotic showed that AAE could be classified into three types (Bhattacharyya et al., 2016). Type 1 AAE causes seizures, myoclonus, and EEG abnormalities within the first few days of administration and is often associated with elderly patients and patients with renal dysfunction. Antibiotics classified as Type 1 AAE are penicillin and cephalosporins. Penicillin is the oldest antibiotic in history and has encephalitic side effects. In 1947, Reuling and Cramer reported that penicillin injected into a patient’s spinal canal caused convulsions (Reuling and Cramer, 1947). It has also been suggested that intravenous injections cause convulsions (Conway et al., 1968). A β-lactam structure that inhibits GABAergic neurotransmission and causes activation of the excitatory nerve causes penicillin-induced convulsions (Sugimoto et al., 2002). Cephalosporins are antibiotics used to treat bacterial infections of the CNS. While cephalosporins can maintain high concentrations in the brain, they can cause epileptic seizures. Cephalosporins inhibit GABA A receptors (De Sarro et al., 1995) and elicit inflammatory responses by increasing cytokine secretion (Alkharfy et al., 2000). All cephalosporins from the first to the fourth generation are at risk of convulsions. Cefepime, a fourth-generation cephalosporin antibiotic, has a higher risk of convulsions than other recently used drugs (Sutter et al., 2015). Type 2 is a quinolone, macrolides, and sulfamethoxazole/trimethoprim (S/T) composition that causes psychiatric symptoms such as hallucinations and delusions to appear within a few days of treatment. Quinolones are more permeable to blood-brain barrier than other antibiotics, resulting in delirium, hallucinations, and extrapyramidal symptoms (Bhattacharyya et al., 2016). The mechanism of quinolone-induced AAE pathogenesis may involve inhibition of GABA receptors and activation of dopamine (Thomas and Reagan, 1996) and NMDA receptors (Schmuck et al., 1998). Patients treated with clarithromycin, a macrolide antibiotic, developed neurotoxicity five days after initiation (Bandettini di Poggio et al., 2011). Although the pathogenesis is unclear, the S/T mixture can also cause delirium, neurosis, and hallucinations. 5-nitroimidazole derivatives cause Type 3 AAE, which is characterized by cerebellar ataxia. The mechanism of Type 3 AAE involves neurotoxicity because of the accumulation of free radicals (Rao et al., 1987). Metronidazole, a nitroimidazole derivative, has also been linked to thiamine deficiency in the brain, leading to symptoms similar to Wernicke’s encephalopathy (Alston and Abeles, 1987). Metronidazole encephalopathy is well-known for cerebellar dentate nucleus lesions and abnormalities in the midbrain lid, corpus callosum, bridges, and medulla oblongata on MRI images (Kim et al., 2007).
An EEG known as Tri-HNC (Triphasic Wave-like Generalized Periodic Discharges with a High Negative Component) has been shown in cefepime encephalopathy (Tamune et al., 2019), suggesting that the characteristic electrical activity of each antibiotic may be involved in AAE. Although EEG analysis in rats and humans has been used to analyze antibiotic-induced encephalopathy, it remains unclear how each antibiotic affects the neural network.
The discovery of human iPS cells (Takahashi et al., 2007) has enabled the use of toxicity assessment systems in nonclinical studies that ensure extrapolation to humans. The functions of neurons are evaluated using calcium imaging, patch clamp, and the extracellular recording method using a planar microelectrode array (MEA). Among the evaluation systems that can measure neuron function in vitro, MEA has recently been actively performed to evaluate toxicity. MEA is appropriate because it is non-invasive, has high time resolution, can measure network electrophysiological activity at multiple points simultaneously, and evaluates neural network activity changes due to compound administration with high accuracy and throughput. We reported human iPS cell-derived neurons’ first electrical activity record using MEA (Odawara et al., 2014). We have earlier reported on the pharmacological response of human iPS cell-derived neural networks to seizurogenic drugs and the analysis method for MEA-measured electrical activity (Odawara et al., 2018). We have also made it possible to predict toxicity in vivo from in vitro analysis results using multivariate analysis to select parameters for predicting drug toxicity and mechanisms from the waveform data gained (Ishibashi et al., 2021).
In this study, we will solve the problem of AAE evaluation in antibiotics development by making it possible to evaluate the AAE type antibiotic in clinical settings using MEA measurements of a human iPS cell-derived neural network’s electrical activity. The electrophysiological characteristics of the administration of antibiotics were extracted, and each antibiotic was classified into three types based on clinically reported AAE by performing principal component analysis (PCA) using the synchronous activity of the neural network as an index. Efficient selection of highly safe drug candidate compounds is important in drug development. The assessment system is useful as an in vitro screening method based on the mechanism of side effects.
Glutamatergic neurons, GABAergic neurons, and Astrocyte (SynFire Co-Culture Kit, Neucyte Inc.) differentiated from human iPS cells were mixed at a ratio of 7:3:3.5 and seeded onto MEA plates (MEA; M768-tMEA-48B, AXION Biosystems) at 8.0 x 105 cells/cm2. MEA plates were coated with polyethyleneimine (Sigma, 0.01%, overnight) and laminin-511 (Nippi, 20 µg/mL, 60 min). SynFire Complete Media Kit (2010–20; Neucyte, Inc.) was used as media. A Seeding Medium was also used for seeding. Short-Term Medium was used on days 1–6 of culture, and Long-Term Medium was used on day-seven and onward. We exchanged half the media every 3–4 days.
Pharmacological testsPharmacological tests were performed after five weeks of culture. The following agents were used to evaluate the effect of antibiotics administration on the neural network established on the MEA: cefepime (C242750, Toronto Research Chemicals Inc.; n = 5), cefoxitin (1098107, United States Pharmacopeial Convention, Inc.; n = 6), and ampicillin (016-23301, FUJIFILM Wako; n = 6) as Type 1 AAE; norfloxacin (142-06391, FUJIFILM Wako; n = 5), ofloxacin (157-02981, FUJIFILM Wako; n = 6), nalidixic acid (147-09641, FUJIFILM Wako; n = 7), clarithromycin (032-17871, FUJIFILM Wako; n = 6), ST mixture [sulfamethoxazole (S0361, TCI) and trimethoprim (T2286, TCI; n = 5) were mixed in a weight ratio of 5: 1; n = 4], trimethoprim (n = 5) and penicillin G procaine (1502552 United States Pharmacopeial Convention, Inc.; n = 5) as Type 2 AAE; metronidazole (132-18061, FUJIFILM Wako; n = 8), secnidazole (S1810, LKT Labs, Inc.; n = 6) and tinidazole (T3454, LKT Labs, Inc.; n = 6) as Type 3 AAE. Each drug was administered cumulatively at concentrations of 3, 10, 30, 100, 300, and 1000 µM. GABA A receptor antagonist, picrotoxin (P1675-1G, Sigma-Aldrich) was administered cumulatively at concentrations of 0.1, 0.3, 1, 3, and 10 µM. Each drug was dissolved in dimethyl sulfoxide (DMSO; D2660-100ML; Sigma-Aldrich) and diluted with a culture medium. We adjusted the final concentrations of these drugs to contain 0.1% DMSO. We administered DMSO (0.1%) in all wells as a vehicle control prior to cumulative administration of the compound. Spontaneous activity of each well was recorded for 10 minutes before the drug administration, after administration of the vehicle (0.1% DMSO), and after antibiotics administration, respectively.
Measurement of the spontaneous activity of neurons and burst analysisSpontaneous extracellular potentials were obtained using an MEA system with 16 electrodes in each well of a 48-well plate (768 electrodes) at 5% CO2 and 37°C. The electrophysiological activity was analyzed using AxIS software (AXION Biosystems) and MATLAB. A spike was counted when the extracellular recorded signal exceeded a threshold of ±5.3σ (σ is the standard deviation of the baseline noise during the resting phase). The “four-step method” was used to detect synchronized burst firing, the main spasm-like activity (Matsuda et al., 2018).
Statistical analysisWe used one-way ANOVA followed by Dunnett’s test to calculate the significant difference between each concentration and the vehicle in burst analysis. One-way MANOVA was used for classification of antibiotic types in human iPS cell-derived neurons.
To evaluate the effect of each antibiotic on the neural network, human iPS cell-derived neurons were cultured on MEA (Fig. 1A), and spontaneous activity was measured. At five weeks of culture, electrical activity with a high signal-to-noise ratio was detected in the neural network (Fig. 1B). Fig. 1C shows the electrophysiological response to antibiotics treatment. Network burst (NB) via synaptic transmission was observed before and after drug administration (Fig. 1D). Seizures characterize antibiotics classified as Type 1 AAEs (cefoxitin, ampicillin). Administration of cefoxitin and ampicillin dose-dependently increased the frequency of synchronous activity in the neural network. Although antibiotics classified as Type 2 AAEs (norfloxacin, clarithromycin) cause psychotic symptoms, these agents increase synchronous activity frequency at low-to-moderate doses.

Electrophysiological response of human iPS cell-derived neurons to antibiotics treatment. (A) Phase contrast of neurons cultured on a microelectrode array (MEA) at eight weeks in vitro (WIV). (B) Typical action potential waveform of the spontaneous activity measurement at eight WIVs. (C) Raster plots of spikes and array-wide spike detection rate (AWSDR, spikes/second, bin size = 10 ms) for 10 min before drug administration, after vehicle administration, and cumulative administration of each antibiotic (cefoxitin (Type 1 AAE), ampicillin (Type 1 AAE), norfloxacin (Type 2 AAE), clarithromycin (Type 2 AAE), metronidazole (Type 3 AAE). (D) No. of NBs with 0 µM as 100% (one-way ANOVA and Dunnett’s test, *p < 0.05, **p < 0.01 vs. 0 µM).
Moreover, at higher doses, norfloxacin at 1 mM and clarithromycin at 100 μM decreased synchronous activity and firing frequencies, demonstrating different effects on the neural network. Cerebellar symptoms characterize antibiotics classified as Type 3 AAE. Metronidazole treatment increased synchronous activity frequency at low-to-moderate doses and decreased it at higher doses (1 mM). These results imply that drugs classified as Type 1 AAE and those classified as Type 2 and 3 AAE have different effects on the neural network depending on the drug concentration.
Analysis using the synchronous activity of neural networksTo analyze the electrical response of each type of AAE acquired in the above experiments, we identified the analysis parameters effective in detecting the characteristics of each antibiotic. Nine analysis parameters were used to create a heat map comparing the drug administration with the DMSO at 100% and Table 1 shows descriptions. Fig. 2A shows drugs classified as Type 1 AAE (cefepime, cefoxitin, ampicillin), Fig. 2B shows drugs classified as Type 2 AAE (ofloxacin, norfloxacin, nalidixic acid, clarithromycin, ST mixture, trimethoprim, penicillin G procaine), Fig. 2C shows drugs classified as Type 3 AAE (metronidazole, secnidazole, tinidazole), and Fig. 2D shows a heat map of picrotoxin. Antibiotics classified as Type 1 AAE and picrotoxin had increased No. of NBs compared with other types. Duration, Spikes in an NB, and MF did not change for Type 1 AAE and Type 3 AAE, but changed significantly for Type 2 AAE. All types also showed an increase in Total Spikes, No. of NBs, but Type 2 AAE showed a decrease in Total Spikes and No. of NBs at higher doses. These results imply that antibiotics belonging to each type have different effects on each analysis parameter. The results also indicate that it is possible to detect and classify the characteristics of electrical activity in each AAE using nine analysis parameters.
| Analytical parameter | Description |
| Total spikes (TS) | The total number of spikes detected on all channels in 10 minutes. |
| No. of network bursts (No. of NBs) | The number of network bursts in 10 minutes. |
| Inter burst interval (IBI) | Average time from the end point of network burst to the start point of the next network burst. |
| Duration of network burst (Duration) | Average duration of network burst. |
| Spikes in a network burst (Spikes) | Average the number of spikes contained in a network burst. |
| Max frequency (MF) | Average peak value of the histogram during a network burst. |
| Inter MF interval (IMFI) | Average time from the peak of network burst to the peak of the next network burst. |
| CV of MF | Coefficient of variance of MF. |
| CV of IMFI | Coefficient of variance of IMFI. |
9 analytical parameters calculated by burst analysis.

Dose dependence of each parameter of the antibiotics analysis. (A) Heat map of Type 1 AAE. (B) Heat map of Type 2 AAE. (C) Heat map of Type 3 AAE. (D) Heat map of picrotoxin as GABA A receptor antagonist. Heat maps were created for cefepime (n = 5), cefoxitin (n = 6), ampicillin (n = 6), ofloxacin (n = 6), norfloxacin (n = 5), nalidixic acid (n = 7), clarithromycin (n = 6), ST mixture (n = 4), trimethoprim (n = 5), penicillin G procaine (n = 6), metronidazole (n = 8), secnidazole (n = 6), tinidazole (n = 6), and picrotoxin (n = 6). Heat map with DMSO as 100% (one-way ANOVA and Dunnett’s test, *p < 0.05, **p < 0.01 vs. vehicle).
To verify whether it is possible to classify each antibiotic based on the characteristics of electrical activity in the neural network using analytical parameters, we performed a PCA based on previous studies (Ishibashi et al., 2021). Of the nine analysis parameters, No. of NBs and Spikes in an NB were used to classify each antibiotic. Table 2 shows principal component loadings. Figure 3A depicts a PCA map with 13 antibiotics (cefepime, cefoxitin, ampicillin, norfloxacin, ofloxacin, nalidixic acid, clarithromycin, S/T mixture, trimethoprim, penicillin G procaine, metronidazole, secnidazole, and tinidazole) and picrotoxin plotted for PC1 and PC2. The results for cephalosporins (cefepime, cefoxitin), quinolones (norfloxacin, ofloxacin, nalidixic acid), and nitroimidazoles (metronidazole, secnidazole, tinidazole) indicate that those with similar pharmacological effects are distributed closer together. Figure 3B shows the PC1 and PC2 plots for 13 antibiotics classified by Types 1–3 AAE, confirming that the drugs of each type were distributed separately (Table 3). When focusing on PC2, there were no significant differences between Type 1 and picrotoxin (Table 4). These findings revealed that antibiotics classification based on electrophysiological response characteristics in human iPS cell-derived neural networks is consistent with the classification based on AAE characteristics in clinical practice.
| Parameter | Principal component loadings | |
|---|---|---|
| PC1 | PC2 | |
| No. of NBs | 0.71 | 0.71 |
| Spikes in an NB | −0.71 | 0.71 |
No. of NBs and Spikes in an NB were effective parameters in detecting AAE type.
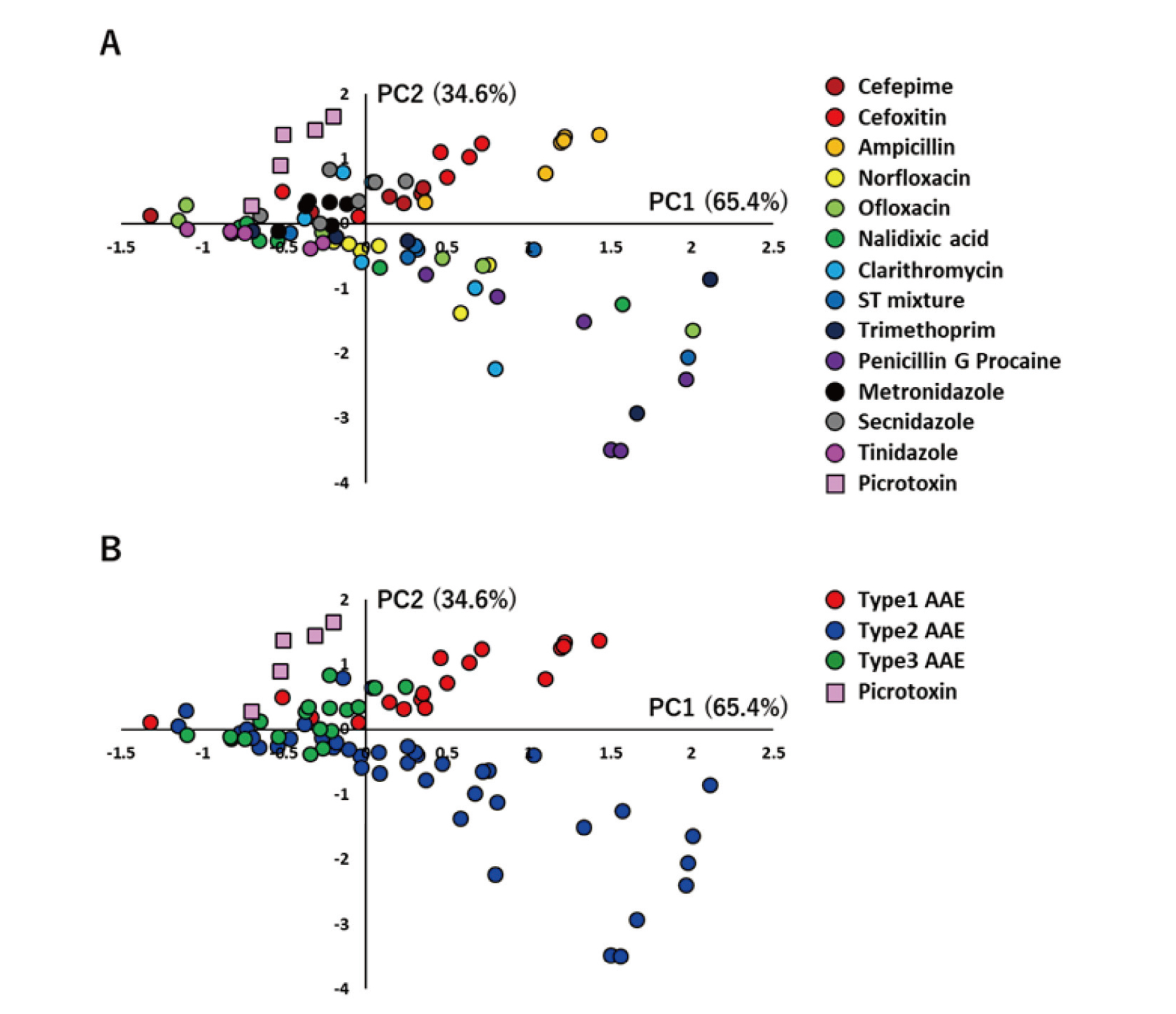
Classification of antibiotic agents in human iPS cell-derived neurons using PCA. (A) Centroid plots of PC1 and PC2 in the PCA using the effective parameter set (No. of NBs, Spikes in an NB) for detecting AAE type. (B) Centroid plots of PC1 and PC2 in a PCA classified each antibiotic as Type 1, Type 2, or Type 3. Changed the color of the plot in Fig. 3A to the AAE type.
| vs. Type 1 AAE | vs. Type 2 AAE | vs. Type 3 AAE | vs. Picrotoxin | ||||
|---|---|---|---|---|---|---|---|
| p value | p value | p value | p value | ||||
| Type 1 AAE | - | ** p < 0.01 | ** p < 0.01 | ** p < 0.01 | |||
| Type 2 AAE | ** p < 0.01 | - | ** p < 0.01 | ** p < 0.01 | |||
| Type 3 AAE | ** p < 0.01 | ** p < 0.01 | - | ** p < 0.01 | |||
| Picrotoxin | ** p < 0.01 | ** p < 0.01 | ** p < 0.01 | - |
Type 1 AAE: cefepime (n = 5), cefoxitin (n = 6), ampicillin (n = 6), Type 2 AAE: ofloxacin (n = 6), norfloxacin (n = 5), nalidixic acid (n = 7), clarithromycin (n = 6), ST mixture (n = 4), trimethoprim (n = 5), penicillin G procaine (n = 6), Type 3 AAE: metronidazole (n = 8), secnidazole (n = 6), tinidazole (n = 6), and picrotoxin (n = 6). One-way MANOVA, *p < 0.05, **p < 0.01.
| vs. Type 1 AAE | vs. Type 2 AAE | vs. Type 3 AAE | vs. Picrotoxin | ||||
|---|---|---|---|---|---|---|---|
| p value | p value | p value | p value | ||||
| Type 1 AAE | - | ** p < 0.01 | ** p < 0.01 | p = 0.27 | |||
| Type 2 AAE | ** p < 0.01 | - | ** p < 0.01 | ** p < 0.01 | |||
| Typ e3 AAE | ** p < 0.01 | ** p < 0.01 | - | ** p < 0.01 | |||
| Picrotoxin | p = 0.27 | ** p < 0.01 | ** p < 0.01 | - |
Type 1 AAE: cefepime (n = 5), cefoxitin (n = 6), ampicillin (n = 6), Type 2 AAE: ofloxacin (n = 6), norfloxacin (n = 5), nalidixic acid (n = 7), clarithromycin (n = 6), ST mixture (n = 4), trimethoprim (n = 5), penicillin G procaine (n = 6), Type 3 AAE: metronidazole (n = 8), secnidazole (n = 6), tinidazole (n = 6), and picrotoxin (n = 6). One-way ANOVA followed by Holm’s test, *p < 0.05, **p < 0.01.
This study tested whether it is possible to classify antibiotics into three groups based on clinically reported AAE by using electrical activity after antibiotics administration in human iPS cell-derived neural networks cultured in MEA. Various changes in neural activity for each antibiotic were detected in human iPS cell-derived neurons after administration of the antibiotic. PCA using analytical parameters showed that the electrophysiological antibiotics classification was consistent with the clinical classification of AAE.
This study used cephalosporins (cefepime, cefoxitin) and penicillins (ampicillin) as Type 1 AAE agents. These drugs have a β-lactam ring in their structure and act by stopping the elongation of peptidoglycan, a bacterial cell wall component; these agents may exhibit seizures by inhibiting GABA A receptors involved in inhibitory signaling (Wallace, 1997). This is noted because the hydrolysis of the β-lactam ring eliminates penicillin’s excitatory effects (Gutnick and Prince, 1971). Benzodiazepine, a GABA A receptor agonist, is used to treat β-lactam antibiotic seizures. In a previous study using MEA and iPS cell-derived neurons to assess the toxicity of seizure compounds, administration of pentylenetetrazole, a GABA A receptor antagonist, dose-dependently increased NB (Odawara et al., 2018), implying that β-lactam antibiotics may also have a similar mechanism and increase the frequency of synchronous activity (Fig. 2). The PCA results in Fig. 3 also showed that cefepime, cefoxitin, and ampicillin could be classified as Type 1 AAEs. When focusing on PC2, there were no significant differences between Type 1 and picrotoxin as GABA A receptor antagonist. Antibiotics have not only a GABA A receptor inhibitory action but also various actions in combination. It is likely that PC2 accounts for GABA A receptor inhibitory action and PC1 accounts for the other effects. The difference between picrotoxin and Type 1 AAE is the result of whether these are antibiotics or not, and may be possible to separate antibiotics and other compounds.
Drugs classified as Type 2 AAEs are quinolones, macrolides, and an ST mixture. Although these drugs are not similar in pharmacological and structural characteristics, they could be classified as Type 2 AAEs by analysis using electrical response properties and PCA. Quinolones act by inhibiting DNA gyrase and topoisomerase, unique to bacteria. Moreover, because of their GABA-like structure, quinolones exhibit convulsant-inducing effects by inhibiting GABA A receptors (Kushner et al., 2001; Neame et al., 2020). It has also been proposed that quinolones interact with GABA B receptors and GABA A receptors because baclofen, a GABA B receptor agonist, suppresses seizures induced by levofloxacin and ciprofloxacin (Akahane et al., 1993). Furthermore, quinolones are structurally similar to quinolinic acid, an endogenous ligand for glutamate receptors. Previous studies using rat hippocampal slices have shown that they enhance neural activity in a concentration-dependent manner by activating NMDA receptors (Schmuck et al., 1998). Figure 2 shows that norfloxacin administration increased NB at low-to-moderate doses and decreased NB and firing frequency at higher doses. There was also a trend toward reduced duration spikes in an NB and MF at higher doses in Type 2 AAE than in Type 1 AAE and Type 3 AAE (Fig. 2B). These results may reflect the combined effects of quinolone antibiotics on GABA A receptors and GABA B and NMDA receptors. Detailed studies in the future on the receptors on which quinolone antibiotics act are needed.
Other drugs classified as Type 2 AAE include macrolides and ST mixture. For example, clarithromycin, a macrolide antibiotic, binds to the bacterial ribosome and inhibits protein synthesis. The ST mixture combines sulfamethoxazole, which inhibits folate synthesis, and trimethoprim, which blocks folate activation. Although the mechanism of macrolide- and ST mixture-induced AAE is unknown, a previous study using a patch clamp to measure the electrical activity of CA3 pyramidal neurons in rat hippocampal slices found that clarithromycin administration at 30–300 μM increased the firing frequency and that a decrease in GABAergic signaling is involved in neuronal activity activation (Bichler et al., 2017). In this study, a dose-dependent increase in the frequency of synchronous activity was observed from 3 μM to 30 μM, whereas it decreased at higher doses above 100 μM (Fig. 2). This may reflect species differences between rats and humans and the ratio of neurons in the neural network constructed in the MEA (Glutamatergic neurons: GABAergic neurons = 7:3). Because macrolide and ST have similar electrical activity characteristics to quinolone antibiotics, they may have multiple action points. Detailed studies in the future on the receptors on which these drugs act are needed.
Drugs classified as Type 3 AAE include nitroimidazole antibiotics, such as metronidazole, secnidazole, and tinidazole. Metronidazole is reduced in pathogenic microorganisms to nitroso compounds (R-NO), which act by cleaving the DNA of bacteria and protozoa. Unlike other AAEs, metronidazole poisoning produces characteristic abnormalities in the dentate nucleus of the cerebellum and corpus callosum on MRI. Additionally, metronidazole increases the risk of AAE seizures due to intermediate metabolite denaturation of GABA (Rao et al., 1987), inhibition of protein synthesis through direct protein synthesis binding to RNA, radical production, and mitochondrial dysfunction (de Oliveira Vilaça et al., 2018). Although it is unclear how each of these mechanisms of toxicity onset contributes to AAE, the current study demonstrates that they can be distinguished as agents with distinct mechanisms of action from Type 1 and Type 2 AAE, and the utility of an in vitro experimental system combining MEA and iPS cells in AAE evaluation.
In summary, we could classify 13 antibiotics into three types based on the clinical characteristics of AAEs using the extracellular recording method using MEA, which can evaluate neural network function. Furthermore, the electrophysiological response of the neural network used in this study, combined with multivariate analysis methods, could be applied as an evaluation system for AAE risk. The assessment system is useful as an in vitro screening method based on the mechanism of side effects.
AMED, Grant Number 20be0304401h0401, supported this study.
Conflict of interestThe authors declare that there is no conflict of interest.