2022 年 47 巻 11 号 p. 467-482
2022 年 47 巻 11 号 p. 467-482
Acrylamide (AA) is a neurotoxicant that causes synaptic impairment in distal axons. We previously found that developmental exposure to AA decreased proliferation of late-stage neural progenitor cells (NPCs) in the hippocampal neurogenesis of the dentate gyrus (DG) in rats. To investigate whether hippocampal neurogenesis is similarly affected by AA exposure in a general toxicity study, AA was administered to 7-week-old male rats via oral gavage at dosages of 0, 5, 10, and 20 mg/kg for 28 days. In the subgranular zone (SGZ) and granule cell layer, AA decreased the densities of doublecortin-positive (+) cells and TOAD-64/Ulip/CRMP protein 4b+ cells per SGZ length. In addition, AA decreased the neurite length of doublecortin+ cells and downregulated genes related to neurite outgrowth (Ncam2 and Nrep) and neurotrophic factor (Bdnf and Ntrk2) in the DG. These results suggest that AA exposure for 28 days decreases type-3 NPCs and immature granule cells in neurogenesis of granule cell lineages involving the impairment of neurite outgrowth in young-adult rats. In the DG hilus, AA increased the density of cholinergic receptor nicotinic beta 2 subunit+ cells. AA also downregulated Reln related to the control of neuronal migration by interneurons in the DG. Furthermore, AA decreased the density of glial fibrillary acidic protein (GFAP)+ astrocytes in the DG hilus and downregulated Gfap and the genes of oligodendrocyte progenitor cells (Cspg4 and Pdgfra). Thus, AA decreased granule cell lineage subpopulations in the late-stage differentiation of hippocampal neurogenesis after young-adult stage exposure, exhibiting a pattern similar to the developmental exposure.
Acrylamide (AA), a water-soluble monomer, has long been produced as a raw material for polyacrylamide, which is used in various industrial applications, and AA itself is used in the field of molecular biology. AA is well known as a distal axon terminal toxicant, and it has been reported that axonal degeneration precedes neuronal degeneration (Lehning et al., 2003; Lee et al., 2005). The mechanism of the neurotoxic effects of AA in distal axon terminals was suggested to be as follows: AA forms chemical adducts with proteins involved in the transport of presynaptic vesicles, resulting in impaired presynaptic function (Graham, 1999; LoPachin and Gavin, 2012). In the early 2000s, it became clear that AA is generated by the Maillard reaction during the cooking of foods (Mottram et al., 2002), and many experimental studies on AA revealed that AA induced various types of toxicity such as genotoxicity, reproductive toxicity, hepatotoxicity, immunotoxicity, and carcinogenicity, in addition to neurotoxicity (Rifai and Saleh, 2020). It has been reported that the estimated average AA intake from diet in humans was 0.001–0.004 mg/kg body weight per day (Joint FAO/WHO Expert Committee on Food Additives, 2011).
The dentate gyrus (DG) is a unique substructure of the hippocampus where neurogenesis occurs during postnatal life and continues throughout life in most mammals (Amrein, 2015; Kempermann et al., 2015). Neural stem cells (NSCs, type-1 neural stem cells, radial glial cells) and neural progenitor cells (NPCs, type-2a and type-2b neural progenitor cells) in the subgranular zone (SGZ) proliferate and differentiate into type-3 NPCs, then gradually migrate into the granule cell layer (GCL) while differentiating into immature granule cells after the final cell division, and finally mature into granule cells (Knoth et al., 2010; Kempermann et al., 2015). In the process of postmitotic maturation, most newborn neurons are eliminated by apoptosis without being integrated into the functional neural network (Kempermann et al., 2015). These developing granule cells in the SGZ/GCL express cell-lineage markers sequentially according to their developmental stages as follows: glial fibrillary acidic protein (GFAP), which is expressed in type-1 NSCs; SRY-box transcription factor 2 (SOX2), which is expressed in type-1 NSCs and type-2a NPCs; T-box brain protein 2 (TBR2), which is expressed in type-2b NPCs; doublecortin (DCX), which is expressed in type-2b and type-3 NPCs and immature granule cells; TOAD-64/Ulip/CRMP protein 4b (TUC4), which is expressed in immature granule cells; and neuronal nuclei (NeuN), which is expressed in postmitotic immature and mature granule cells (Hodge et al., 2008; Knoth et al., 2010; Kempermann et al., 2015). The target cells of neurotoxicants in neurogenesis can be inferred by confirming the changes in the expressions of these neuronal markers using immunohistochemistry.
We previously found that neurotoxicants that target mature neural tissues (named as ‘adult neurotoxicants’), such as cuprizone (a demyelinating agent) and glycidol (an axon terminal toxicant), show similar patterns in the disruption of adult neurogenesis in the DG when developmental exposure and young-adult stage exposure were compared (Akane et al., 2013, 2014; Abe et al., 2015a, 2015b). This finding suggests that monitoring adult neurogenesis in a 28-day general toxicity study using rodents may be useful for predicting developmental neurotoxicity caused by adult neurotoxicants. On the other hand, neurotoxicants that target developing immature neural tissues (named as ‘developmental neurotoxicants’), such as 6-propyl-2-thiouracil (an anti-thyroid agent), valproic acid (an antiepileptic drug), and N-methyl-N-nitrosourea (a cytocidal DNA alkylating agent), have a wide range of effects on various processes of hippocampal neurogenesis and show different effects in the granule cell lineage and γ-aminobutyric acid (GABA)-ergic interneuron subpopulations when developmental exposure and young-adult stage exposure were compared (Shiraki et al., 2016a, 2016b; Watanabe et al., 2017a, 2017b, 2019). These results suggest that developmental neurotoxicants might also affect adult neurogenesis even by young-adult stage exposure, while toxicity targets may differ from that of developmental exposure.
AA is an adult neurotoxicant because it injures mature neural tissues, including neuropathy in the distal ends of axons of peripheral nerves (Lehning et al., 2003). We previously showed that developmental exposure to AA caused a decrease in the density of immature granule cells of hippocampal neurogenesis due to decreased proliferation of late-stage NPCs in rat offspring (Ogawa et al., 2012). In hippocampal neurogenesis, the postmitotic differentiation process of immature granule cells involves neuronal migration, neurite outgrowth, and synaptogenesis (Kempermann et al., 2015). Considering that the target mechanism of AA-induced neurotoxicity affects nerve terminal processes and presynaptic function, it was considered that developmental exposure to AA causes disruption of neurogenesis targeting neuronal migration, neurite outgrowth, and synaptogenesis in our previous study (Ogawa et al., 2012). Moreover, according to our hypothesis, AA might disrupt neurogenesis in the DG while showing similar cellular targets in the granule cell lineages, irrespective of the life stage during the exposure period.
To clarify whether hippocampal neurogenesis is similarly affected by AA exposure in a general toxicity study, the present study investigated cellular targets in the granule cell lineages of hippocampal neurogenesis and the corresponding molecular mechanisms in young-adult rats after 28 days of exposure to AA. For this purpose, we focused on changes in the distribution of granule cell lineage subpopulations in the SGZ/GCL, as well as their proliferation, apoptosis, and synaptic plasticity, using immunohistochemistry and real-time reverse transcription (RT)-PCR. Moreover, we analyzed the changes in the distribution of GABAergic interneuron subpopulations in the DG hilus, as well as changes in the transcript levels of genes functionally related to disruption of neurogenesis.
Acrylamide (AA, CAS No. 79-06-1; purity: ≥ 98%) was purchased from MilliporeSigma (Burlington, MA, USA). Sixty-four 6-week-old male Sprague-Dawley (Crl:CD [SD]) rats were obtained from the Jackson Laboratory Japan Inc. (Yokohama, Japan) and housed individually in polycarbonate cages with wood chip bedding in an air-conditioned animal room (temperature 23°C ± 3°C, relative humidity 50% ± 20%) with a 12-hr light/dark cycle. They were given a pelleted basal diet (MF; Oriental Yeast Co., Ltd., Tokyo, Japan) and tap water ad libitum throughout the experimental period. Since neurogenesis and synaptic plasticity in the DG are influenced by circulating levels of steroid hormones during the female estrous cycle (Sheppard et al., 2019), male animals were used in the present study.
Experimental designAnimals were randomly divided into 4 groups (N = 16/group) and were administered with 0, 5, 10, or 20 mg/kg of AA via oral gavage from the age of 7 weeks for 28 consecutive days (Day 1: the day of first dosing; Supplementary Fig. 1). In each group, ten animals were used for immunohistochemistry, and 6 animals were used for RNA extraction for real-time RT-PCR. The animals used for gait abnormality scoring and body weight measurement during experiment were subjected to immunohistochemistry. Based on the results of a previous report (Lehning et al., 2003) and our preliminary dose-finding study consisting of groups of 20 and 30 mg/kg for 28 days (N = 5/group), the highest dose of 20 mg/kg was set as the dose level at which mild to moderate gait abnormality would be induced due to peripheral nerve injury by 28 days of repeated dosing of AA.
Physical examinations were performed during the dosing period, and gait abnormalities were scored into the following 4 categories according to the previous reports (Lee et al., 2005; Ogawa et al., 2012): Grade 1 as normal gait; Grade 2 as slightly abnormal gait with slight degrees of ataxia, foot splay, and hopping; Grade 3 as moderately abnormal gait with moderate degrees of ataxia, foot splay, and limb abduction; Grade 4 as severely abnormal gait with inability to support the body weight. Body weight was measured 3 times per week during dosing period and on the day of necropsy. On Day 29, animals were sacrificed by exsanguination from the abdominal aorta under deep isoflurane anesthesia and then necropsied to collect the brain. The brain was weighed using an electric balance and immersed in Bouin’s solution (picric acid saturated aqueous solution, 1500 mL; 37% formaldehyde, 500 mL; glacial acetic acid, 100 mL) for 24 hr at 4°C. The removed brain for real-time RT-PCR was immersed in methacarn solution (methanol, 1000 mL; chloroform, 500 mL; glacial acetic acid, 167 mL) for fixation for 5 hr at 4°C. Animal experiments including preliminary study in the present study were performed in accordance with the protocols approved by the Institutional Animal Care and Use Committee at Taisho Pharmaceutical Co., Ltd (approved No.: AN12128-Z00).
Immunohistochemistry and apoptotic cell detection in the hippocampal DGBrain sections of 10 animals/group were subjected to staining with hematoxylin and eosin (HE), immunohistochemistry, and apoptotic cell detection. Coronal brain slices were prepared at the position of –3.5 mm from the bregma with the aid of ruler, paraffin embedded, and sectioned at 3 μm for HE staining and immunohistochemistry. Immunohistochemistry was performed using the primary antibodies against the following antigens (Supplementary Table 1), and one section per animal was subjected to immunohistochemistry of each antigen: GFAP, which is expressed in type-1 NSCs in the SGZ and astrocytes; SOX2, which is expressed in type-1 NSCs and type-2a NPCs in the SGZ; TBR2, which is expressed in type-2b NPCs in the SGZ; DCX, which is expressed in type-2b and type-3 NPCs in the SGZ and immature granule cells in the GCL; TUC4, which is expressed in immature granule cells in the SGZ/GCL; NeuN, which is expressed in immature and mature granule cells and hilar interneurons; proliferating cell nuclear antigen (PCNA), a cell proliferation marker in the SGZ; phosphorylated H2A histone family, member X (γ-H2AX), which is a DNA double-strand break marker (He et al., 2016); reelin (RELN), parvalbumin (PVALB), somatostatin (SST), calbindin-D-29K (CALB2), and cholinergic receptor nicotinic beta 2 subunit (CHRNB2), which are expressed in the hilar interneuron (Pesold et al., 1998; Son and Winzer-Serhan, 2008; Catavero et al., 2018); activity-regulated cytoskeleton-associated protein (ARC), Fos proto-oncogene, AP-1 transcription factor subunit (FOS), early growth response 1 (EGR1), and cyclooxygenase 2 (COX2), which are synaptic plasticity-related immediate-early gene (IEG) products (Yagami et al., 2016; Duclot and Kabbaj, 2017; Jaworski et al., 2018; Nikolaienko et al., 2018). Deparaffinized sections were incubated in absolute methanol containing 0.3% hydrogen peroxide for 30 min to block endogenous peroxidase. Antigen retrieval was carried out in 10 mM citrate buffer (pH 6.0) by autoclaving at 121°C for 10 min or by incubating in water bath at 100°C for 20 min for some primary antibodies before blocking of endogenous peroxidase (Supplementary Table 1). Sections were incubated overnight in the primary antibodies at 4°C. Immunodetection was performed using a VECTASTAIN® Elite ABC kit (Vector Laboratories Inc., Burlingame, CA, USA). Sections were rinsed with phosphate-buffered saline (PBS; pH 7.4) followed by visualization of immunoreactivity with 3,3’-diaminobenzidine/H2O2 in PBS as chromogen. Sections were then rinsed with distilled water, counterstained with hematoxylin for 1 min, immersed in running water for 10 min, dehydrated, and cover-slipped for morphometry.
To evaluate apoptosis in the SGZ, a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was performed using the commercial kit (ApopTag® Peroxidase In situ Apoptosis Detection kit; MilliporeSigma) with 3,3’-diaminobenzidine/H2O2 as chromogen (Mohan et al., 2015). One section per animal was subjected to TUNEL assay. The sections were counterstained with hematoxylin and cover-slipped for morphometry.
Morphometry of immunoreactive and apoptotic cells in the hippocampal DGAll of the HE-stained sections, and immunohistochemistry and TUNEL assay sections were scanned at 2 pixels per micron with 20× objective using Leica Aperio Scanscope XT (Leica Microsystems, Wetzlar, Germany) and the length of SGZ or area unit of DG hilus of the bilateral hippocampi were measured using Leica Aperio Imagescope digital slide viewer (Leica Microsystems). All of the HE-stained sections were subjected to measurement of the SGZ length and DG hilar area. All of the immunohistochemistry and TUNEL assay sections were subjected to positive cell counting in the SGZ/GCL or DG hilus. All of the numbers of GFAP-positive (+) cells, SOX2+ cells, TBR2+ cells, PCNA+ cells, TUNEL+ cells, and γ-H2AX+ cells in the SGZ, the numbers of DCX+ cells and TUC4+ cells in the SGZ/GCL, and all of the numbers of NeuN+ cells, ARC+ cells, FOS+ cells, EGR1+ cells, and COX2+ cells in the GCL were bilaterally counted and normalized for the length of the SGZ. All of the numbers of RELN+ cells, PVALB+ cells, SST+ cells, CALB2+ cells, NeuN+ cells, CHRNB2+ cells, and GFAP+ cells in the DG hilus were bilaterally counted and normalized for the hilar area unit (Supplementary Fig. 2). The average of the values in both sides was used as the individual value. With regard to the cytoplasmic immunoreactivity, positive cells without nucleus were excluded from counting. GFAP+ NSCs were morphologically identified as those having cell bodies that reside in the SGZ and vertical processes that extend into the molecular layer of the DG and distinguished from GFAP+ astrocytes extending their processes in multiple directions in the DG (Fukuda et al., 2003). Cornu Ammonis (CA) 3 neurons distributed within the DG hilus were excluded from quantification. Except for NeuN+ cells in the GCL, the numbers of immunoreactive cells and apoptotic cells were manually counted while blinded to the treatment conditions using a microscope (BX53; Olympus Corporation, Tokyo, Japan). The number of NeuN+ cells in the GCL was counted in the digital images using Leica Aperio Imagescope digital slide viewer.
Measurement of the neurite length of DCX+ cellsThe neurite length of DCX+ cells in the SGZ/GCL of the bilateral hippocampi was measured in the control and all AA-treated groups using Leica Aperio Imagescope digital slide viewer, and the mean neurite length of all DCX+ cells was calculated in each animal. The length of the longest neurite extending toward the molecular layer in each DCX+ cell was measured as the representative neurite.
Transcript-level expression analysis in the hippocampal DGReal-time RT-PCR analysis on mRNA expression in the hippocampal DG was performed. The methacarn-fixed brains were subjected to dehydration 3 times for 1 hr in fresh 99.5% ethanol on ice with agitation, and then stored at 4°C. Following the previous report (Akane et al., 2013), 2-mm-thick coronal cerebral slices were prepared at the position of –2.8 mm from the bregma and portions of hippocampal DG were excised using punch-biopsy devices with a bore-size diameter of 1 mm (BPP-10F; Kai Industries Co., Ltd., Gifu, Japan). The excised DG tissues were stored at –80°C in 99.5% ethanol until RNA extraction. Total RNA was extracted using RNeasy Plus Universal Mini kit (Qiagen, Hilden, Germany) and cDNA was synthesized using iScriptTM cDNA Synthesis Kit (Bio-Rad Laboratories Inc., Hercules, CA, USA) according to the manufacturer’s protocol. Analysis of mRNA expression levels was performed with the PCR primers shown in Supplementary Table 2. Real-time RT-PCR with Power SYBR® Green PCR Master Mix (Thermo Fisher Scientific Inc., Waltham, MA, USA) was performed using DNA Engine Opticon® 2 System (Bio-Rad Laboratories Inc.). The relative differences in gene expression between the 0-mg/kg control and each AA-treated group were calculated using the 2-ΔΔCT method (Livak and Schmittgen, 2001). In brief, threshold cycle (CT) values were normalized by housekeeping genes (Gapdh or Hprt1), and then calculated relative to the values of control group.
Statistical analysisFor each group, the mean and standard deviation were calculated for numerical data (body weights, brain weights, SGZ length and DG hilar area, morphometry of immunoreactive and apoptotic cells, neurite length, and transcript-level expression), and data of the control group and AA-treated groups were analyzed by the multiple comparison test. The homogeneity of variance was first analyzed by the Bartlett’s test, followed by a one-way analysis of variance (one-way ANOVA) for homogeneous data. In cases where significant difference was found among the groups, Dunnett’s test (parametric) was applied for multiple comparison between the control group and each AA-treated group. When the variance was heterogeneous, Kruskal-Wallis’s H-test was applied, and in cases where significant difference was found among the groups, the Steel’s test was applied for multiple comparison between the control group and each AA-treated group. Regarding the gait score data, Mann-Whitney’s U-test was applied to compare between the control group and each AA-treated group. Differences from the control group were evaluated at 5% level of significance and presented as P < 0.05 or P < 0.01 in the figures and tables. All statistical analyses were performed using MiTOX (SAS ver. 9.4; Mitsui E&S Systems Research Inc. Chiba, Japan).
No death was observed during the dosing period but decreased body weight and changes indicating neurotoxicity were observed in the AA-treated groups. Body weight was significantly decreased from Day 15 to Day 29 in the 20-mg/kg AA group, and the final body weight on Day 29 was –16%, compared with the weight in the control group (Fig. 1A). Other AA-treated groups did not change the body weight, compared with the control group.
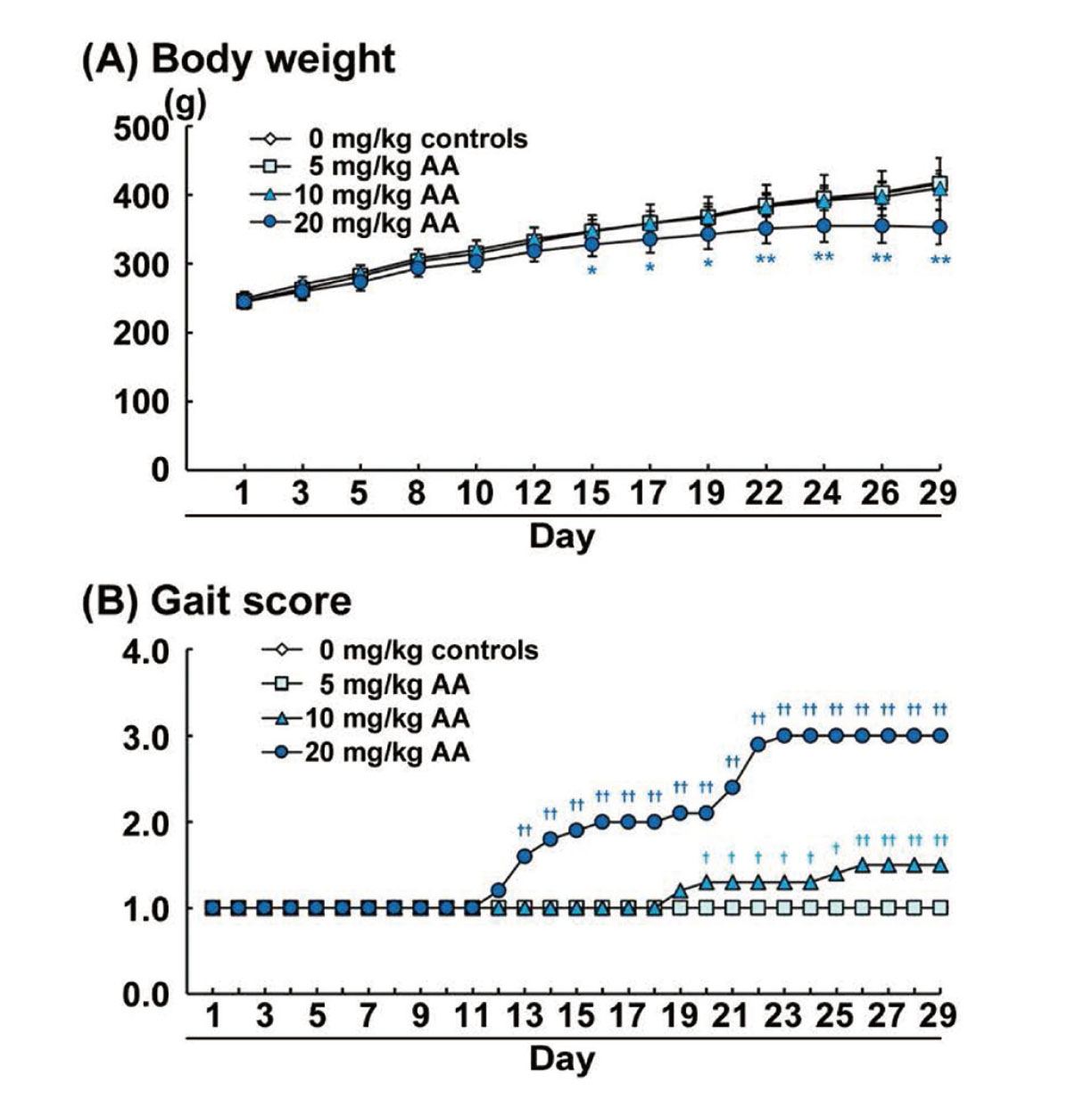
(A) Body weight and (B) gait score during the exposure period of acrylamide (AA). *P < 0.05, **P < 0.01, compared with the control group by Dunnett’s test or Steel’s test. †P < 0.05, ††P < 0.01, compared with the control group by Mann-Whitney’s U-test.
To evaluate the gait abnormalities, we observed gait and scored the degree of gait abnormalities. Gait scores of the 10- and 20-mg/kg AA groups were gradually increased during the latter half of the dosing period. Gait score was significantly increased in the 10-mg/kg AA group from Day 20 to Day 29 and in the 20-mg/kg AA group from Day 13 to Day 29, compared with the score in the control group (Fig. 1B). The gait scores of animals on Day 29 were either Grade 1 or 2 in the 10-mg/kg AA group and Grade 3 in the 20-mg/kg AA group. The animals in the 5-mg/kg AA group as well as the control group did not show any gait abnormality.
Brain weights at the necropsy significantly decreased in the 10- and 20-mg/kg AA groups, compared with the weight in the control group (Table 1).
 Morphological changes in the DG
Morphological changes in the DG
Since the brain weights were decreased in AA-treated groups, we investigated whether the size of hippocampal DG was also decreased. In HE-stained sections, there were no significant differences in the SGZ length and DG hilar area in any of the AA-treated groups, compared with the control group (Supplementary Table 3). There were also no histopathological changes in the DG of AA-treated groups.
Immunoreactive cells and apoptotic cells in the DGThe results of the immunohistochemistry and TUNEL assay are summarized in Supplementary Table 4. The location, pattern, and extent of immunoreactivity for each marker or TUNEL-assay reactivity were similar to those in the age-matched animals in the previous studies (Akane et al., 2014; Abe et al., 2015b; Shiraki et al., 2016a; Watanabe et al., 2017b). Densities of immunoreactive cells and apoptotic cells in the AA-treated groups were compared with those in the control group.
Among the cellular markers of granule cell lineages, GFAP showed immunoreactivity in the cytoplasm of the SGZ cells, which extend single foot process toward the molecular layer (Fig. 2A). Immunoreactivity of SOX2 and TBR2 was similarly observed in the nucleus of the SGZ cells (Fig. 2B and 2C). SOX2+ cells were diffusely scattered in the SGZ, and TBR2+ cells were solitary or in clusters in the SGZ. DCX immunoreactivity was observed in the cytoplasm including neurites of the SGZ cells and GCL cells (Fig. 2D). TUC4 immunoreactivity was observed in the cytoplasm of the immature granule cells in the SGZ/GCL (Fig. 2E). NeuN immunoreactivity was observed in the nucleus and cytoplasm, and most of granule cells were positive for NeuN (Fig. 2F). Regarding the effect of AA on granule cell lineage subpopulations in the SGZ/GCL, the densities of DCX+ cells and TUC4+ cells in the SGZ/GCL per SGZ length were significantly decreased in the 20-mg/kg AA group (Fig. 2D and 2E). In the 20-mg/kg AA group, DCX+ cells showed DCX+ short neurites. The densities of GFAP+ cells, SOX2+ cells, TBR2+ cells, and NeuN+ cells per SGZ length were not significantly different in any of the AA-treated groups.
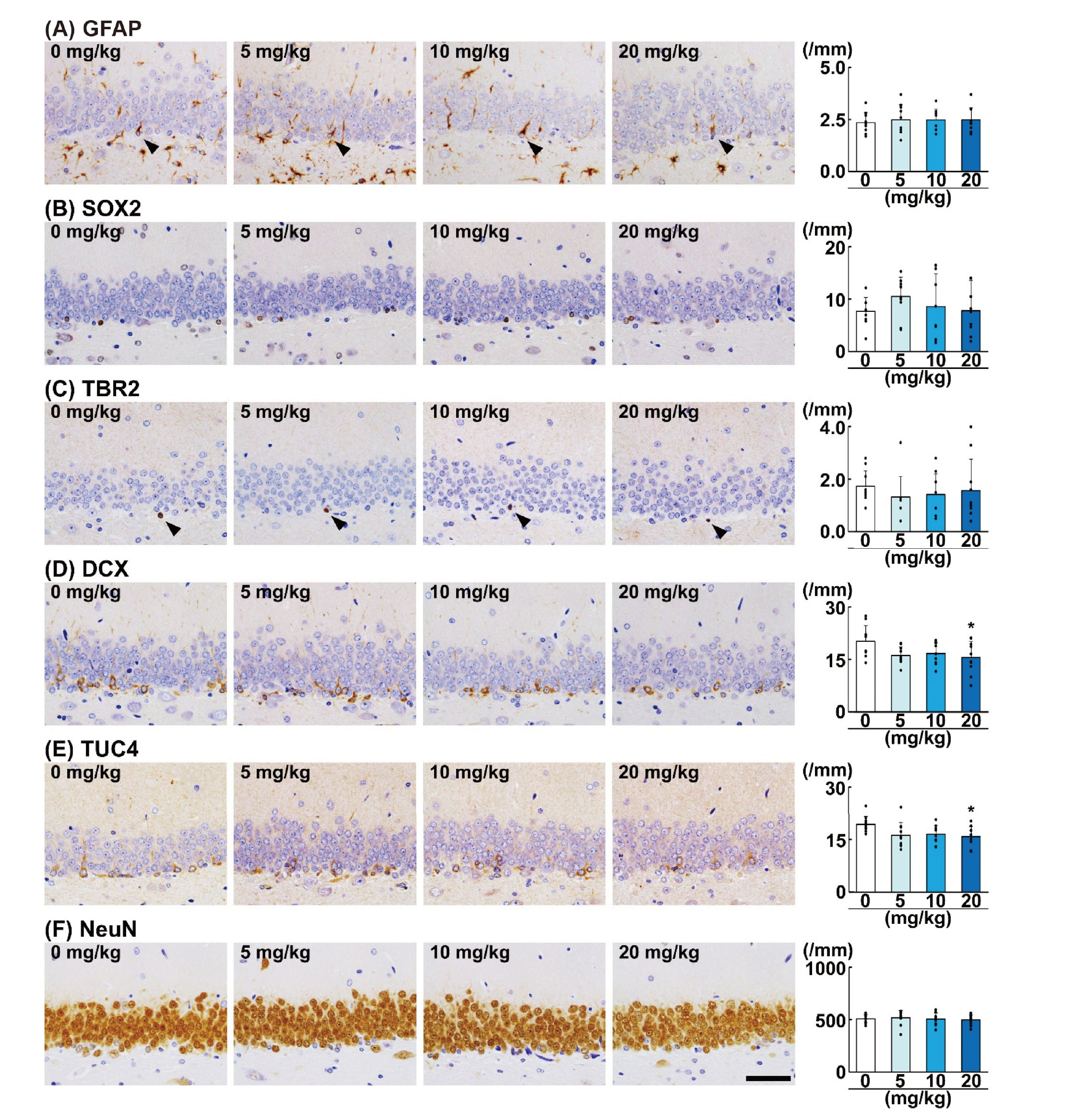
Distribution of immunoreactive cells for (A) glial fibrillary acidic protein (GFAP), (B) SRY-box transcription factor 2 (SOX2), and (C) T-box brain protein 2 (TBR2) in the subgranular zone (SGZ), (D) doublecortin (DCX) and (E) TOAD-64/Ulip/CRMP protein 4b (TUC4) in the SGZ and granule cell layer (GCL), and (F) neuronal nuclei (NeuN) in the GCL of the hippocampal dentate gyrus (DG). Arrowheads indicate immunoreactive cells. Magnification × 400; bar 50 µm. Graphs show the density of immunoreactive cells in the SGZ and GCL per SGZ length. N = 10/group. *P < 0.05, compared with the control group by Dunnett’s test or Steel’s test.
Among the GABAergic interneuron subpopulations in the DG hilus, immunoreactivity of RELN, PVALB, SST, and CALB2 was observed in the cytoplasm (Fig. 3). Immunoreactivity of CHRNB2 was observed in dots in the perinuclear cytoplasm of the interneurons (Fig. 4). NeuN immunoreactivity of interneurons in the DG hilus was observed in the nucleus and cytoplasm (Fig. 3E). Regarding the effect of AA on interneuron subpopulations in the DG hilus, the density of RELN+ cells, PVALB+ cells, SST+ cells, and CALB2+ cells, and NeuN+ postmitotic interneurons were not significantly different in any of the AA-treated groups (Fig. 3). On the other hand, the density of CHRNB2+ cells was significantly increased in the 20-mg/kg AA group (Fig. 4).
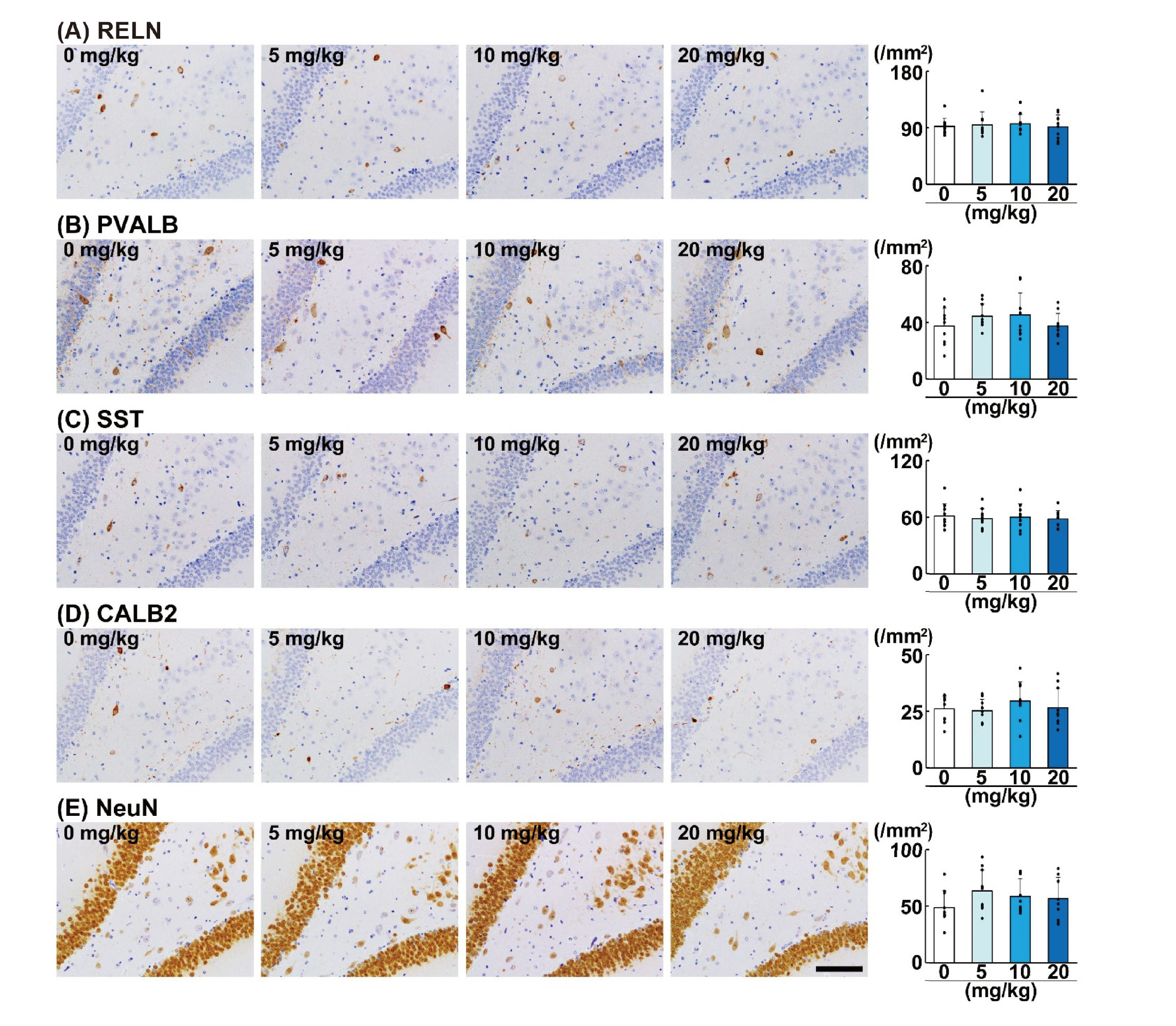
Distribution of immunoreactive cells for (A) reelin (RELN), (B) parvalbumin (PVALB), (C) somatostatin (SST), (D) calbindin-D-29K (CALB2), and (E) neuronal nuclei (NeuN) in the hilus of the hippocampal dentate gyrus (DG). Magnification × 200; bar 100 µm. Graphs show the density of immunoreactive cells in the DG hilus. N = 10/group.
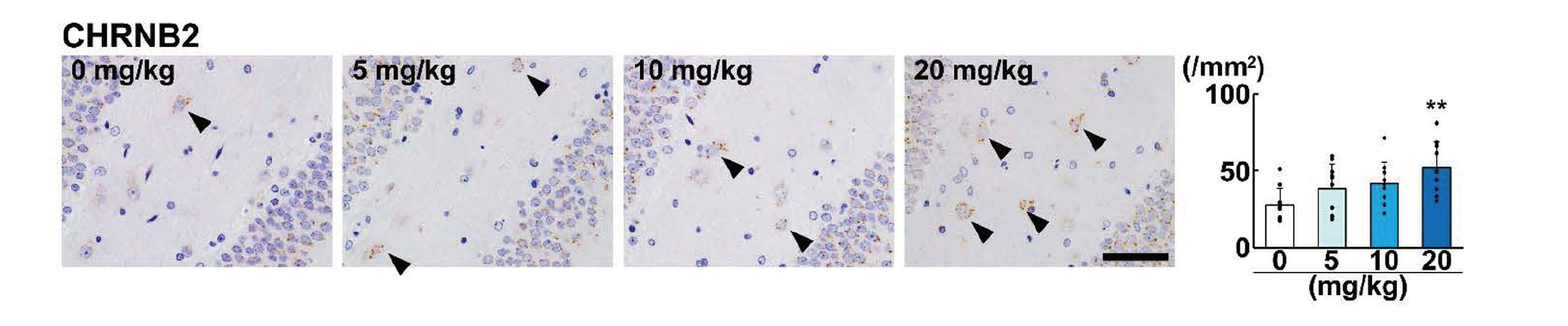
Distribution of immunoreactive cells for cholinergic receptor nicotinic beta 2 subunit (CHRNB2) in the hilus of the hippocampal dentate gyrus (DG). Arrowheads indicate immunoreactive cells. Magnification × 400; bar 50 µm. Graphs show the density of immunoreactive cells in the DG hilus. N = 10/group. **P < 0.01, compared with the control group by Dunnett’s test or Steel’s test.
To investigate the cellular kinetics in the neurogenesis, PCNA immunohistochemistry (cell proliferation activity) and TUNEL assay (apoptosis) were performed. In the SGZ, PCNA+ cells were scattered and TUNEL+ cells were rarely observed (Fig. 5). The densities of PCNA+ proliferating cells and TUNEL+ apoptotic cells per SGZ length were not significantly different in any of the AA-treated groups. Since AA is a genotoxic compound (Rifai and Saleh, 2020), we also investigated γ-H2AX, the marker of DNA double-strand breaks (He et al., 2016). γ-H2AX+ cells were scattered in the SGZ (Fig. 5C), but the density of γ-H2AX+ cells per SGZ length was not significantly different in any of the AA-treated groups.

Distribution of (A) proliferating cell nuclear antigen (PCNA)-positive (+) proliferating cells, (B) terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)+ apoptotic cells, and (C) phosphorylated H2A histone family, member X (γ-H2AX)+ cells in the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG). Magnification × 400; bar 50 µm. Graphs show the densities of immunoreactive cells and TUNEL+ cells in the SGZ per SGZ length. N = 10/group.
To examine changes in synaptic plasticity in the GCL, immunohistochemistry of synaptic plasticity-related IEG products was performed (Fig. 6). The immunoreactivity of IEG products was scattered in the GCL. Among them, ARC was observed in the nucleus and cytoplasm of granule cells (Fig. 6A). FOS and EGR1 were observed in the nucleus (Fig. 6B and 6C), and COX2 was observed in the cytoplasm of granule cells (Fig. 6D). However, the densities of ARC+ cells, FOS+ cells, EGR1+ cells, and COX2+ cells per SGZ length were not statistically different in any of the AA-treated groups.

Distribution of immunoreactive cells for (A) activity-regulated cytoskeleton-associated protein (ARC), (B) Fos proto-oncogene, AP-1 transcription factor subunit (FOS), (C) early growth response 1 (EGR1), and (D) cyclooxygenase 2 (COX2) in the granule cell layer (GCL) of the hippocampal dentate gyrus (DG). Magnification × 400; bar 50 µm. Graphs show the density of immunoreactive cells in the GCL per SGZ length. N = 10/group.
We further examined GFAP+ astrocytes in the DG hilus. The density of GFAP+ cells was significantly decreased in the 20-mg/kg AA group (Fig. 7).

Distribution of immunoreactive cells for glial fibrillary acidic protein (GFAP) in the hilus of the hippocampal dentate gyrus (DG). Magnification × 200; bar 100 µm. Graph shows the density of immunoreactive cells in the DG hilus. N = 10/group. *P < 0.05, compared with the control group by Dunnett’s test or Steel’s test.
Since the neurites of DCX+ cells in the 20-mg/kg AA group were shorter than that in the control group (Fig. 2D), the mean neurite length was quantified in all groups. As a result, the mean neurite length was significantly decreased in the 20-mg/kg AA group (Table 2).
 Transcript-level expression changes in the DG
Transcript-level expression changes in the DG
Relative transcript levels in the AA-treated groups in comparison with the control group are summarized in Table 3. Along with the immunohistochemical analysis, expression changes of the genes for granule cell lineage subpopulations, GABAergic interneuron subpopulations, synaptic plasticity, and astrocytes were investigated. Regarding the genes for granule cell lineage subpopulations, the transcript levels of Dcx and Dpysl3 were significantly decreased in the 10-mg/kg AA group after normalization with Hprt1 and in the 20-mg/kg AA group after normalization with Gapdh and Hprt1. The transcript level of Nes was significantly decreased in the 10- and 20-mg/kg AA groups after normalization with Gapdh. The transcript level of Rbfox3 was significantly increased in the 5-, 10-, and 20-mg/kg AA groups after normalization with Gapdh. As for the genes for GABAergic interneuron subpopulation, the transcript level of Reln was significantly decreased in the 20-mg/kg AA group after normalization with Gapdh and Hprt1. The transcript level of Sst was significantly increased in the 5- and 10-mg/kg AA groups after normalization with Gapdh. In case of the synaptic plasticity-related IEGs, the transcript levels of Arc, Fos, Egr1, and Ptgs2 (encoding COX2) were not statistically different in the 10- and 20-mg/kg AA groups after normalization with Gapdh and Hprt1. The transcript level of Egr1 was significantly increased in the 5-mg/kg AA group after normalization with Gapdh. Regarding the genes for astrocytes, the transcript level of Gfap was significantly decreased in the 20-mg/kg AA group after normalization with Gapdh.
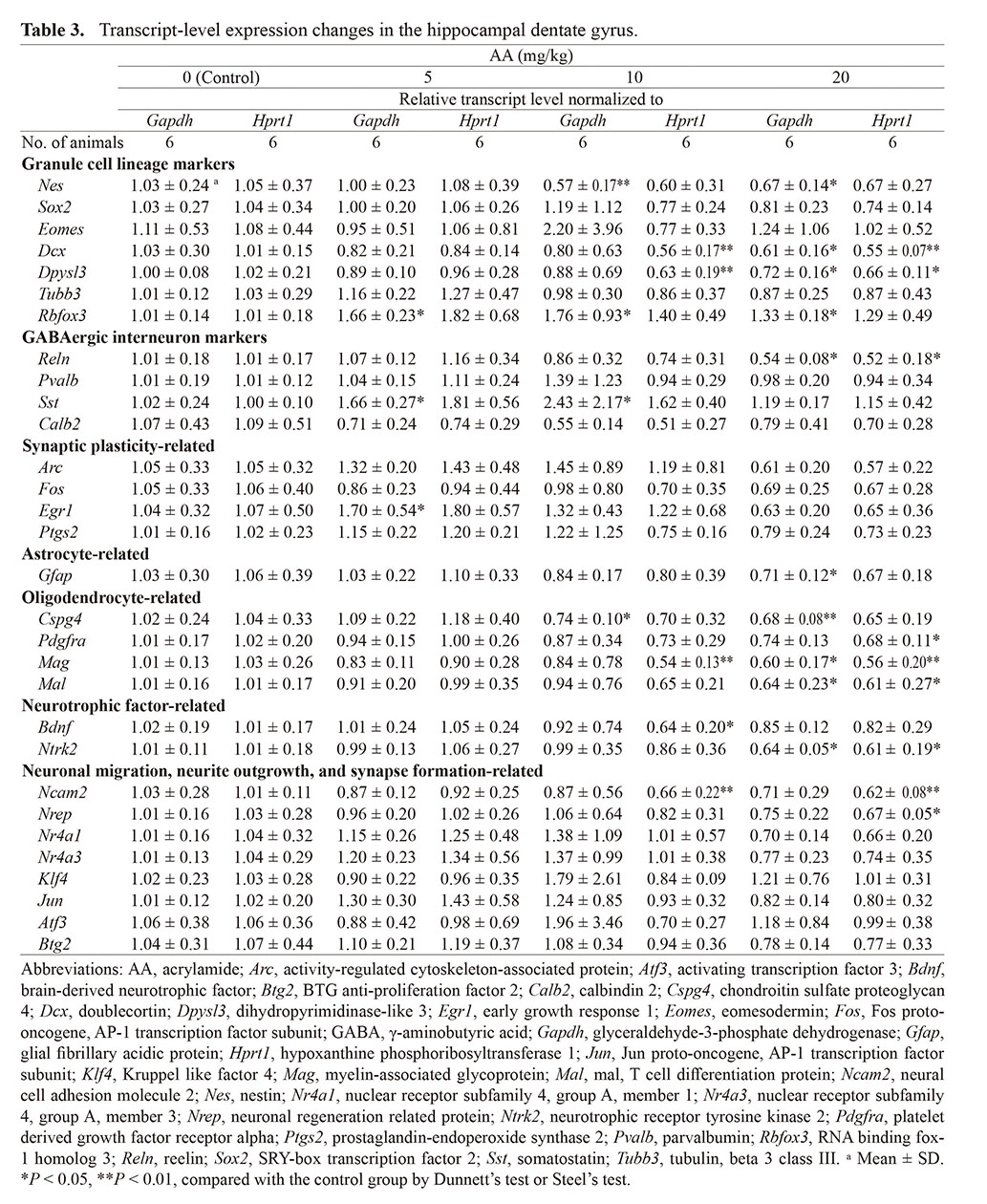
We additionally examined expression changes of oligodendrocyte-related genes. The transcript levels of Cspg4 and Pdgfra were significantly decreased in the 10- and 20-mg/kg AA group after normalization with Gapdh or Hprt1. The transcript level of Mag was significantly decreased in the 10-mg/kg AA group after normalization with Hprt1 and in the 20-mg/kg AA group after normalization with Gapdh and Hprt1. The transcript level of Mal was significantly decreased in the 20-mg/kg AA group after normalization with Gapdh and Hprt1.
To investigate toxicity mechanism of AA, expression changes of neurotrophic factor-related genes and neurite outgrowth-related genes were investigated. The transcript level of Bdnf was significantly decreased in the 10-mg/kg AA group after normalization with Hprt1, and the transcript level of Ntrk2 was significantly decreased in the 20-mg/kg AA group after normalization with Gapdh and Hprt1. Regarding the neurite outgrowth-related genes, the transcript level of Ncam2 was significantly decreased in the 10- and 20-mg/kg AA group after normalization with Hprt1. The transcript level of Nrep was significantly decreased in the 20-mg/kg AA group after normalization with Hprt1.
We have previously reported that developmental exposure to AA causes decrease in the density of immature granule cells of hippocampal neurogenesis in rat offspring (Ogawa et al., 2012). To clarify whether hippocampal neurogenesis in developed rats is similarly affected after young-adult stage exposure of AA, we investigated changes in the granule cell lineage subpopulations as well as their proliferation, apoptosis, and synaptic plasticity, and the interneuron and glial cell lineage subpopulations in the hippocampal DG by means of immunohistochemical and gene expression analysis. Here, we report that AA decreased the granule cell lineage subpopulations in the late-stage differentiation accompanying impaired neurite outgrowth in young-adult rats, exhibiting a pattern similar to the developmental exposure.
It has been reported that suppressed body weight gain and gait abnormality were observed in rats by AA-treatment with the similar dose levels and exposure period employed in the present study (Lehning et al., 2003). Consistent with the previous study, body weight gain was suppressed in the 20-mg/kg AA group; the body weight was significantly decreased from Day 15, compared with the weight in the control group. At the necropsy, the body weight was significantly decreased in the 20-mg/kg AA group and the absolute brain weight was significantly decreased in the 10- and 20-mg/kg AA groups, compared with the control group. During the dosing period, the gait score was dose-relatedly increased in the 10- and 20-mg/kg AA groups. These results suggest that the AA doses used in this study induced toxic effects suggestive of peripheral neuropathy, as reported in previous studies (Lehning et al., 2003; Lai et al., 2017).
Immunohistochemical results in the present study revealed that the densities of DCX+ cells and TUC4+ cells in the SGZ/GCL were decreased in the 20-mg/kg AA group, whereas the densities of GFAP+ cells, SOX2+ cells, TBR2+ cells, and NeuN+ cells were not different in the AA-treated groups, compared with the control group. In accordance with the immunohistochemical results, the transcript levels of Dcx and Dpysl3, the gene encoding TUC4, in the DG were decreased in the 10- and 20-mg/kg AA groups. Since TBR2 is expressed mainly in type-2b NPCs, DCX is expressed mainly in type-2b and type-3 NPCs and immature granule cells, TUC4 is expressed mainly in immature granule cells, and NeuN is expressed mainly in immature and mature granule cells (Hodge et al., 2008; Knoth et al., 2010; Kempermann et al., 2015), the results suggest that AA decreased the densities of type-3 NPCs and immature granule cells. On the other hand, the density of PCNA+ cells did not change in the SGZ in AA-treated groups. Although PCNA is a general-purpose cell proliferation marker, it has been reported that PCNA is produced not only in DNA replication but in DNA damage and PCNA accumulates at sites of DNA damage (Essers et al., 2005). AA and its metabolite, glycidamide are genotoxic compounds and form adducts with DNA (Manière et al., 2005; Klaunig, 2008; Rifai and Saleh, 2020), and it has been reported that a single dose of AA at the similar dose level with the highest dose in the present study causes DNA damage and forms DNA adducts with glycidamide in rat brain (Manière et al., 2005). Therefore, the changes in PCNA+ cells may reflect changes in DNA damage as well as cell proliferation. Considering that AA did not change the density of immunoreactive SGZ cells for γ-H2AX, a marker of DNA double-strand breaks (He et al., 2016), in the present study, AA-induced DNA damage in the brain may not lead to DNA double-strand breaks. Therefore, we could not rule out the possibility of suppressed proliferation activity in the SGZ in AA-treated groups. We also observed a decrease in the transcript level of Nes, the gene encoding nestin, and an increase in the transcript level of Rbfox3, the gene encoding NeuN. Nestin is a marker for type-1 NSCs and type-2a and type-2b NPCs and NeuN is a marker for mature neurons (Kempermann et al., 2015), but the densities of GFAP+ cells, SOX2+ cells, TBR2+ cells in the SGZ, NeuN+ granule cells in the GCL, or NeuN+ interneurons in the DG hilus did not change as aforementioned. Although examination of single time point can be misleading with respect to protein synthesis and transcript levels do not always reflect protein expression levels, these results might suggest changes in expression levels per single cell. Since Rbfox3 is a postmitotic neuron marker and is involved in the promotion of the differentiation of postmitotic neurons (Kim et al., 2013), the increase in the Rbfox3 transcript level in the present study might be observed in mature granule cells rather than interneurons as a compensatory response for the decrease in the densities of type-3 NPCs and immature granule cells by AA.
Neurite outgrowth is a complex process involving various molecules, such as cell adhesion molecules, cytoskeletons, and neurotrophic factors (Fujitani et al., 2004; De Vincenti et al., 2019; Parcerisas et al., 2021). In this study, we found that the neurite length of DCX+ cells was decreased in the 20-mg/kg AA group, compared with the control group. Among DCX+ cell populations, those extending neurites are identified as immature granule cells (Knoth et al., 2010; Kempermann et al., 2015). Real-time RT-PCR also revealed the decreases in the transcript levels of Ncam2 in the 10- and 20-mg/kg AA groups and Nrep in the 20-mg/kg AA group. Since neural cell adhesion molecule 2 (NCAM2) and neuronal regeneration related protein (NREP, also known as P311) are known to be involved in neuronal migration and neurite outgrowth (Fujitani et al., 2004; Parcerisas et al., 2021), these results suggest that AA suppressed the neurite outgrowth of immature granule cells via the downregulation of Ncam2 and Nrep. The decrease in the transcript level of Nes might also be related to the impairment of neurite outgrowth of the DCX+ cells since it has been reported that nestin forms a complex with DCX to regulate the growth cone morphology in developing cortical neurons (Bott et al., 2020). In our previous study, exposure to glycidol, a neurotoxicant causing distal axonopathy similar to that induced by AA, also resulted in gait abnormality and a decrease in the densities of type-3 NPCs and immature granule cells and suppressed the gene expressions of Ncam2, suggesting that AA share a similar target mechanism with glycidol for disruption of hippocampal neurogenesis (Akane et al., 2013, 2014).
In addition to its role in neuronal migration and neurite outgrowth, NCAM2 is known to be involved in synapse formation (Parcerisas et al., 2021). In the present study, the transcript levels of synaptic plasticity-related IEGs, such as Arc, Fos, Egr1, and Ptgs2, the gene encoding COX2 (Yagami et al., 2016; Duclot and Kabbaj, 2017; Jaworski et al., 2018; Nikolaienko et al., 2018), tended to be decreased in the DG of the 20-mg/kg AA group, although the differences were not statistically significant. The densities of ARC+ cells and EGR1+ cells also tended to be decreased in this group. These results suggest that AA decreased synaptic plasticity in the DG. Since functional synapses begin to be made at the dendrites of newborn granule cells after neurite outgrowth and migration (Toni and Schinder, 2015), impairment of neurite outgrowth also affects subsequent synapse formation (Guzmán-Salas et al., 2022). Because AA impaired neurite outgrowth of immature granule cells in the present study, AA might decrease synaptic plasticity through the suppression of synapse formation by the impairment of neurite outgrowth. We also observed decreases in the transcript levels of Bdnf in the 10-mg/kg AA group and Ntrk2, the gene encoding tropomyosin receptor kinase B (TrkB), in the 20-mg/kg AA group. Since BDNF-TrkB signaling contributes to dendritic and spine growth during the differentiation of immature granule cells (De Vincenti et al., 2019), AA might impair neurite outgrowth and synapse formation of immature granule cells by suppressing BDNF-TrkB signaling. Thus, AA decreased the late-stage progenitor cells and immature granule cells and impaired neurite outgrowth as well as subsequent synaptic plasticity.
GABAergic interneurons in the DG widely regulate hippocampal neurogenesis from differentiation and maturation to synaptic integration (Catavero et al., 2018). In the present study, we observed a decrease in the transcript level of Reln in the DG of the 20-mg/kg AA group, while the density of RELN+ cells in the DG hilus of the corresponding animals was not changed. Although it is necessary to discuss carefully the obtained results due to the data of gene expression change at a single time point, this result might suggest that the expression level of Reln was decreased per RELN-expressing cell. In the DG, RELN is expressed in GABAergic interneurons, and its main role is in migration and the correct positioning of developing neurons (Pesold et al., 1998). The involvement of RELN in adult neurogenesis through its support of not only neuronal migration, but also neurite outgrowth and subsequent synapse formation was recently reported (Faini et al., 2021). These findings suggest that Reln downregulation might be causally related to the decrease in the densities of type-3 NPCs and immature granule cells by AA in the present study.
CHRNB2+ cells in the DG hilus are GABAergic interneurons (Son and Winzer-Serhan, 2008). In the present study, AA at 20 mg/kg increased the density of CHRNB2+ cells in this region. CHRNB2 is a nicotinic acetylcholine receptor subunit and is involved in neuroprotection (Zoli et al., 1999). It has been reported that AA increases acetylcholinesterase activity in cholinergic synapses in rats and mice (Kopanska et al., 2018), suggesting the suppression of cholinergic neurotransmitter signaling. Therefore, the increase in CHRNB2+ cells in the present study was considered to be a compensatory response of GABAergic interneurons for the suppression of cholinergic neurotransmitter signaling induced by AA.
Regarding changes in glial cell lineage subpopulations, AA at 20 mg/kg decreased the density of GFAP+ astrocytes in the DG hilus accompanying decrease in the Gfap transcript level in the DG in the present study. In addition, we found that the transcript levels of Cspg4 and Pdgfra (marker genes of oligodendrocyte progenitor cells; Gallo and Deneen, 2014), and Mag and Mal (genes encoding myelin component proteins; Schaeren-Wiemers et al., 2004; Nguyen et al., 2009) were decreased in the DG of the 10- and 20-mg/kg AA groups. These results may suggest suppressed gliogenesis, while further study will be necessary to be addressed on this point.
In the present study, we estimated the number of immunoreactive cells per unit length of the SGZ or unit area of the DG hilus. On the other hand, the absolute brain weight was decreased in the AA-treated groups as aforementioned. Therefore, the normalized cell number as density may be overestimated if brain volume is decreased. In such case, it may be recommended to estimate the total cell number of immunoreactive cells in specific brain region without applying normalization for comparison. However, it is difficult to judge whether the total cell number or the density of cells are critical for disease conditions. Because the purpose of the normalization was to exclude interindividual variability, we had to estimate the cell density in the present study. We also did not observe any changes in the length of the SGZ and area of the DG hilus in AA-treated groups, suggesting that cell density may not be overestimated even if the total cell number is rather critical than the cell density for development of diseases.
As mentioned above, exposure to AA during the young-adult stage decreased the densities of type-3 NPCs and immature granule cells in the present study. In the previous study, we also observed decreased density of immature granule cells after developmental AA exposure in rats (Ogawa et al., 2012), suggesting that the toxicity pattern of AA in hippocampal neurogenesis is similar between developmental exposure and young-adult stage exposure, as observed with other adult neurotoxicants such as cuprizone and glycidol (Akane et al., 2013, 2014; Abe et al., 2015a, 2015b). In contrast, developmental neurotoxicants, such as 6-propyl-2-thiouracil, valproic acid, and N-methyl-N-nitrosourea, have different targets in neurogenesis during developmental and young-adult stage exposure (Shiraki et al., 2016a, 2016b; Watanabe et al., 2017a, 2017b, 2019). Differences in the sensitivity of embryo-fetal neurogenesis and postnatal hippocampal neurogenesis, including neurogenic regulatory systems, to neurotoxicants may be responsible for these conflicting observations. Developmental neurotoxicants exposed during the developmental stage may disrupt both embryo-fetal neurogenesis and postnatal neurogenesis, including hippocampal neurogenesis. On the other hand, even with developmental exposure, adult neurotoxicants might only affect postnatal neurogenesis, primarily targeting differentiation processes and/or regulatory systems of neurogenesis.
At the seventy-second meeting of the Joint FAO/WHO Expert Committee on Food Additives, the committee concluded that based on the epidemiological studies, the estimated average AA exposure in humans was 0.001 mg/kg body weight per day for the general population and 0.004 mg/kg body weight per day for consumers with high dietary exposure (Joint FAO/WHO Expert Committee on Food Additives, 2011). The committee also concluded that the no-observed adverse effect level (NOAEL) was determined to be 0.2 mg/kg body weight per day for the ultrastructural changes in peripheral nerves in rats. In this study, 10 mg/kg body weight per day was determined to be the NOAEL based on the aberrations in hippocampal neurogenesis by means of immunohistochemical analysis, which was a 2,500- to 10,000-fold margin from the estimated average intake in humans.
In conclusion, we have shown that AA exposure for 28 days decreased the densities of type-3 NPCs and immature granule cells and impaired neurite outgrowth, involving suppression of signaling of NCAM2, NREP, RELN, and BDNF-TrkB. Thus, AA reduced late-stage progenitor cells and immature granule cells in the adult hippocampal neurogenesis after young-adult stage exposure, showing a pattern similar to that observed for developmental exposure.
We thank the general toxicity group (Tomoko Ishihara, Aya Izaki, and Yusuke Nishioka) for dosing and other in-life examination, Kohga Hirano for technical advice about real-time RT-PCR, and Tomoya Hasegawa for preparation of the histopathological sections.
Conflict of interestThe authors declare that there is no conflict of interest.