2022 年 47 巻 8 号 p. 327-336
2022 年 47 巻 8 号 p. 327-336
We used an abortion-prone mouse model, generated by mating female CBA/J mice with male DBA/2JJcl mice, to examine the effects of changes in the Th1/Th2 cell ratio and the percentage of regulatory T (Treg) cells on the maintenance of pregnancy. We subcutaneously injected female CBA/J mice once each with 50 μg/mouse of Dermatophagoides farinae (Df) extract and the squalene-based adjuvant (SquA); 10 days later, these mice were mated with male DBA/2JJcl mice. Compared with injection of vehicle or adjuvant, the Df treatment decreased the Th1/Th2 cell ratio and concomitantly increased the percentage of Treg cells in the spleen. In addition, fetal death rates were decreased. We then explored a substance which shifted the Th1/Th2 balance toward Th1 side. We found that 50 μg/mouse of keyhole limpet hemocyanin (KLH) increased the splenic Th1/Th2 cell ratio of nonpregnant female CBA/J mice. We subcutaneously injected female CBA/J mice with KLH and SquA; 10 days later, these mice were mated with male DBA/2JJcl mice. Compared with injection of vehicle or adjuvant, treatment with KLH enhanced the Th1 bias during pregnancy and increased the fetal death rate. The percentage of Treg cells, however, was increased in these KLH-injected pregnant mice contrary to our presumption. All collected data showed strong positive correlation between the Th1/Th2 cell ratio and fetal death rate. The increase in Treg cells independent of effects on the fetal death rate suggests that Treg cells do not necessarily induce maternal tolerance to the fetus but may prevent excessive Th1/Th2 imbalance during pregnancy.
Recurrent pregnancy loss is defined as a disorder in maintaining a fetus after implantation in the uterus, causing repeated abortion. For example, uterine malformation and parental chromosomal aberration can lead to abortion. Inadequate maternal immunological status during pregnancy may also cause rejection of the semi-alloantigen of fetus. The complex immune system network cooperates with the reproductive system for the maintenance of pregnancy. The cytokine pattern associated with successful pregnancy led to the hypothesis known as the “T helper 2 (Th2) phenomenon” (Wegmann et al., 1993), i.e., skewing of the Th1/Th2 balance toward the Th2 side especially at early pregnancy. Furthermore, Treg cells have been thought to play pivotal roles to promote acceptance of the fetus by suppressing maternal immune responses (Aluvihare et al., 2004; Sasaki et al., 2004). Given that the suppressive activities of Treg cells contribute to immunological homeostasis throughout the body (Sakaguchi et al., 2008), we extend this idea to the function of Treg cells in modulating the Th1/Th2 balance for pregnancy. In the current study, we used Dermatophagoides farinae (Df) extract to cause a Th2 bias because house dust mite antigen is implicated in Th2-type allergic conditions, such as asthma, allergic rhinitis, and atopic dermatitis (Xie et al., 2008; Hinz et al., 2015; Jang et al., 2017; Rahimi et al., 2020). In contrast, keyhole limpet hemocyanin (KLH), which is highly immunogenic in humans, induces Th1-type immune responses under various conditions and is considered as an immunotherapeutic in some cases, such as in cancer therapy (Moltedo et al., 2006). Paradoxically, KLH can be used to induce Th2-type responses to treat Th1 type autoimmune disease (Kirwin et al., 2006). The effect of KLH to switch the Th1/Th2 pathway depends on the dose used (Rizzo et al., 1995).
In the present study, we used Df and KLH to intentionally modulate the immunological status (Th1/Th2 balance and Treg cell proportion) of female CBA/J mice mated with male DBA/2 mice, a widely used abortion-prone mouse model for human spontaneous abortion (Clark, 2016), and examined the effects on fetuses. We analyzed spleen lymphocytes instead of decidual lymphocytes after subcutaneous injection of Df or KLH with squalene-based adjuvant. Treg cells were enriched in decidua and displayed more suppressive phenotypes than in blood (Mjösberg et al., 2010), but they are generated outside the uterus and migrate into there for pregnancy (Thuere et al., 2007). Above all, the spleen is considered one of the primary immunotoxicological target organs, and flow cytometry of spleen lymphocytes can be conducted for routine toxicology or immunotoxicology studies. We, herein, discuss the immunotoxicological and immunopharmacological effects of xenobiotics on pregnancy and the “regulatory” role of Treg cells in the differentiation of Th1 and Th2 cells during pregnancy.
Female CBA/J mice were obtained from Charles River Laboratories Japan (Yokohama, Japan); male DBA/2JJcl mice from CLEA Japan (Tokyo, Japan). All animals were housed under controlled environmental conditions, with temperature at 23 ± 3°C, relative humidity at 40% to 60% and a 12:12-hr dark:light cycle. Tap water passed through a 5-μm filter and standard rodent chow (CE-2, CLEA Japan) were provided ad libitum. At dissection of animals, they were euthanized by cardiac puncture under anesthesia by intraperitoneal injection of medetomidine (0.3 mg/kg of body weight), midazolam (4 mg/kg) and butorphanol (5 mg/kg) mixture in physiological saline. Animal experimental procedures were approved by the Animal Experimental Committee of the School of Veterinary Medicine, Kitasato University (approval number: 19-100).
Abortion-prone mouse modelFemale CBA/J mice (age, 8 to 12 weeks) were mated overnight with male DBA/2JJcl mice (age, 8 to 12 weeks) at 1:1 ratio. The presence of a vaginal plug on the following morning was considered to be day 0.5 of gestation.
Treatment of mice by using Df and KLHWe obtained Df from Institute of Tokyo Environmental Allergy (Tokyo, Japan) and KLH from G-Biosciences/Geno Technology (St. Louis, MO, USA). Both substances were dissolved in physiological saline (PS) at 500 μg/mL. We used squalene-based adjuvant (SquA) as an adjuvant, which we prepared by mixing 4.3% squalene (Tokyo Chemical Industry, Tokyo, Japan) into 10 mM sodium citrate buffer solution containing surfactants (Tween 80 and Span 85). SquA was emulsified with an equal volume of PS, Df in PS, or KLH in PS to obtain a final concentration of Df or KLH in the emulsion at 250 μg/mL. For the Df+SquA and KLH+SquA groups, each female CBA/J mouse was subcutaneously injected once with 50 μg/0.2 mL of Df or KLH, respectively (Fig. 1). For the PS and PS+SquA groups, each female CBA/J mouse was subcutaneously given 0.2 mL of PS or PS+SquA, respectively. The female mice were euthanized or mated with male DBA/2JJcl mice at 10 days after immunization; pregnant CBA/J mice were euthanized on gestational days 7.5 and 14.5 (Fig. 1).

Schedules of immunization, mating, and sampling. On day 0, female CBA/J mice were immunized with physiologic saline (PS), PS+SquA (adjuvant), Dermatophagoides farinae (Df) +SquA, or keyhole limpet hemocyanin (KLH) +SquA. On day 10, the mice were euthanized (Fig. 1, a) or mated with male DBA/2JJcl mice (Fig. 1, b and c). When a vaginal plug was present in mated female mice on the morning of day 11, we designated day 11 as gestational day 0.5 (GD 0.5). Pregnant CBA/J mice were euthanized on day 17.5 (GD 7.5) and day 24.5 (GD 14.5).
For flow cytometry, spleens harvested from pregnant or naïve female CBA/J mice treated with PS, PS+SquA, Df+SquA, or KLH+SquA were dispersed through a mesh cup to obtain single cells in RPMI1640 containing 5 × 10–5 M 2-mercaptoethanol and 1% penicillin–streptomycin (Nacalai Tesque, Kyoto, Japan) as the basal medium. The single spleen cells were treated with 0.375% ammonium chloride (FUJIFILM Wako Pure Chemical, Osaka, Japan) aqueous solution to eliminate erythrocytes. The cells were centrifuged (300 × g) at 4°C for 5 min and washed twice with the basal medium with 10% fetal calf serum (MP Bio Japan, Tokyo, Japan) which had been inactivated at 56°C for 30 min. For determination of Th1 and Th2 cells, the single spleen cells suspended in 5 mL of the basal medium with 10% fetal calf serum were activated with 5 μg/mL (final concentration) of concanavalin A (FUJIFILM Wako Pure Chemical) for 24 hr in a 37°C incubator maintained at 5% CO2. At 4 hr before the end of the concanavalin A treatment, 5 μL of eBioscience monensin solution (1000 ×; Invitrogen/Thermo Fisher Scientific, Waltham, MA, USA) was added to inhibit release of cytokines. Cells were washed with and resuspended in 1% fetal calf serum in 11.8 mM phosphate buffered saline (PBS) (-) (pH 7.4), PBS (-). Half μL of fluorescein isothiocyanate (FITC)-labeled rat anti-mouse CD4 monoclonal antibody (clone RM4-5, Invitrogen/Thermo Fisher Scientific) was added to 1 mL of the cell suspension at 2 × 106 to 1 × 107 cells/mL and incubated at 4°C for 30 min in the dark. Cells were washed again and then incubated with diluted eBioscience fixation/permeabilization concentrate (Invitrogen/Thermo Fisher Scientific) and eBioscience permeabilization buffer (10 ×; Invitrogen/Thermo Fisher Scientific) followed by reacting with 0.3 μL of allophycocyanin (APC)-labeled rat anti-mouse IFN-γ monoclonal antibody (clone XMG1.2, Invitrogen/Thermo Fisher Scientific) and 5 μL of phycoerythrin–cyanin7 (PC7)-labeled anti-mouse IL-4 monoclonal antibody (clone BVD6-24G2, Invitrogen/Thermo Fisher Scientific) at room temperature for 30 min in the dark.
For determination of Treg cells, ammonium chloride-treated 2 × 106 to 1 × 107 spleen cells in 1 mL of 1% fetal calf serum in PBS (-) were stained with 0.5 μL of FITC-labeled rat anti-mouse CD4 monoclonal antibody and 0.65 μL of APC-labeled rat ant-mouse CD25 monoclonal antibody (clone PC61.5, Invitrogen/Thermo Fisher Scientific) at 4°C for 30 min in the dark. Cells were washed again, and permeabilized cells were incubated with 5 μL of phycoerythrin (PE)-conjugated mouse Foxp3 monoclonal antibody (clone FJK-16s, Invitrogen/Thermo Fisher Scientific) at room temperature for 1 hr in the dark.
Flow cytometry was conducted using a CytoFLEX flow cytometer (Beckman Coulter, Brea, CA, USA). Lymphocytes were gated according to forward and side scatters (Fig. 2, a); CD4+ cells were identified within the lymphocyte region (Fig. 2, b). Among the CD4+ cells, those positive for intracellular IFN-γ were defined as Th1 cells, and those expressing intracellular IL-4 were defined as Th2 cells (Fig. 2, c and d). Treg cells were identified as cells positive for both cell surface CD25 and intracellular Foxp3 within the CD4+ cell population (Fig. 2, e and f). The obtained data were analyzed using Kaluza 1.5 (version 1.0) software (Beckman Coulter).
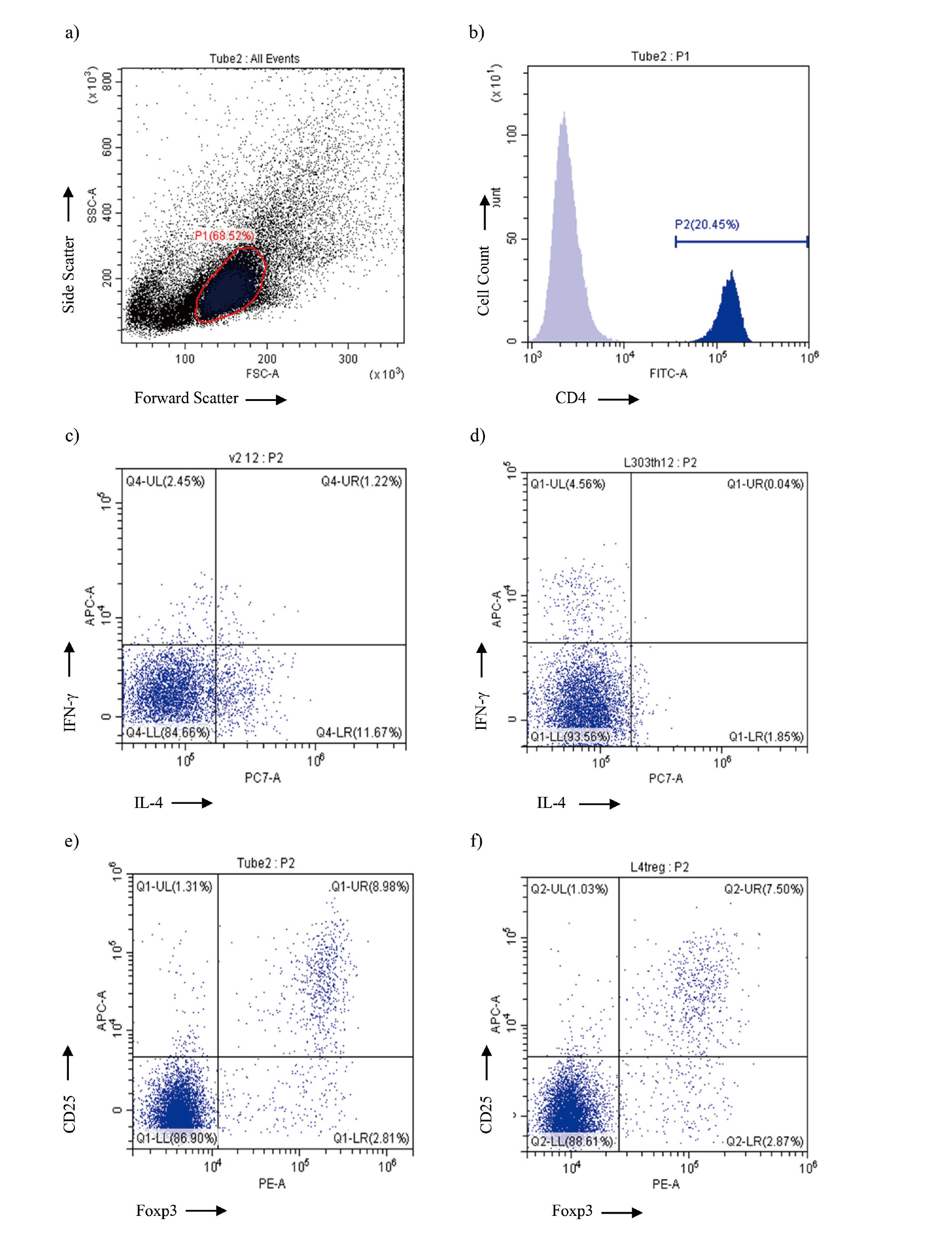
Flow cytometric analysis of spleen lymphocytes obtained from female CBA/J mice immunized with Df+SquA or KLH+SquA. a) Example of forward and side scatter gate to define lymphocytes in the spleen. b) Example of CD4 T cell identification within spleen lymphocytes. c, d) Representative dot plots of CD4+ T cells substratified into IFN-γ+ Th1 cells and IL-4+ Th2 cells in the spleen from female CBA/J mice immunized with Df+SquA (c) or KLH+SquA (d). e, f) Representative dot plots of CD4+ T cells to identify CD25/Foxp3 double positive Treg cells in the spleen from female CBA/J mice immunized with Df+SquA (e) or KLH+SquA (f).
On day 24.5 (gestational day 14.5), we counted surviving and resorbed fetuses (Fig. 3) in the uteri of pregnant CBA/J mice treated with PS, PS+SquA, Df+SquA, or KLH+SquA.
The fetal death rate (%) was expressed as
(Number of resorbed fetuses / Number of surviving and resorbed fetuses) × 100%
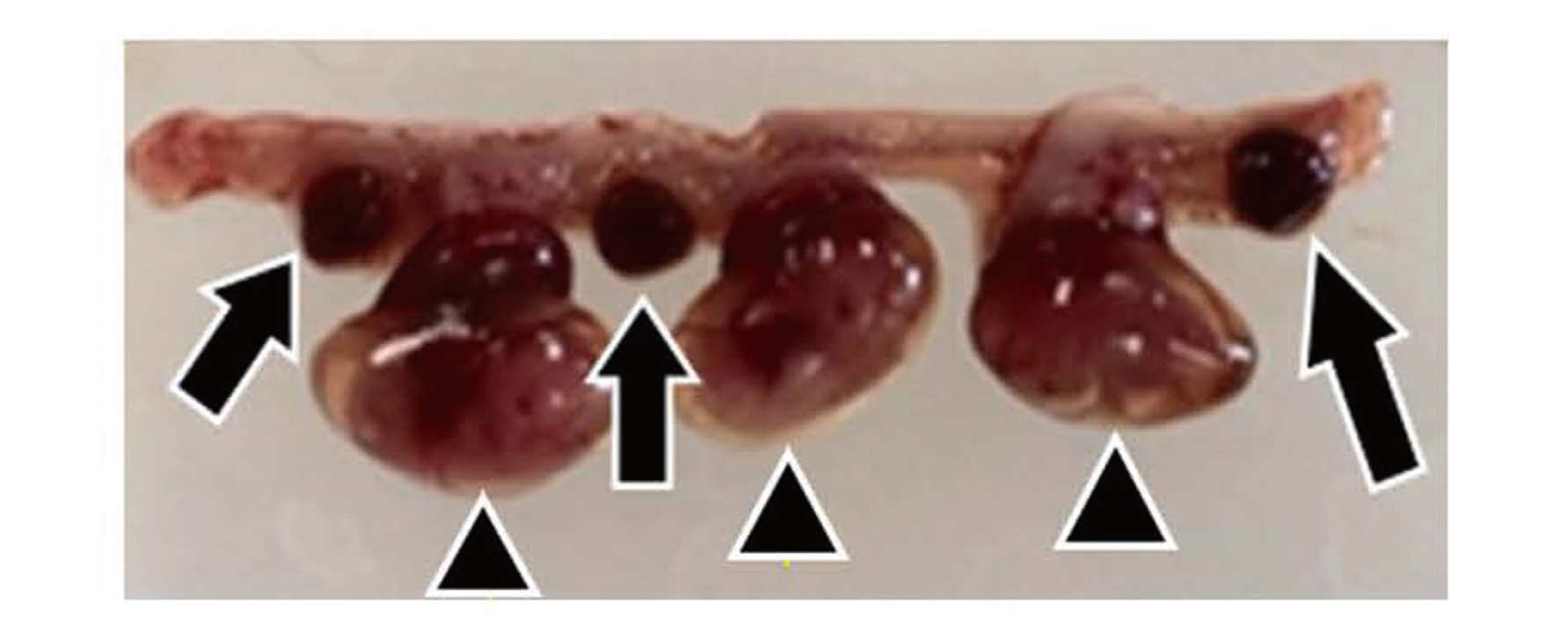
Uterus of pregnant CBA/J mouse. Pregnant CBA/J mice were euthanized and dissected to observe the uterus on gestational day 14.5. Triangles indicate surviving fetuses; arrows indicate resorbed fetuses.
All data are presented as mean ± 1 standard deviation. The Th1/Th2 cell ratios and the percentages of Treg cells in the spleens of female CBA/J mice were analyzed using one-way analysis of variance with the Tukey–Kramer test. We calculated Pearson’s correlation coefficient to evaluate the correlation between Th1/Th2 cell ratios and fetal death rates and between Treg cell percentages and fetal death rates. A P value less than 0.05 was considered statistically significant. All statistical analyses were performed using BellCurve for Excel (Social Survey Research Information, Tokyo, Japan).
At 10 days after female CBA/J mice were immunized with PS, PS+SquA, Df+SquA, or KLH+SquA, we performed flow cytometry to determine Th1/Th2 cell ratio and percentage of Treg cells in the spleen cells (Fig. 1, a). The Th1/Th2 cell ratio was lower in Df+SquA mice compared with that in the PS group (P < 0.05) but higher in the KLH+SquA group than in PS+SquA group (P < 0.01) (Fig. 4). Treatment with Df shifted the Th1/Th2 cell ratio toward the Th2 side, whereas KLH led to Th1 bias. We here noted lower (but not significantly) Th1/Th2 cell ratio of PS+SquA group compared with that in the PS group (Fig. 4). In the same spleens as for the Th1/Th2 analysis, the percentage of Treg cells was higher in both the Df+SquA and KLH+SquA groups than in PS group (P < 0.01) (Fig. 5), and the Df+SquA group had more Treg cells than the PS+SquA group (P < 0.01). These findings show that Df treatment of female CBA/J mice caused a Th2 bias, but administration of KLH shifted the balance toward Th1. However, the proportion of splenic Treg cells increased regardless of the antigen (Df or KLH) used to immunize the mice.

Th1/Th2 cell ratio in the spleen of nonpregnant female CBA/J mice at 10 days after immunization with Df or KLH. Female CBA/J mice were immunized with 50 μg/animal of Df or KLH plus SquA (adjuvant). Spleen cells were analyzed by flow cytometry at 10 days after immunization. Th2 cells were dominant over Th1 cells after immunization with Df, whereas Th1 cells predominated over Th2 cells after KLH treatment. The difference was statistically significant at *P < 0.05 or **P < 0.01. Data are presented as mean ± 1 standard deviation.
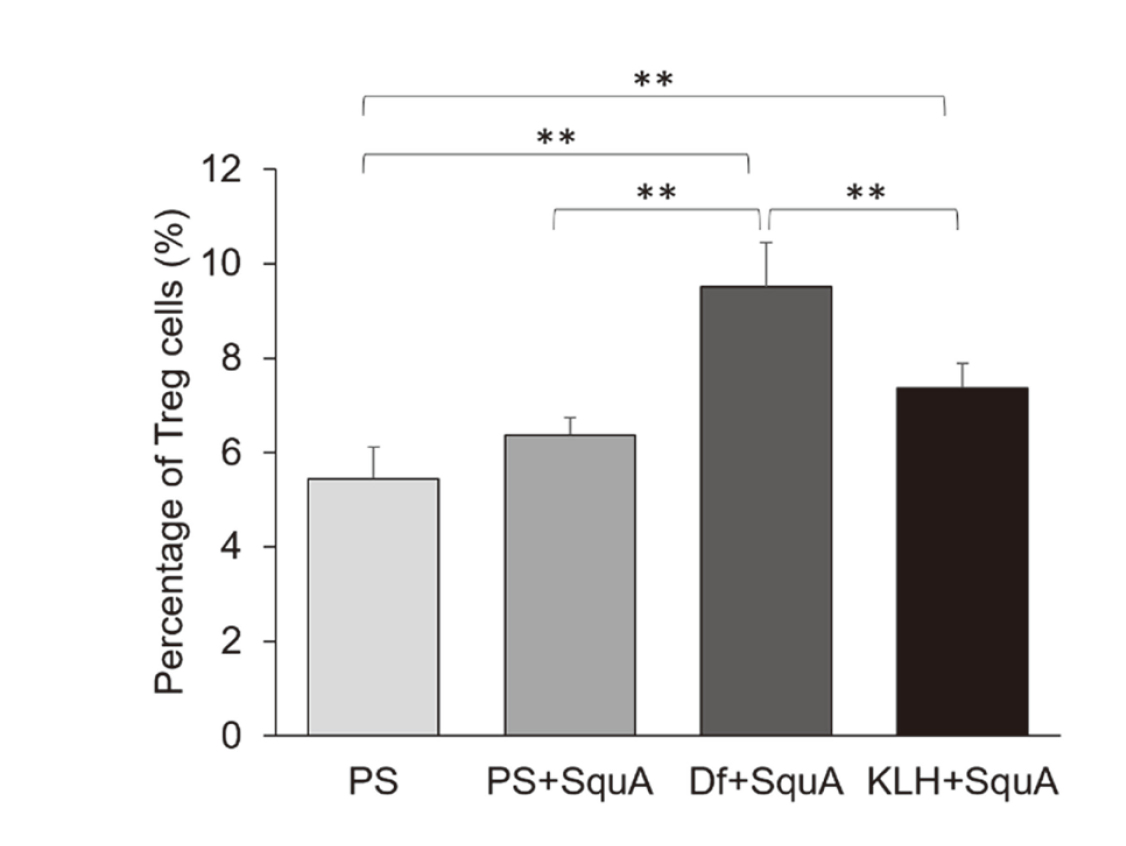
Percentages of Treg cells in the spleen of nonpregnant female CBA/J mice 10 days after immunization with Df or KLH. By using the same nonpregnant female CBA/J mice as for determination of the Th1/Th2 cell ratios, spleen cells were examined by flow cytometry for the percentage of Treg cells. At 10 days after immunization, Treg cell percentages were increased in both Df+SquA and KLH+SquA groups. The difference was statistically significant at **P < 0.01. Data are presented as mean ± 1 standard deviation.
At 10 days after their immunization with Df+SquA, female CBA/J mice were mated with male DBA/2JJcl mice (Fig. 1, b and c). The immunophenotypic pattern of splenic Th cells remained consistent throughout pregnancy after Df immunization. Representative dot plots of flow cytometry for identification of Th1/Th2 cells and Treg cells are shown in Fig. 2, c and e, respectively. In pregnant mice, the Th1/Th2 cell ratio was lower in the Df+SquA group compared with that in the PS group on day 17.5 (i.e., gestational day 7.5) and compared with that in the PS and PS+SquA groups on day 24.5 (i.e., gestational day 14.5) (P < 0.01) (Fig. 6). In addition, the percentages of Treg cells on gestational days 7.5 and 14.5 were higher in Df+SquA mice compared with those in the PS and PS+SquA groups (P < 0.01) (Fig. 7). Furthermore, the fetal death rate on gestational day 14.5 was decreased in DF+SquA group (which showed Th2 bias and increased Treg percentage) compared with PS group (P < 0.05) (Fig. 8).
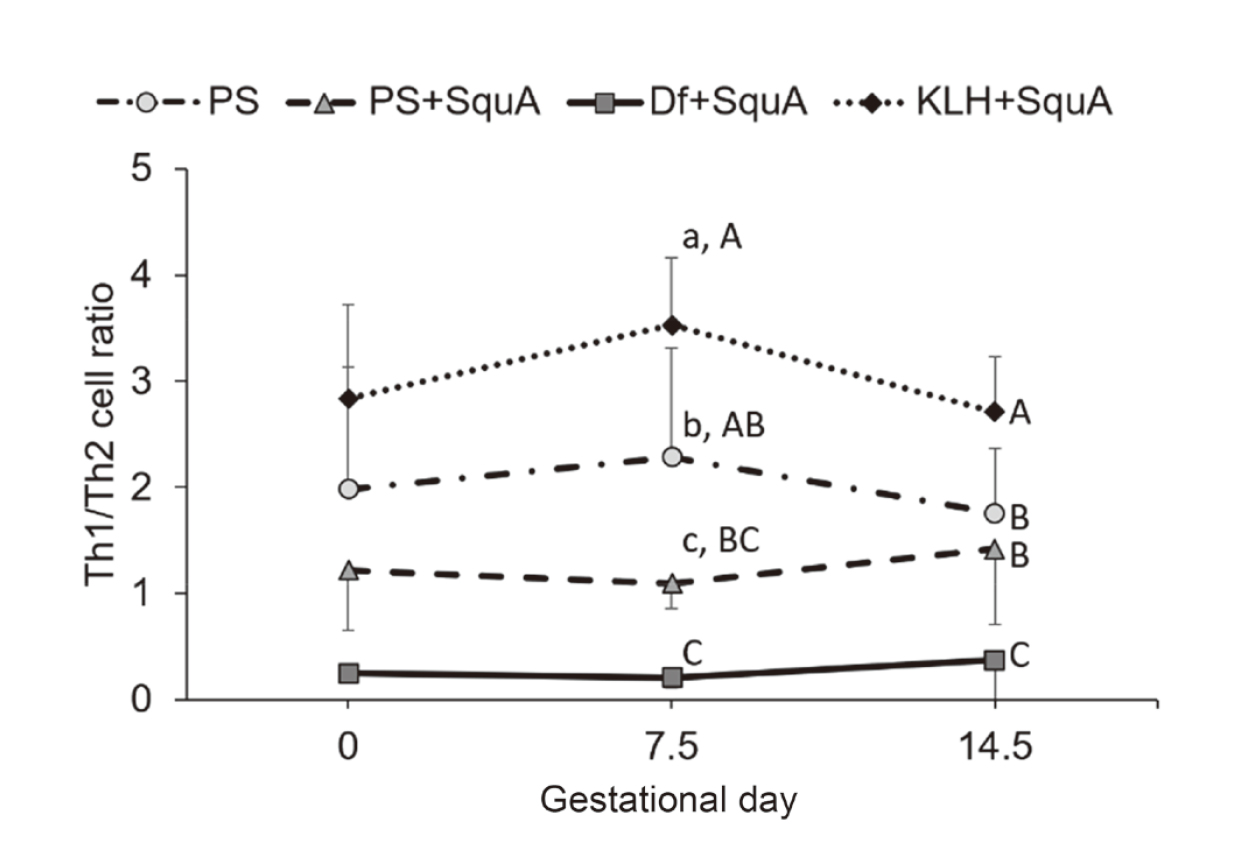
Variation of the splenic Th1/Th2 cell ratio during pregnancy in CBA/J mice immunized with Df or KLH. Female CBA/J mice were immunized with 50 μg/animal of Df or KLH plus SquA (adjuvant) at 10 days before mating. Flow cytometry was performed on gestational days 0, 7.5, and 14.5. Th1/Th2 cell ratios during gestation were lower in Df+SquA group and higher in KLH+SquA group than in PS and PS+SquA groups. Solid line, Df+SquA group; dotted line, KLH+SquA group; dash-dotted line, PS group; dashed line, PS+SquA group. Values indicated by different lowercase letters differed significantly at P < 0.05; those indicated by different uppercase letters differed at P < 0.01. Data are presented as mean ± 1 standard deviation.
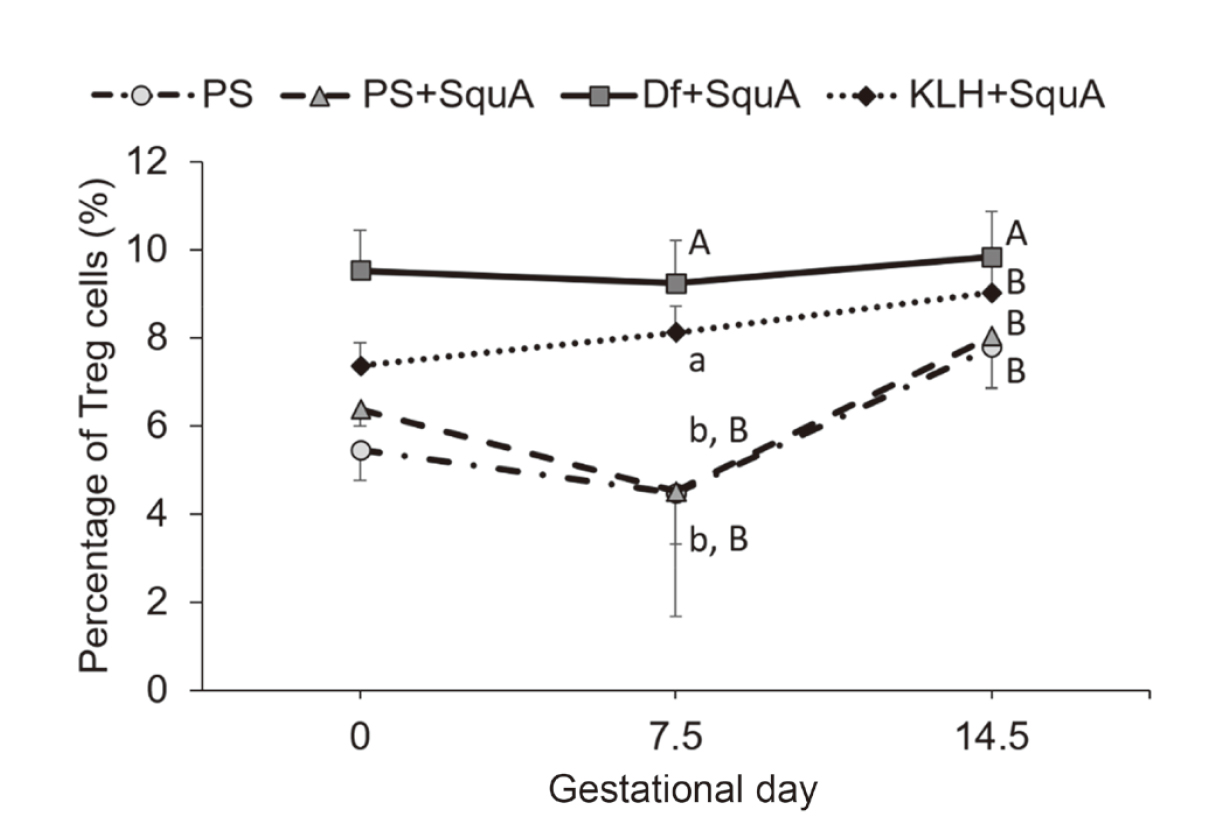
Variation of the percentage of splenic Treg cells during pregnancy in CBA/J mice immunized with Df or KLH. In the same pregnant female CBA/J mice as used for determination of the Th1/Th2 cell ratio, the percentages of splenic Treg cells were higher in both Df+SquA and KLH+SquA mice than in PS and PS+SquA groups. Solid line, Df+SquA group; dotted line, KLH+SquA group; dash-dotted line, PS group; dashed line, PS+SquA group. Values indicated by different lowercase letters differed significantly at P < 0.05; those indicated by different uppercase letters differed at P < 0.01. Data are presented as mean ± 1 standard deviation.

Fetal death rates in pregnant CBA/J mice immunized with Df or KLH. Female CBA/J mice were immunized with 50 μg/animal of Df or KLH plus adjuvant (SquA) at 10 days before mating. Fetal death rates (determined on gestational day 14.5 as the number of resorbed fetuses divided by the number of surviving and resorbed fetuses) were lower in Df+SquA group but higher in KLH+SquA mice than in PS or PS+SquA group. The difference was statistically significant at *P < 0.05 or **P < 0.01. Data are presented as mean ± 1 standard deviation.
At 10 days after immunization with KLH+SquA, female CBA/J mice were mated with male DBA/2JJcl mice (Fig. 1, b and c). No obvious changes were also seen in the immunophenotypic pattern of splenic Th cells during pregnancy after KLH immunization. Representative dot plots of flow cytometry for identification of Th1/Th2 cells and Treg cells are shown in Fig. 2, d and f, respectively. This immunization with KLH+SquA maintained higher the Th1/Th2 cell ratio compared with that after PS injection (P < 0.05 on gestational day 7.5; P < 0.01 on gestational day 14.5) or PS+SquA immunization (P < 0.01 on gestational days 7.5 and 14.5) (Fig. 6). In addition, the percentage of Treg cells in KLH+SquA group was higher than that in the PS and PS+SquA groups on gestational day 7.5 (P < 0.05) (Fig. 7). The fetal death rate was increased in KLH+SquA-treated pregnant mice, which had a Th1-skewed Th1/Th2 balance and an increased proportion of splenic Treg cells, compared with that in PS+SquA-treated mice (P < 0.05) (Fig. 8).
Correlation between the splenic Th cells and the fetal death rateWe analyzed the data from all mice in the PS, PS+SquA, Df+SquA, and KLH+SquA groups for correlation between the Th1/Th2 cell ratio and the fetal death rate and between the Treg percentage and the fetal death rate. An increased Th1/Th2 cell ratio was strongly correlated with a higher fetal death rate (r = 0.69, P < 0.001) (Fig. 9). In contrast, no significant correlation was shown between the Treg percentage and the fetal death rate (r = −0.11, P = 0.445) (Fig. 10).
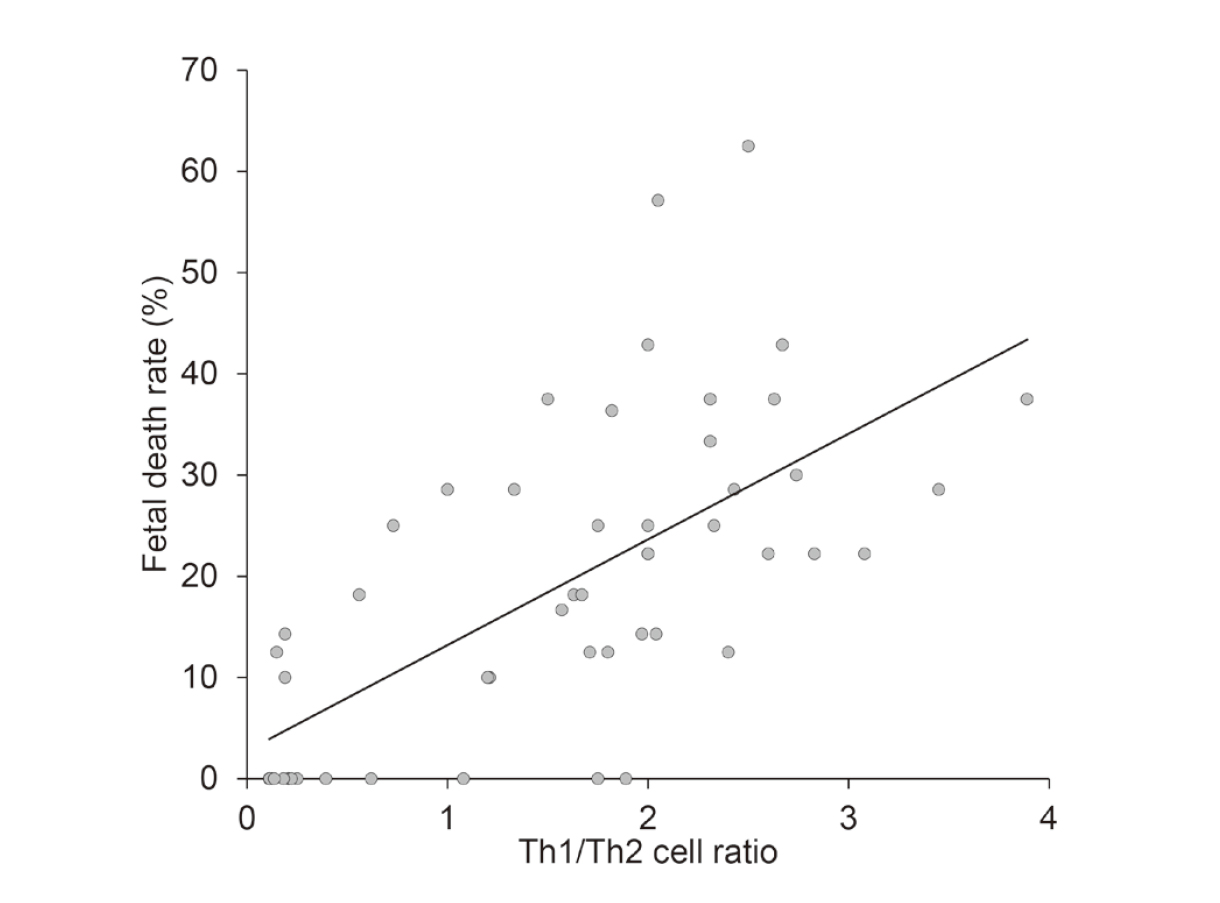
Correlation between Th1/Th2 cell ratio and fetal death rate. All data collected from PS, PS+SquA, Df+SquA, and KLH+SquA mice were analyzed for correlation between the Th1/Th2 cell ratio and fetal death rate. The Th1/Th2 cell ratio was strongly correlated with the fetal death rate (r = 0.69, P < 0.001).
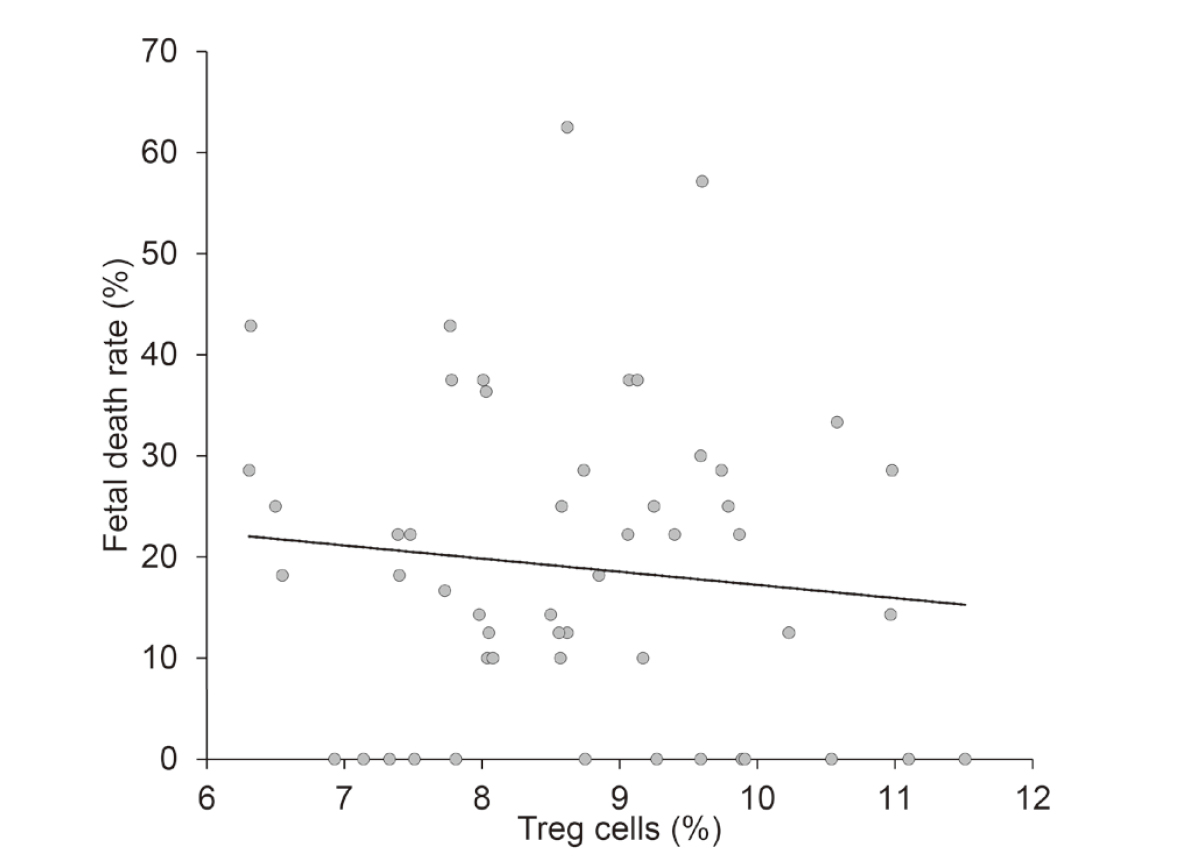
Correlation between Treg cell percentage and fetal death rate. All data collected from PS, PS+SquA, Df+SquA, and KLH+SquA mice were analyzed for correlation between the Treg cell percentage and fetal death rate. There was no significant correlation between the Treg cell percentage and the fetal death rate (r = −0.11, P = 0.445).
Normal pregnancy is associated with high levels of Th2 cytokines and a low Th1/Th2 cell ratio (Lin et al., 1993; Saito et al., 1999; Hayakawa et al., 2000). In very early pregnancy, the corpus luteum produces progesterone to promote the growth of the uterine endometrium to prepare it for embryonic implantation. In addition, progesterone favors the development of Th2 cells and stimulates IL-4 production (Piccinni et al., 1995). Progesterone-induced blocking factor (PIBF), which is produced by lymphocytes that express progesterone receptors, mediates the immunomodulatory effects of progesterone (Szekeres-Bartho and Wegmann, 1996). In humans, the IL-4 secreted from Th2 cells induces the production of human chorionic gonadotropin (hCG) from fetal trophoblasts; hCG works on the uterus to promote the formation of a sturdy placenta (Saito et al., 1997). Using Df and KLH immunization to intentionally modulate the Th1/Th2 cell ratio in an abortion-prone mouse model, we here examined the effect of the skewed Th1/Th2 balance on pregnancy. Whereas Df immunization significantly decreased both the fetal death rate and Th1/Th2 cell ratio, KLH immunization increased both of these parameters in spite of using SquA as an adjuvant although squalene-based MF59 per se reportedly induces Th2 immune responses (Yang et al., 2012). Accordingly, the Th1/Th2 cell ratio and fetal death rate were well correlated. These data suggest that Th2 cells help to maintain pregnancy, whereas Th1 cells are detrimental. In another study using the (CBA/J × DBA/2J) mouse model, infusion using the microosmotic pump filled with recombinant Dirofilaria immitis polyproteins (100 μg/body) for 14 days before mating improved fetal survival, but decreased serum levels of Th2 cytokines, such as IL-4, and had no effect on IL-5 and IL-10 levels on gestational day 13.5 (Komine-Aizawa et al., 2011). In contrast, we noted that a low Th1/Th2 cell ratio persisted throughout pregnancy after Df immunization. Therefore, in our experimental system, Th2 cytokines might be beneficial during early pregnancy.
Treg cells are a type of Th cells that express CD4 on their cell surface. Unlike other Th cell subsets (e.g., Th1, Th2, Th17 cells), Treg cells regulate the immune system in regard to immunological tolerance and autoimmunity (Sakaguchi et al., 1995; Suri-Payer et al., 1999). During pregnancy, Treg cells mediate maternal immunological tolerance to the father’s alloantigen in the fetus (Aluvihare et al., 2004). Paternal antigen-specific Treg cells are primed by seminal fluid and proliferate just before implantation to achieve successful allogeneic pregnancy (Shima et al., 2015). Because the proportion of decidual Treg cells is significantly lower in cases of spontaneous abortion than in induced abortions (Sasaki et al., 2004), we anticipated that, in our abortion-prone mouse model, the proportion of Treg cells would decrease, followed by an increase in the fetal death rate. Contrary to our hypothesis, both the Treg cell proportion and fetal death rate increased after KLH immunization, which created a Th1-dominant status in pregnant mice. Our data also indicated that, after Df immunization, the Treg cell percentage was increased in the context of Th2 dominance, which decreased the fetal death rate. We attribute these findings to the elaborate mechanism of Treg cells for the regulation of particular populations of Th cells. T-bet (T-box expressed in T cells) and GATA3 (GATA binding protein 3) are regarded as transcription factors associated with the development of Th1 and Th2 cells, respectively. In Treg cells, both transcription factors are expressed in the steady state, but local environmental conditions influence the expression of T-bet and GATA3 to regulate polarized immune conditions (Yu et al., 2015). During Th1-type inflammation, upregulation of T-bet expression in Treg cells is required for immune homeostasis by suppressing Th1 responses (Koch et al., 2009). Without T-bet-expressing Treg cells, severe Th1 autoimmunity may occur, such that, conversely, T-bet-expressing Treg cells inhibit the activation of Th1 and CD8+ T cells (Levine et al., 2017). Furthermore, Treg cells expressing IRF4 (interferon regulatory factor-4), essential for the differentiation of Th2 cells, work for selective suppression of Th2 responses (Zheng et al., 2009). In the present study, we manipulated the Th1/Th2 balance of female CBA/J mice by immunizing them with Df or KLH prior to pregnancy. Treg cells were increased during pregnancy, regardless of whether Th1 cells (leading to increased abortion) or Th2 cells (which is beneficial to pregnancy maintenance) predominated. Treg cells might modulate Th1- or Th2-type immune responses during conditions of Th1- or Th2-bias, respectively.
The present study provides several insights. First, from a toxicological point of view, xenobiotics such as drugs and environmental substances that disrupt the Th1/Th2 balance in the mother may cause abortion; this outcome can be designated as reproductive immunotoxicity. Second, regarding the development of therapeutics, substances that modify the Th1/Th2 balance may be candidates for the treatment of recurrent pregnancy loss. Finally, in the diagnosis of recurrent pregnancy loss, the Th1/Th2 ratio is likely a more informative biomarker when compared with the percentage of Treg cells.
We thank Dr. Ryo Kamata (School of Veterinary Medicine, Kitasato University) for editing a draft of this manuscript.
Conflict of interestThe authors declare that there is no conflict of interest.