Abstract
A reactive oxygen species (ROS) assay has been widely used for photosafety assessment; however, the phototoxic potential of complex materials, including plant extracts, essential oils, and functional polymers, is unevaluable because of their undefined molecular weights. The present study was undertaken to modify the ROS assay protocol for evaluating phototoxic potentials of those materials with use of their apparent molecular weight (aMw). On preparing sample solutions for the ROS assay, aMw ranging from 150 to 350 was tentatively employed for test substances. The modified ROS assays were applied to 45 phototoxic and 19 non-phototoxic substances, including 44 chemicals and 20 complex materials (plant extracts) for clarification of the predictive performance. Generation of ROS from photo-irradiated samples tended to increase as aMW grew, resulting in the largest number of false-positive predictions at aMW of 350. Some false-negative predictions were also observed when aMW was set at 200 or less. At aMw of 250, all tested phototoxic substances could be correctly identified as photoreactive with no false-negative predictions. Based on these observations, aMw of 250 was found to be suitable for the ROS assay on complex materials, and the sensitivity, specificity, and positive and negative predictivity for the proposed ROS assay were calculated to be 100, 52.6, 83.3, and 100%, respectively. Thus, the proposed approach may be efficacious for predicting phototoxic potentials of complex materials and contribute to the development of new products with a wide photosafety margin.
INTRODUCTION
Drug-induced phototoxicity refers to abnormal skin reactions, including photoirritant and photoallergic events, caused after topical or systemic administration of chemicals, and can be induced by several chemicals such as drugs, cosmetics, and food ingredients (Moore, 2002). As an in chemico photoreactivity assessment tool, the reactive oxygen species (ROS) assay was developed to predict the phototoxic risk of new drug entities (Onoue and Tsuda, 2006; Onoue et al., 2008a, 2008b). The ROS assay has been recommended as a photosafety evaluation method in the International Council for Harmonization of Technical Requirements of Pharmaceuticals for Human Use (ICH) S10 guidelines (ICH, 2014) and adopted as the Organization for Economic Co-operation and Development (OECD) test guidelines for photoreactivity/photosafety assessment of various industrial materials (Onoue et al., 2013b, OECD, 2019). In the development of cosmetic products, complex materials, including plant extracts and polymers, have been widely used. Since the ROS assay was originally designed to evaluate phototoxic potentials of chemicals at a fixed concentration, it is still challenging to apply the ROS assay to complex materials without a defined molecular weight. Therefore, partial modification of the assay protocol would be required to improve applicability of the ROS assay for complex ingredients.
In the validated protocol of the ROS assay (Onoue et al., 2013a, 2014), a molar concentration is employed as a unit of the test chemical concentration, and the photoreactivity of test chemicals is judged on the basis of ROS generation from test chemicals at a concentration of 200 μM on exposure to simulated sunlight. In our previous study, the mass concentration (μg/mL) was tentatively employed as the concentration unit, and then the ROS assay was applied to 11 phototoxic and 9 non-phototoxic plant extracts or oils at a chemical concentration of 50 μg/mL (Nishida et al., 2015). All the phototoxic chemicals tested were correctly identified as photoreactive, and the phototoxic potential of complex materials was also partially evaluable in the ROS assay at the concentration of 50 μg/mL. ROS generation from photo-irradiated chemicals generally occurs in a concentration-dependent manner (Onoue and Tsuda, 2006), so that further optimization and verification would be required to strengthen the rationale for setting the concentration for the ROS assay on complex materials.
The present study was undertaken to modify the protocol of the ROS assay to use an optimum concentration facilitating reliable prediction of the photosafety of materials with no defined molecular weights. In this study, 45 phototoxic and 19 non-phototoxic samples, including 44 chemicals and 20 complex materials, were selected as model samples and evaluated by the ROS assay in which the apparent molecular weight (aMW) of test samples was tentatively defined ranging from 150 to 350 (equivalent to the ROS assay at the concentration of 30 to 70 μg/mL). To clarify the prediction capacity of the ROS assay under each condition, specificity, sensitivity, and positive and negative predictivity were calculated.
MATERIALS AND METHODS
Materials
Plant materials
Bergamot oil (Sigma, 36), celery seed oil (38), cumin seed oil (39), lemon oil (41), lime oil (42), tagetes oil (45), verbena oil (46), carrot oil (56), ginger oil (58), and lemongrass oil (59) were obtained from Sigma-Aldrich Japan (Tokyo, Japan). Bergamot oil (Wako, 37), grapefruit oil (40), corn oil (57), olive oil (60), rape seed oil (62), safflower oil (63), and soybean oil (64) were purchased from Fujifilm-Wako Pure Chemical Industries (Osaka, Japan). Parsley oil (43), St. John’s wort powder (44), and orange oil (61) were obtained from Nacalai Tesque (Kyoto, Japan).
Chemicals and reagents
Dimethyl sulfoxide (DMSO, 49), p-nitrosodimethylaniline, imidazole, nitroblue tetrazolium (NBT), quinine HCl (1), 4-methyl-7-ethoxycoumarin (3), 7-methoxycoumarin (5), 8-methoxypsoralen (6), acridine (7), diclofenac Na (11), hexachlorophene (16), indomethacin (19), ketoprofen (21), methyl β-naphthyl ketone (22), piroxicam (29), promethazine HCl (31), sulfanilamide (34), tetracycline HCl (35), 1,3-butylene glycol (47), 2-propanol (48), ethanol (50), and glycerin (51) were obtained from Fujifilm-Wako Pure Chemical Industries. Sulisobenzone (2), bithionol (9), doxycycline HCl (12), enoxacin (13), furosemide (14), glibenclamide (15), hydrochlorothiazide (16), ibuprofen (18), isoniazid (20), mequitazine (24), nalidixic acid (25), octyl dimethyl PABA (26), ofloxacin (27), omadine Na (28), prochlorperazine maleate (30), pyridoxine HCl (32), sparfloxacin (33), lactic acid (52), and penicillin G (54) were purchased from Sigma-Aldrich Japan. Benzophenone (8), lauric acid (53), and propylene glycol (55) were obtained from Junsei Chemical Co. (Tokyo, Japan), and 6-methylcoumarin (4) was purchased from Nacalai Tesque. Chlorpromazine HCl (10) and methyl-N-methylanthranilate (23) were purchased from Tokyo Chemical Industry (Tokyo, Japan).
ROS assay
Irradiation conditions
Simulated sunlight was used to irradiate samples for ROS assay systems by applying Atlas Suntest CPS plus (Atlas Material Technology LLC, Chicago, IL, USA) equipped with a xenon arc lamp (1500 W) and a UV special filter to adapt the spectrum of the artificial light source to that of natural daylight. The irradiation test was carried out at 25°C with an irradiance of ca. 2.0 mW/cm2 (300–800 nm).
Assay procedures
The ROS assay was carried out according to the validated protocol with minor modifications (Onoue et al., 2013a, 2014). To evaluate the photoreactivity of tested substances by the ROS assay when the average molecular weights of test compounds were tentatively defined as 150 to 350 (equivalent to the ROS assay at the concentration of 30 to 70 μg/mL), tested substances were dissolved in DMSO at 1.5, 2.0, 2.5, 3.0, and 3.5 mg/mL and these were used as stock solutions. Singlet oxygen generated from samples in 20 mM sodium phosphate buffer (NaPB; pH 7.4) after irradiation with light was measured by monitoring the bleaching of p-nitrosodimethylaniline at 440 nm in the presence of imidazole as a selective acceptor of singlet oxygen. Test substances (30, 40, 50, 60, or 70 μg/mL), p-nitrosodimethylaniline (50 μM), and imidazole (50 μM) in 20 mM NaPB were mixed in a tube. Aliquots of 200 μL of the samples were transferred into wells of a plastic 96-well plate (Asahi Glass Co., Ltd., Tokyo, Japan; code number 3881-096; clear, untreated, flat-bottomed) in triplicate and checked for precipitation before light exposure. The absorbance at 440 nm of each well was measured with a SAFIRE unit (TECAN, Männedorf, Switzerland). The plate was fixed in a quartz reaction container with a quartz cover (Ozawa Science, Aichi, Japan), and then irradiated with simulated sunlight for 1 hr. After agitation on a plate shaker, the absorbance at 440 nm was measured. For the determination of superoxide anions, reduction of NBT by irradiated test substances was monitored in terms of the increase of absorbance at 560 nm. Test substances (30, 40, 50, 60, or 70 μg/mL) and NBT (50 μM) in 20 mM NaPB were mixed in a tube, and the absorbance at 560 nm was measured in the same manner as described for singlet oxygen determination. To avoid false predictions due to spectral interference at 440 nm for singlet oxygen determination and at 560 nm for detection of superoxide generation, experimental controls in which test substances alone were exposed to simulated sunlight were conducted, and the control values were subtracted from sample values.
Criteria for data acceptance and judgment in the ROS assay
Photoreactivity of samples (30, 40, 50, 60 and 70 μg/mL) was evaluated according to the same criteria as used in the validated protocol. The photoreactivity of each tested substance should be judged as follows, based on the mean value of triplicate determinations in the ROS assay: (i) positive for singlet oxygen (ΔA440 nm × 103): 25 or more; and/or superoxide (ΔA560 nm × 103): 20 or more, or (ii) negative for singlet oxygen (ΔA440 nm × 103): less than 25, and superoxide (ΔA560 nm × 103): less than 20. The final determination should be made as follows: (i) positive: above the threshold level for either singlet oxygen or superoxide; or (ii) negative: below the threshold level for both singlet oxygen and superoxide.
RESULTS
Experimental design
Chemical selection
On the basis of the previous studies and literature searches (Onoue et al., 2017, 2009; Onoue and Tsuda, 2006; Onoue et al., 2013b, EFSA, 2012; EMA, 2021; IFRA, 2021; Nishida et al., 2015), 20 plant extracts and 44 chemicals were selected as model substances (Table 1). To clarify the impact of MW of test materials on prediction accuracy of the ROS assay, chemicals with various MW ranging from 60 to 606 were used as test chemicals. These materials have been topically or systemically applied to humans as drugs, cosmetics, or food ingredients, and clinical or experimental phototoxic/non-phototoxic information has also been reported. Two chemicals and 8 plant extracts were employed as model phototoxic substances referring to the globally-accepted International Fragrance Association (IFRA) standards (IFRA, 2021), and 32 chemicals and 3 plant extracts were also selected according to the previous reports (Onoue et al., 2017, 2009; Onoue and Tsuda, 2006; Onoue et al., 2013b, EFSA, 2012; EMA, 2021). As reported previously (Onoue et al., 2013b, Nishida et al., 2015), 10 chemicals and 9 plant extracts were defined as non-phototoxic materials. Thus, 44 chemicals, including 34 phototoxic and 10 non-phototoxic chemicals, and 20 complex materials (plant extracts), including 11 phototoxic and 9 non-phototoxic materials, were selected for the present study.
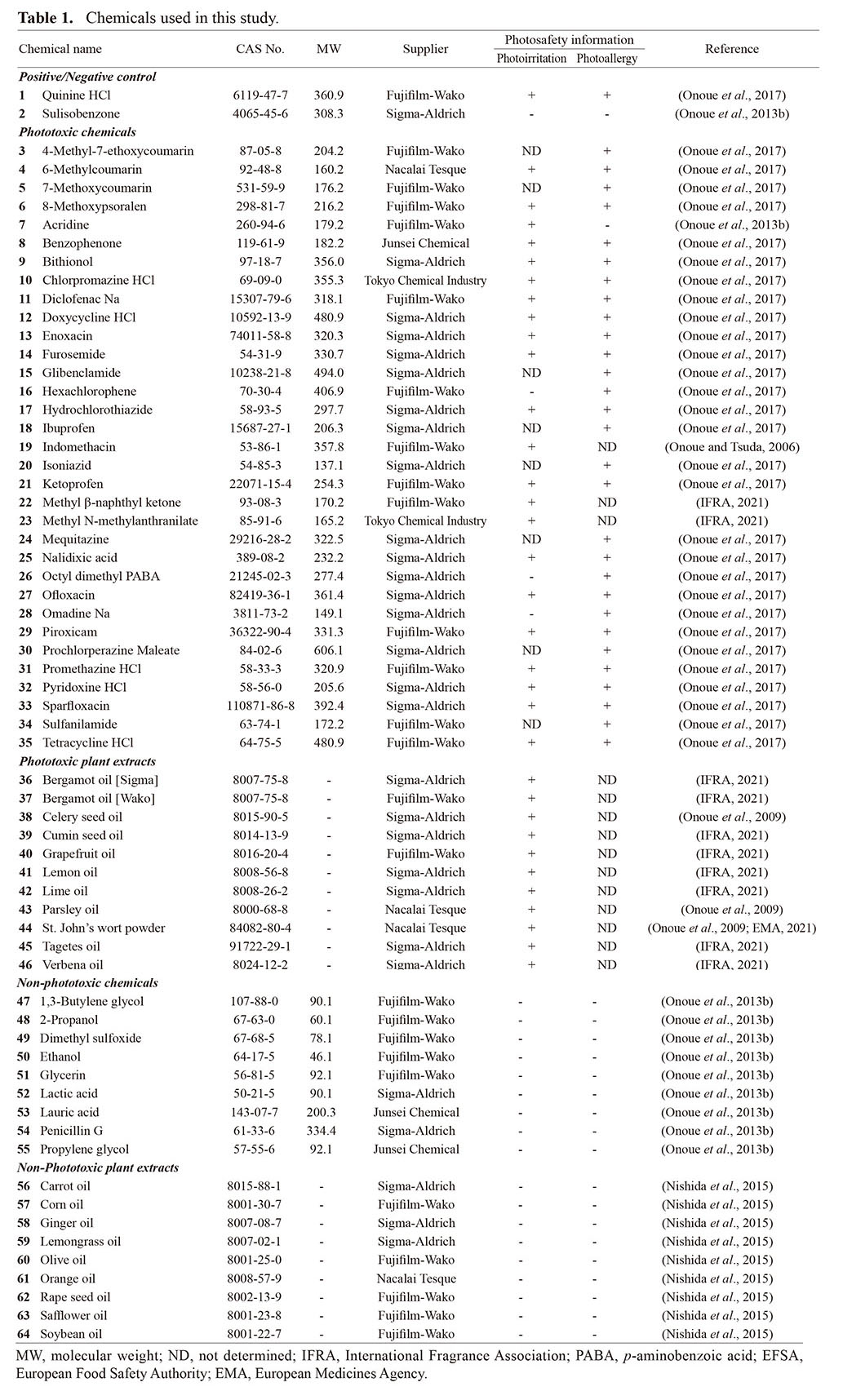
Table 1. Chemicals used in this study.
 Assay concentration of test substance
Assay concentration of test substance
The concentration of test chemicals for the ROS assay were defined in accordance with OECD test guidelines and a previous report (Nishida et al., 2015; OECD, 2019). For reliable photosafety prediction of the complex materials with no defined molecular weight, the concentrations of test material were optimized with the use of aMw. According to a previous report (Nishida et al., 2015), the mode value in the average molecular weight distribution of phototoxic chemicals was found to range from 200 to 300. Therefore, for comparison, the ROS assay was conducted with the use of aMw ranging from 150 to 350, in increments of 50, to identify the appropriate concentration range of materials with an undefined molecular weight.
Outcomes from ROS assay
Under the optimized conditions, the ROS assay was carried out to evaluate the photoreactivity of 34 phototoxic chemicals, 11 phototoxic plant materials, 10 non-phototoxic chemicals, and 9 non-phototoxic plant materials (Table 2). For the ROS assay, quinine (1) and sulisobenzone (2) were treated as positive and negative controls, respectively. In the present study, no significant generation of ROS was observed for sulisobenzone (2) under any conditions (aMw: 150–350). In contrast, quinine (1) was found to be highly photoreactive, as evidenced by potent ROS generation in a sample concentration manner. Even at the lowest concentration tested (30 μg/mL at aMw of 150), quinine (1) exhibited significant generation of singlet oxygen (ΔA440 nm × 103: 347) and superoxide (ΔA560 nm × 103: 151).
Table 2. ROS data for 20 plant extracts and 44 chemicals.
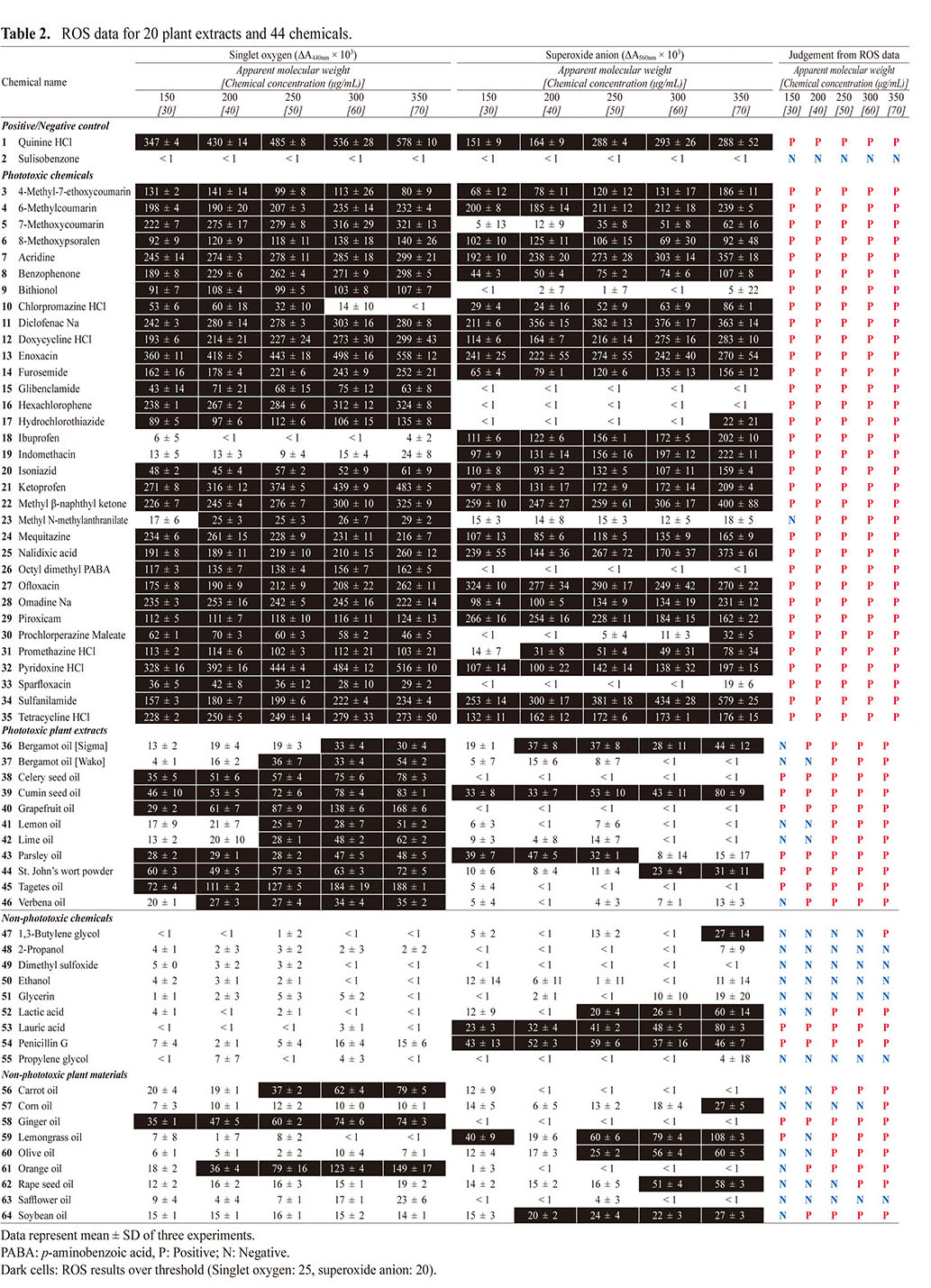
At aMw from 150 (equivalent to 30 μg/mL) to 350 (equivalent to 70 μg/mL), all phototoxic chemicals (3–35) except for methyl N-methylanthranilate (23) showed a predicted significant ROS generation under exposure to simulated sunlight, and they could be correctly identified as photoreactive on the basis of the defined threshold {25 (ΔA440 nm × 103) for singlet oxygen and 20 (ΔA560 nm × 103) for superoxide} (Onoue et al., 2008a). In general, ROS generation from photo-irradiated chemicals tends to increase in a concentration-dependent manner. As compared with other phototoxins, methyl N-methylanthranilate (23) was relatively less photoreactive, and the outcome from the ROS assay at aMw of 150 was a false-negative prediction for this phototoxin. Interestingly, there appeared to be very limited generation of singlet oxygen from photo-irradiated chlorpromazine (10) at aMw of 300 and 350, although it showed potent generation at aMw of below 250. In theory, photochemical properties of chemicals can be variable depending on microenvironment such as pH, heat, solvent system, and inter-/intra-molecular interaction (Onoue et al., 2008c). A previous study also demonstrated that successive increase of the solution concentration led to significant transition in UV/VIS-spectral patterns of some chemicals (Tong et al., 2020). The major reason for this data discrepancy is still unclear; however, transition of photochemical properties at higher concentration might explain them in part. Further investigation would be needed to clarify the possible reasons for data discrepancy, and it might lead to the reliable photosafety prediction.
In addition to the phototoxic chemicals (3–35), all the phototoxic plant materials (36–46) tended to show generation of ROS when exposed to the simulated sunlight, while generation of superoxide anions was negligible for some samples. It should be noted that bergamot oil from two different sources exhibited inconsistent outcomes of the ROS assay, possibly due to different specifications of these two extracts. Among the phototoxic plant materials tested (36–46), 5 samples: bergamot oil [Sigma] (36), bergamot oil [Wako] (37), lemon oil (41), lime oil (42), and verbena oil (46), were partially judged to be non-photoreactive based on the ROS assay at aMw of 200 (equivalent to 40 μg/mL) or less. In order not to provide false-negative predictions for plant materials and other complex materials, the ROS assay should be carried out with aMw of 250 (equivalent to 50 μg/mL) or higher.
With regard to the ROS assay on non-phototoxic samples (47–64), they were correctly identified as non-photoreactive; however, false-positive predictions were given for some samples, including lauric acid (53), penicillin G (54), ginger oil (58), and lemongrass oil (59). Higher false-positive predictions tended to be seen as aMw increased, and the ROS assay at aMw of 350 (equivalent to 70 μg/mL) resulted in false-positive judgments for all non-phototoxic plant extracts tested (56–64), except for safflower oil (63). Based on these findings, aMw of 300 (equivalent to 60 μg/mL) or less might be suitable for reliable photosafety prediction of complex materials.
Prediction capacity and suitable apparent molecular weight
Using the defined criteria for the ROS assay (Onoue et al., 2008a), most phototoxic chemicals and plant extracts were correctly identified as phototoxic at all aMw (150–350), whereas 3 and 6 phototoxins were in the subthreshold region at aMw of 150 and 200, respectively (Fig. 1). Higher negative predictivity was suggested for the ROS assay at lower aMw, although false-positive results were produced at higher aMw. Prediction capacity of the ROS assay at various aMw is summarized in Table 3. Positive predictivity of the ROS assay ranged from 78.9 to 90.7%, and negative predictivity was very high at aMw of 250–350. Notably, at aMw of 250–350, the phototoxic chemicals and plant materials could be captured with 100% sensitivity, resulting in no false-negative predictions. Individual specificities for ROS assays at each aMw were calculated to be between 36.8–78.9%. For exploratory and regulatory purposes, the ROS assay can be applied for early photosafety screening; therefore, the production of false-negative results should be avoided for the development of new materials with a wide photosafety margin. In this context, aMw of 250 (equivalent to 50 μg/mL) would be preferable for the ROS assay of complex materials, in which both no false-negatives and suppression of false-positives could be achieved.
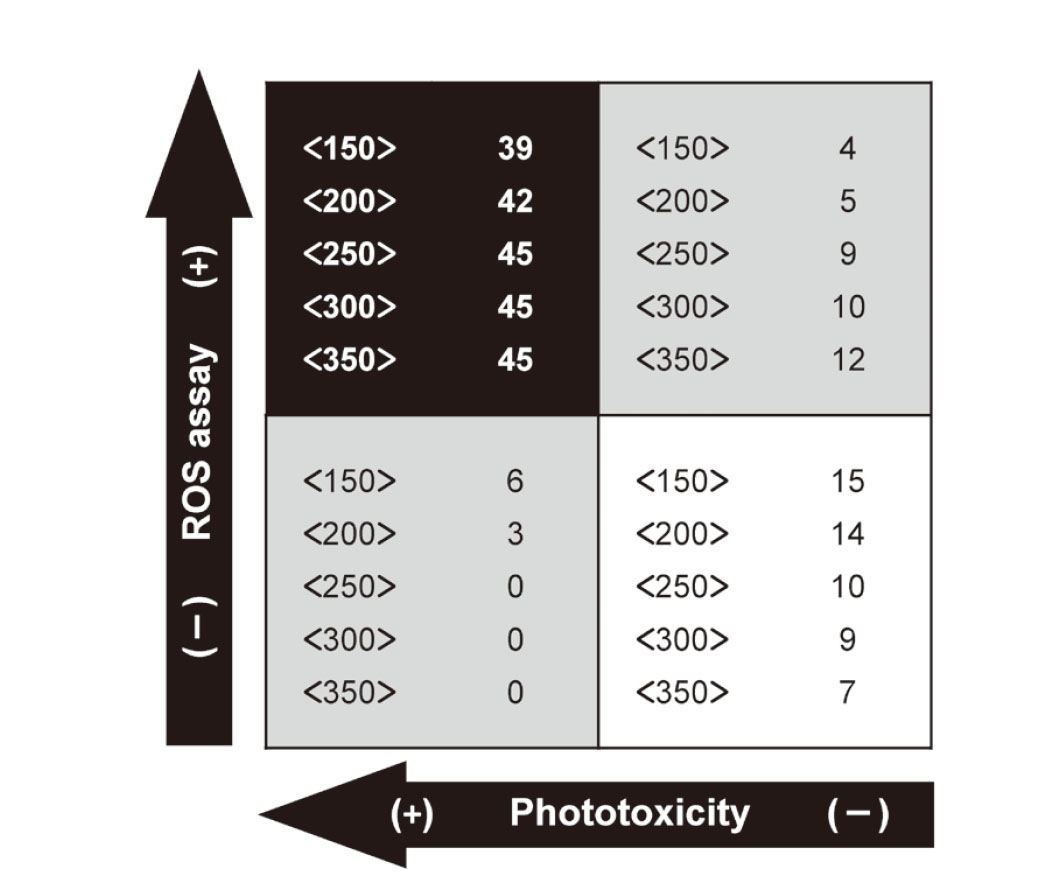
Table 3. Predictive capacity of proposed photosafety screening.

DISCUSSION
The major findings of the present study are that: i) the ROS assay could be used to identify the generation of ROS from photo-irradiated materials with undefined molecular weights, and ii) phototoxic potentials of these complex materials could be correctly predicted with no false-negatives by the ROS assay when their aMw was set at 250 (equivalent to 50 μg/mL). The obtained results may be useful for in chemico phototoxic evaluations of complex materials such as plant extracts, essential oils, and functional polymers.
This study was aimed to verify the applicability of the ROS assay for complex materials with undefined molecular weights, and the outcomes of the present study highlight some points to consider regarding ROS assay-based photosafety testing. Firstly, phototoxic plant materials tended to be judged as negative by the ROS assay at lower concentrations when compared with phototoxic chemicals. Plant extracts can be defined as a mixture of multiple phytochemicals, and not all phytochemicals may have potent phototoxic potential. Because of the limited assay sensitivity, a higher concentration of plant materials may be required for reliable evaluation in the ROS assay. However, it should be noted that the ROS assay at higher concentrations might lead to false-positive predictions, resulting in poor assay specificity and impaired reliability. In addition to the possible issues concerning prediction capacity, some plant extracts at higher concentrations might be unevaluable in the ROS assay because of their poor solubility/dispersibility in the assay buffer. There might be precipitation of hydrophobic components in the ROS assay at high sample concentrations, and then it would be challenging to detect ROS generation precisely, owing to marked spectral interference at 440 and 560 nm (Onoue et al., 2008c). Secondly, generations of ROS from non-phototoxic plant materials tended to exceed those from non-phototoxic chemicals, leading to false-positive predictions for plant extracts. In theory, all photochemically active substances can be assessed by the ROS assay since the principle of the ROS assay is to detect radical species through type I and/or II photochemical reactions by photo-irradiated substances. In this context, there is the probability that, as well as phototoxins, some photolabile substances would also be recognized as phototoxic by the ROS assay. This may be a reason why the ROS assay sometimes leads to false-positive predictions, and having some false-positive predictions for plant extracts is not surprising as they contain a number of photosensitive and photolabile components.
For more accurate prediction of the phototoxic risk of complex materials, it is necessary to fix the appropriate concentration of test samples in consideration of the above characteristics. From the ROS data on 64 samples in the present study, it was suggested that good prediction accuracy with no false-negative prediction could be achieved by the ROS assay when aMw of samples was set at 250. These observations were mostly in agreement with our previous study (Nishida et al., 2015), providing further support for the prediction performance of the ROS assay with aMw of 250 (equivalent to 50 μg/mL). However, the specificity of the ROS assay under this assay condition was found to be 52.6%; thus, false-positive predictions were given for about half of non-phototoxic chemicals upon the ROS assay alone. To improve the reliability of the photosafety testing of complex materials, as well as their photoreactivity, the pharmacokinetic behavior of major components should also be assessed with a focus on absorption, distribution to UV-exposed tissues, and skin penetration and retention. In our previous investigation, a number of potent phototoxins in humans exhibited significant permeation and accumulation in the skin, and even photoreactive chemicals with low transdermal absorbability tended to show a low phototoxic potential in animal testing (Seto et al., 2012, 2020; Iyama et al., 2020, 2019). Thus, the combined use of the ROS assay at aMw of 250 and a pharmacokinetic study would be efficacious to make reliable photosafety predictions on the complex materials.
The ROS assay system even at aMw of 250 still has some limitations in terms of applicability for test substances. Firstly, phototoxic predictions for plant materials may be influenced by the product lot and/or its specification. The ROS assay at aMw of 150–350 demonstrated that the photoreactivity of bergamot oil purchased from Wako Pure Chemicals (36) was likely to be higher than that from Sigma-Aldrich (37). In general, components in plant extracts may vary depending on their harvest time, place, and extraction process. The detailed reasons for the data discrepancy remain unclear; however, possible differences in amounts of phototoxic components might explain the variation in photoreactivity of the two bergamot oils. Secondly, a relatively high solubility of plant extracts (50 μg/mL) in the assay buffer would be required for the ROS assay at aMw of 250. Some plant extracts might be highly lipophilic; in particular, when they are manufactured with subcritical/supercritical fluid extraction, ethanolic extraction, or extraction with other organic solvents. Due to poor solubility, some of them would be unevaluable in the ROS assay because of marked precipitation of components, high turbidity, and thereby analytical interference. To overcome this limitation, our group previously proposed the micellar ROS (mROS) assay for poorly-soluble chemicals (Seto et al., 2013), and the mROS assay would be available for complex materials as the micelles can enhance the apparent solubility of lipophilic components. However, to apply the mROS assay, further investigations will be needed to define the threshold for photosafety evaluation and fix appropriate aMw of complex materials.
In conclusion, the ROS assay system at aMw of 250 (equivalent to 50 μg/mL) exhibited a good predictive capacity in photosafety testing of complex materials with an undefined molecular weight. Photosafety assessment employing the proposed ROS assay would contribute to the development of new drugs, cosmetics, and foods with a wide photosafety margin.
ACKNOWLEDGMENT
This work was supported in part by JSPS KAKENHI [Grant-in-Aid for Scientific Research(C) (No. 20K07158: S. Onoue; and No. 20K07180: H. Sato)].
Conflict of interest
The authors declare that there is no conflict of interest.
REFERENCES
- EFSA. (2012): Compendium of botanicals reported to contain naturally occuring substances of possible concern for human health when used in food and food supplements. EFSA J., 10, 2663.
- EMA. (2021): Assessment report on Hypericum perforatum L., herba 2nd Draft – Revision 1. ((HMPC), C.o.H.M.P., ed.).
- ICH. (2014): ICH guideline S10, Guidance on photosafety evaluation of pharmaceuticals, International Council on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use.
- IFRA (2021): IFRA Standards-50th Amendment.
- Iyama, Y., Sato, H., Seto, Y. and Onoue, S. (2019): A new photosafety screening strategy based on in chemico photoreactivity and in vitro skin exposure for dermally-applied chemicals. Toxicol. Lett., 317, 45-52.
- Iyama, Y., Sato, H., Seto, Y. and Onoue, S. (2020): Strategic photosafety screening system consisting of in chemico photoreactivity and in vitro skin exposure for quinolone derivatives. Eur. J. Pharm. Sci., 146, 105257.
- Moore, D.E. (2002): Drug-induced cutaneous photosensitivity: incidence, mechanism, prevention and management. Drug Saf., 25, 345-372.
- Nishida, H., Hirota, M., Seto, Y., Suzuki, G., Kato, M., Kitagaki, M., Sugiyama, M., Kouzuki, H. and Onoue, S. (2015): Non-animal photosafety screening for complex cosmetic ingredients with photochemical and photobiochemical assessment tools. Regul. Toxicol. Pharmacol., 72, 578-585.
- OECD. (2019): OECD guideline for testing of chemicals, 495, Ros (Reactive Oxygen Species) Assay for Photoreactivity.), The Organisation for Economic Co-operation and Development.
- Onoue, S., Hosoi, K., Toda, T., Takagi, H., Osaki, N., Matsumoto, Y., Kawakami, S., Wakuri, S., Iwase, Y., Yamamoto, T., Nakamura, K., Ohno, Y. and Kojima, H. (2014): Intra-/inter-laboratory validation study on reactive oxygen species assay for chemical photosafety evaluation using two different solar simulators. Toxicol. In Vitro, 28, 515-523.
- Onoue, S., Hosoi, K., Wakuri, S., Iwase, Y., Yamamoto, T., Matsuoka, N., Nakamura, K., Toda, T., Takagi, H., Osaki, N., Matsumoto, Y., Kawakami, S., Seto, Y., Kato, M., Yamada, S., Ohno, Y. and Kojima, H. (2013a): Establishment and intra-/inter-laboratory validation of a standard protocol of reactive oxygen species assay for chemical photosafety evaluation. J. Appl. Toxicol., 33, 1241-1250.
- Onoue, S., Igarashi, N., Yamada, S. and Tsuda, Y. (2008a): High-throughput reactive oxygen species (ROS) assay: an enabling technology for screening the phototoxic potential of pharmaceutical substances. J. Pharm. Biomed. Anal., 46, 187-193.
- Onoue, S., Kawamura, K., Igarashi, N., Zhou, Y., Fujikawa, M., Yamada, H., Tsuda, Y., Seto, Y. and Yamada, S. (2008b): Reactive oxygen species assay-based risk assessment of drug-induced phototoxicity: classification criteria and application to drug candidates. J. Pharm. Biomed. Anal., 47, 967-972.
- Onoue, S., Seto, Y., Gandy, G. and Yamada, S. (2009): Drug-induced phototoxicity; an early in vitro identification of phototoxic potential of new drug entities in drug discovery and development. Curr. Drug Saf., 4, 123-136.
- Onoue, S., Seto, Y., Sato, H., Nishida, H., Hirota, M., Ashikaga, T., Api, A.M., Basketter, D. and Tokura, Y. (2017): Chemical photoallergy: photobiochemical mechanisms, classification, and risk assessments. J. Dermatol. Sci., 85, 4-11.
- Onoue, S., Suzuki, G., Kato, M., Hirota, M., Nishida, H., Kitagaki, M., Kouzuki, H. and Yamada, S. (2013b): Non-animal photosafety assessment approaches for cosmetics based on the photochemical and photobiochemical properties. Toxicol. In Vitro, 27, 2316-2324.
- Onoue, S. and Tsuda, Y. (2006): Analytical studies on the prediction of photosensitive/phototoxic potential of pharmaceutical substances. Pharm. Res., 23, 156-164.
- Onoue, S., Yamauchi, Y., Kojima, T., Igarashi, N. and Tsuda, Y. (2008c): Analytical studies on photochemical behavior of phototoxic substances; effect of detergent additives on singlet oxygen generation. Pharm. Res., 25, 861-868.
- Seto, Y., Hosoi, K., Takagi, H., Nakamura, K., Kojima, H., Yamada, S. and Onoue, S. (2012): Exploratory and regulatory assessments on photosafety of new drug entities. Curr. Drug Saf., 7, 140-148.
- Seto, Y., Kato, M., Yamada, S. and Onoue, S. (2013): Development of micellar reactive oxygen species assay for photosafety evaluation of poorly water-soluble chemicals. Toxicol. In Vitro, 27, 1838-1846.
- Seto, Y., Ohtake, H., Sato, H. and Onoue, S. (2020): Phototoxic risk assessment of dermally-applied chemicals with structural variety based on photoreactivity and skin deposition. Regul. Toxicol. Pharmacol., 113, 104619.
- Tong, A., Tang, X., Zhang, F. and Wang, B. (2020): Study on the shift of ultraviolet spectra in aqueous solution with variations of the solution concentration. Spectrochim. Acta A Mol. Biomol. Spectrosc., 234, 118259.




