2022 年 47 巻 9 号 p. 349-357
2022 年 47 巻 9 号 p. 349-357
Evidence has shown that suppression of the activation of NLRP3 inflammasome could ameliorate surgery/sevoflurane (SEV)-induced post-operative cognitive dysfunction (POCD). However, the underlying mechanisms remain unclear. UAF1 acts as a binding partner of USP1, which inhibits the ubiquitination-mediated degradation of NLRP3, indicating that UAF1 may be implicated in POCD through regulating the NLRP3 inflammasome. Here, we studied the role of UAF1/NLRP3 in SEV-induced cognitive impairment and neurotoxicity in rats. Neonatal rats were randomly divided into control, SEV, SEV+AAV-shNC and SEV+AAV-shUAF1 (UAF1-downregulated) groups. Morris water maze (MWM) test was applied to assess cognitive impairment. TUNEL staining, qRT-PCR and ELISA were used to assess the apoptosis and inflammation markers, respectively. The levels of superoxide dismutase (SOD), catalase (CAT) and malondialdehyde (MDA) were quantified to determine oxidative stress. The results showed that SEV treatment led to significant cognitive impairment, increased apoptosis in hippocampal tissues, upregulation of MDA and inflammatory factors (TNF-α, IL-1β, IL-18), as well as a decrease in SOD and CAT levels. All of the above observations were reversed by UAF1 downregulation. Furthermore, depletion of UAF1 neutralized SEV-mediated increase in p-NLRP3, p-IκBα and p-p65 levels. Altogether, the current study demonstrated that knockdown of UAF1 could alleviate SEV-induced cognitive impairment and neurotoxicity in rats by inhibiting pro-inflammatory signaling and oxidative stress.
Post-operative cognitive dysfunction (POCD), characterized by the impairment of memory, attention, and information processing capacity, is a devastating complication of surgery and anesthesia. Approximately 10% of surgical patients experience POCD, and the incidence rate increases to 40% in elderly patients (≥ 65 years) (Dong et al., 2018). The mortality rises in patients with POCD, together with impaired quality of life (Li et al., 2017; Skvarc et al., 2018). Despite ongoing study in POCD, the prognosis of POCD remains poor. Thus, it is important to understand the pathogenesis underlying POCD in order to achieve effective management of this disease.
Neuroinflammation and oxidative stress are known to play a crucial role in the occurrence and progression of POCD (Netto et al., 2018). Inflammasomes are intracellular multiprotein complexes that trigger the inflammatory responses. Among all types of inflammasomes, including NLRP1, NLRP3, NLRC4 and AIM2, NLRP3 is the most studied inflammasome, especially in the central nervous system (Guo et al., 2018; Vats et al., 2018). Activation of NLRP3 inflammasome increases the activity of caspase-1, resulting in the secretion of mature IL-1β and IL-18, which trigger cell pyrosis (Tang et al., 2017). NLRP3 inflammasomes in the microglia, astrocytes and neurons have been shown to induce neuroinflammation in the animal models of traumatic brain injury (Xu et al., 2018b) and depression (Wang et al., 2018). Ye et al. (2019) reported that through suppressing the activation of NLRP3 inflammasome in the hippocampus, honokiol ameliorated surgery/sevoflurane (SEV)-induced POCD, suggesting an important role of NLRP3 in POCD. However, the underlying mechanisms remain unclear.
Ubiquitin specific peptidase 1 (USP1) belongs to the USP family, which mediates the deubiquitination of a wide range of substrates. USP1-associated factor 1 (UAF1), also known as p80 or WDR48 (WD40 repeat containing protein 48), acts as a binding partner of USP1 and can enhance the deubiquitinase activity of USP1 (Yu et al., 2017; Williams et al., 2011). UAF1 and USP1 form a deubiquitinase complex, which takes part in multiple biological processes, including regulation of DNA repair and tumorigenesis (Liang et al., 2014; Lim et al., 2018). Recently, Song et al. (2020) reported that the UAF1-USP1 deubiquitinase complex could inhibit the ubiquitination-mediated degradation of NLRP3, leading to the accumulation of cellular NLRP3 and the subsequent NLRP3 inflammasome activation. However, whether UAF1 is implicated in POCD through promoting the activation of pro-inflammatory signaling remains unclear.
To this end, this study was carried out with the goal to explore the role of UAF1/NLRP3 in the pathogenesis of POCD. This study may provide an effective method for the treatment of POCD.
Animal experiments were approved by the Institutional Ethical Animal Experimentation Committee of the affiliated Shanghai East Hospital of Tongji University, and were performed in accordance to the National Institutes of Health (NIH) Guidelines for the Use of Laboratory Animals. Pregnant Sprague-Dawley rats (n = 8) were purchased from BetterBiotechnology Co., Ltd. (Nanjing, China), and were fed in specific pathogen-free (SPF) environment in a 12/12 light/dark cycle, with a relative temperature of 22 ± 1°C and a relative humidity of 46 ± 2%. The rats were given free access to diet and water. The adenovirus used to downregulate UAF1 (AAVshUAF1) and the control vector (AAV) were obtained from Guangzhou Yuanjing Biotechnology Co., Ltd. (Guangdong, China).
TreatmentsTwenty neonatal rats, weighing 16–21 gm, were randomly divided into four groups: Control, SEV, SEV+AAV and SEV+AAVshUAF1, with 5 rats in each group. On postnatal day 6 (P6), the neonatal rats in SEV, SEV+AAV-sh-NC (SEV+shNC) and SEV+AAV-shUAF1 (SEV+shUAF1) groups were exposed to the gas mixture containing 2% SEV and 30% oxygen at a flow rate of 2 L/min (Huang et al., 2020). The treatment frequency was 2 hr per day on P6, P8 and P10. After exposure to SEV, rats in SEV+AAV-sh-NC and SEV+AAV-shUAF1 groups received AAV-shNC and AAV-shUAF1 treatment via bilateral infusion in the dorsal hippocampus on P6. The injection site was set at −3.7 mm anteroposterior from the bregma, ± 2.2 mm mediolateral from the bregma and 3.5 mm below the surface of the skull. A total of 2 μL AAV/AAVshUAF1 (3.92 × 1012 v.g./mL, vector genomes/mL) was used in each rat, with an infusion speed of 0.2 μL/min (Ji et al., 2020). Following the Morris water maze (MWM) test, rats (n = 20) were euthanized and the hippocampus was collected for protein, RNA, apoptosis and cytokine analyses.
MWM testMWM tests were performed to assess the cognitive ability of rats (n = 20) as previously described (Song et al., 2019). Briefly, the water maze was equipped with opaque water using titanium dioxide to reach the level of 10 mm above the surface of a platform. In the training phase on P25-P29, rats were trained for 5 days with a frequency of 4 times per day. The escape latency referring to time to reach the platform was recorded to assess the spatial learning. On P30, the testing phase was carried out and the platform was removed, and mean distance from the original platform area, platform-crossing times, and time spent in the fourth quadrant (the platform quadrant) were recorded to evaluate the memory ability.
Fear conditioning testsThe fear conditioning test was applied to explore the spatial memory and learning capacities of rats (n = 20) (Peng et al., 2020). This test was carried out on the 2nd and 3rd days after the final SEV treatment. Following anesthesia, the rats were placed in the chamber for 120 sec, and submitted to a sound stimulus for 20 sec at 80 dB, followed by an electric foot shock for 2 sec at 0.75 mA and a 25 sec interval. The process was repeated twice. Rats were placed in the same chamber applied for training with no stimulus and no foot shock with the same interval used to train the animal. The percentage of freezing time (not moving) was recorded.
Western blottingTotal protein was extracted using the RIPA lysis buffer (Solarbio, Beijing, China) containing 1% protease inhibitor (Solarbio). Subsequently, a total of 20 μg protein was loaded into the 10% SDS-polyacrylamide gel and subjected to electrophoresis. Next, the protein sample was transferred onto the polyvinylidene difluoride membranes (PVDF; Millipore, Billerica, MA, USA). After that, blocking was performed with 5% non-fat milk for 1 hr at room temperature to prevent nonspecific binding, followed by primary antibody incubation at 4°C for 15–18 hr. Primary antibodies used in this study are as follows: anti-GAPDH (1:10,000 dilution; cat no. ab8226, Abcam, Waltham, MA, USA), anti-UAF1 (1:2,000 dilution; cat no. sc-514473, Santa Cruze, Dallas, TX, USA), anti-p65 (1:1,000 dilution; cat no. #8242, Cell Signaling Technology, CST, Danvers, MA, USA), anti-IκBα (1:1,000 dilution; cat no. #9242, CST), anti-p-p65 (1:1,000 dilution; cat no. #3033, CST), p-IκBα (1:1,000 dilution; cat no. #2859, CST) and anti-NLRP3 antibody (1: 2,000 dilution, cat no. #30565, CST). Then, the membranes were probed with HRP-conjugated secondary antibodies at room temperature for 1 hr. Next, membranes were incubated with ECL reagent (Millipore) and ProfiBlot-48 (Tecan, Männedorf, Switzerland) was used to detect protein signals. ImageJ software was used for protein quantification. Each experiment was repeated three times.
Quantitative reverse transcription-PCR (qRT-PCR)Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and was reverse transcribed into cDNA using the PrimeScript RT Master Mix kit (RR036A; Takara) according to manufacturer’s instructions. PCR amplification was performed using the 2 × SYBR Green PCR Mastermix (Solarbio, Beijing, China) on a 7500 Real-Time PCR System (Applied Biosystems, Foster, CA, USA). Primers used in this study are listed in Table 1. Each experiment was repeated three times.
| Gene | Sense (5’-3’) | Anti-sense (5’-3’) |
|---|---|---|
| GAPDH | GCATCTTCTTGTGCAGTGCC | GATGGTGATGGGTTTCCCGT |
| TNF-α | GGCTTTCGGAACTCACTGGA | GGGAACAGTCTGGGAAGCTC |
| IL-1β | AGCTTCAGGAAGGCAGTGTC | TCAGACAGCACGAGGCATTT |
| IL-18 | CTCTTCTACCAGCAAACATCTCACT | CAACTGAGAGGCTGTGCCCT |
| UAF1 | TGCATGCCGTATCTGGTTGT | ACCTCTTGCGAGGGTCTTTG |
| NLRP3 | GTCAACATGCAAGATGGCGG | TGAGGGTGGACATGCAGAAC |
The TUNEL assay was carried out using the One-step TUNEL In situ Apoptosis Kit (Red, AF647, Elabscience, Elabscience Biotechnology Co., Ltd., Wuhan, China) according to the manufacturer’s protocols. Briefly, hippocampal sections were immersed in 0.1% Triton X-100 for 10 min on ice, followed by incubation with the TUNEL reagent at 37ºC for 1 hr. Sections were then incubated with Hoechst 33342 for 20 min. The TUNEL-positive cells were counted under a confocal microscope (TCS SP5, Leica, Mannheim, Germany). Each experiment was repeated three times.
Detection of MDA, SOD and CATThe rat hippocampus was homogenized and centrifuged at 4,000 rpm for 10 min. Then, the levels of superoxide dismutase (SOD) and catalase (CAT) activity and malondialdehyde (MDA) were detected using the biochemical analysis kits (Beyotime, Shanghai, China) according to manufacturer’s instructions. Each experiment was repeated three times.
ELISAHippocampal tissues were homogenized and centrifuged at 10,000 × g at 4°C for 15 min. The levels of the inflammatory factors, including IL-1β, TNF-α and IL-18 were tested using enzyme-linked immunosorbent assay (ELISA) kits (Nanjing KeyGen Biotech Co., Nanjing, China) according to the manufacturer’s protocols. Each experiment was repeated three times.
Statistical analysisSPSS21.0 software (IBM, Armonk, NY, USA) was used for the statistical analysis. Student’s t-test and one-way ANOVA with Tukey’s tests were used to compare differences between two groups and multiple groups, respectively. A p value less than 0.05 was considered statistically significant.
First, we assessed the effect of UAF1 downregulation on cognitive impairment in rats induced by SEV. The MWM test showed that rats in the SEV group had increased mean distance from the platform, a shorter platform-crossing time and a longer escape latency as compared with the control group. These observations were rescued by UAF1 downregulation (Fig. 1A–1D). In addition, downregulation of UAF1 neutralized SEV-mediated decrease in freezing time (Fig. 1E). These results indicated that AAV-mediated downregulation of UAF1 could mitigate SEV-induced cognitive impairment.
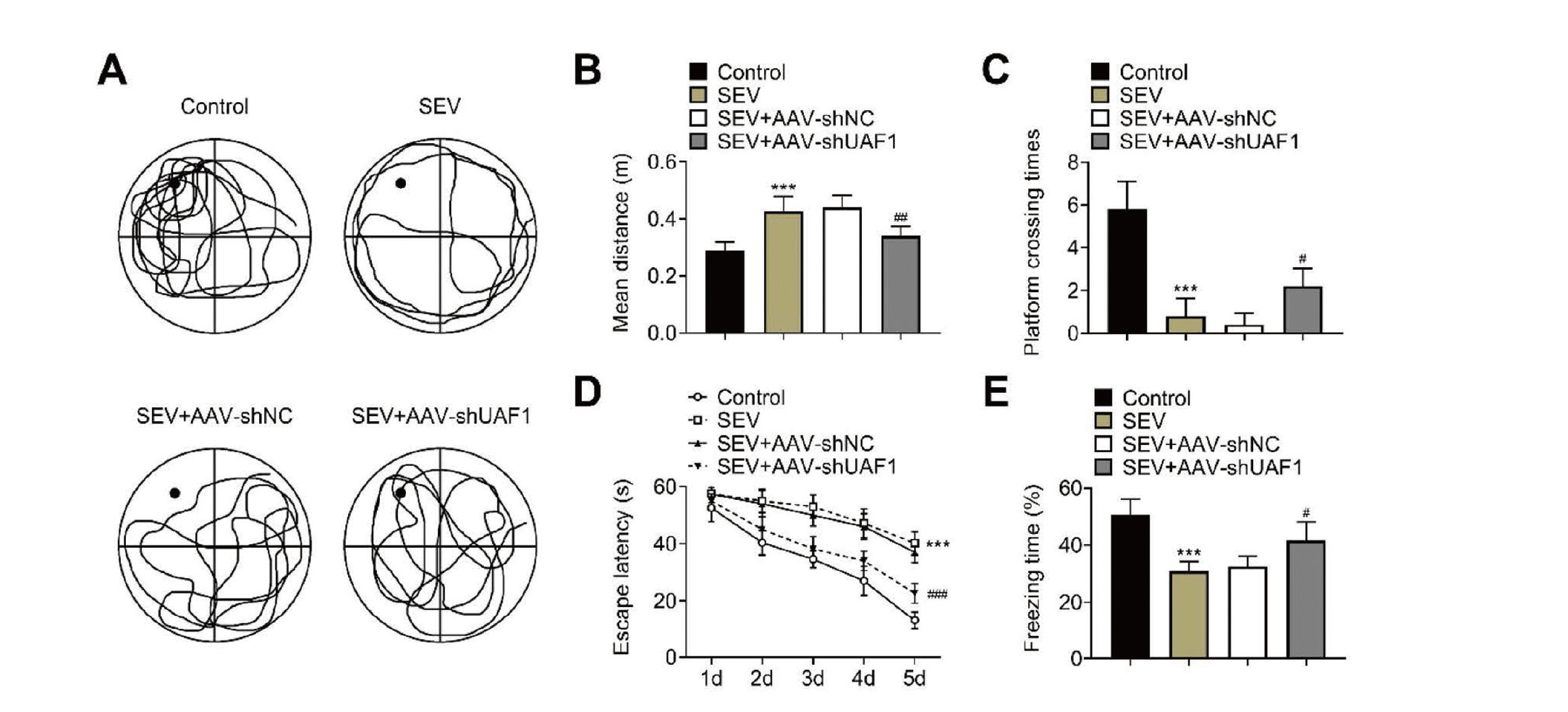
Downregulation of UAF1 mitigates SEV-induced cognitive impairment. (A–C) Morris water maze demonstrating the mean distance from the original platform area and fewer platform-crossing times in different groups. (D) Morris water maze demonstrating the freezing time in different groups. (E) Fear conditioning test showing the freezing time of rats in different times. Five rats per group. (***P < 0.001, vs. Control group; #P < 0.05, ##P < 0.01, ###P < 0.001, vs. SEC+AAV-shNC group).
Next, we assessed the effect of UAF1 depletion on SEV-induced hippocampal apoptosis and oxidative stress. UAF1 expression was significantly enhanced in the hippocampal tissues from rats underwent SEV treatment, and AAV-shUAF1 treatment significantly decreased UAF1 expression (Fig. 2A, 2B). Furthermore, downregulation of UAF1 induced by AAV-shUAF1 neutralized SEV-triggered decrease in SOD and CAT levels, and increase in MDA level (Fig. 2C). These findings suggested that UAF1 depletion could mitigate SEV-induced oxidative stress in hippocampal tissues.
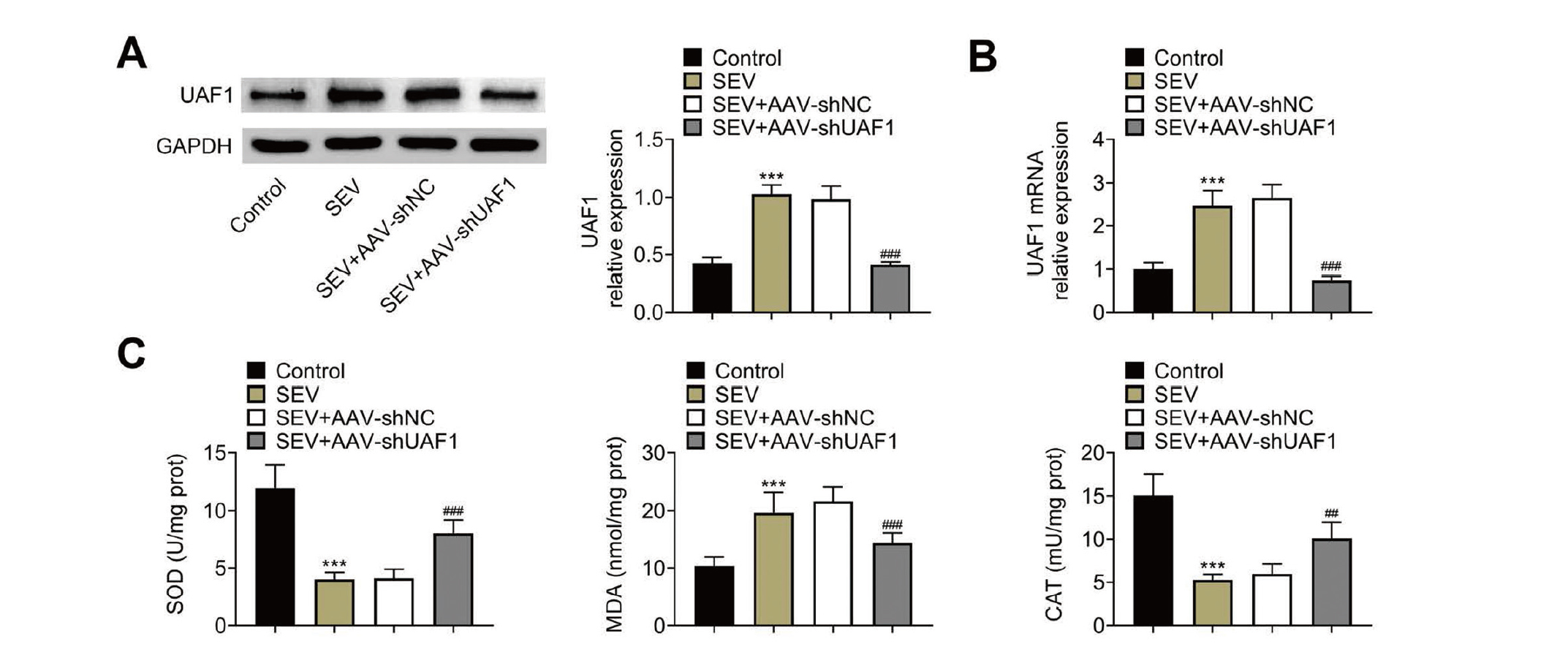
Downregulation of UAF1 inhibits SEV-induced apoptosis and oxidative stress in hippocampal tissues. (A) Western blotting and (B) qRT-PCR were used to assess UAF1 expression in the hippocampus of rats. (C) Histograms showing the levels of SOD, MDA, and CAT in the hippocampus. Five rats per group and each experiment was repeated three times. (***P < 0.001, vs. Control group; #P < 0.05, ##P < 0.01, ###P < 0.001, vs. SEC+AAV-shNC group).
Next, we investigated the role of UAF1 in SEV-induced inflammation in hippocampal tissues. The levels of TNF-α, IL-1β and IL-18 were significantly increased following SEV treatment, as detected by qRT-PCR (Fig. 3A) and ELISA (Fig. 3B). Interestingly, UAF1 silencing decreased the levels of TNF-α, IL-1β and IL-18 (Fig. 3A, 3B). These results demonstrated that knockdown of UAF1 inhibited SEV-induced inflammation in hippocampal tissues.

Downregulation of UAF1 attenuates SEV-mediated inflammatory reactions. (A) qRT-PCR and (B) ELISA were used to detect the levels of TNF-α, IL-1β and IL-18 in the hippocampus of rats. Five rats per group and each experiment was repeated three times. (***P < 0.001, vs. Control group; #P < 0.05, ##P < 0.01, ###P < 0.001, vs. SEV+AAV-shNC group).
In addition, we assessed the role of UAF1 in SEV-mediated hippocampal cell death. The number of dead cells in the hippocampal tissues was significantly increased by SEV treatment, and was decreased when UAF1 was depleted (Fig. 4A). To further confirm which type of cell death was caused by SEV treatment, western blotting was carried out to assess the expression of cleavage of caspase-1 (a pyroptotic marker) and cleaved caspase-3 (an apoptotic marker). The levels of both cleaved caspase-1/caspase-1 and cleaved caspase-3/caspase-3 were increased following SEV treatment, which were neutralized by the downregulation of UAF1 (Fig. 4B), suggesting that knockdown of UAF1 attenuated SEV-induced cell apoptosis and pyroptosis in hippocampal tissues.
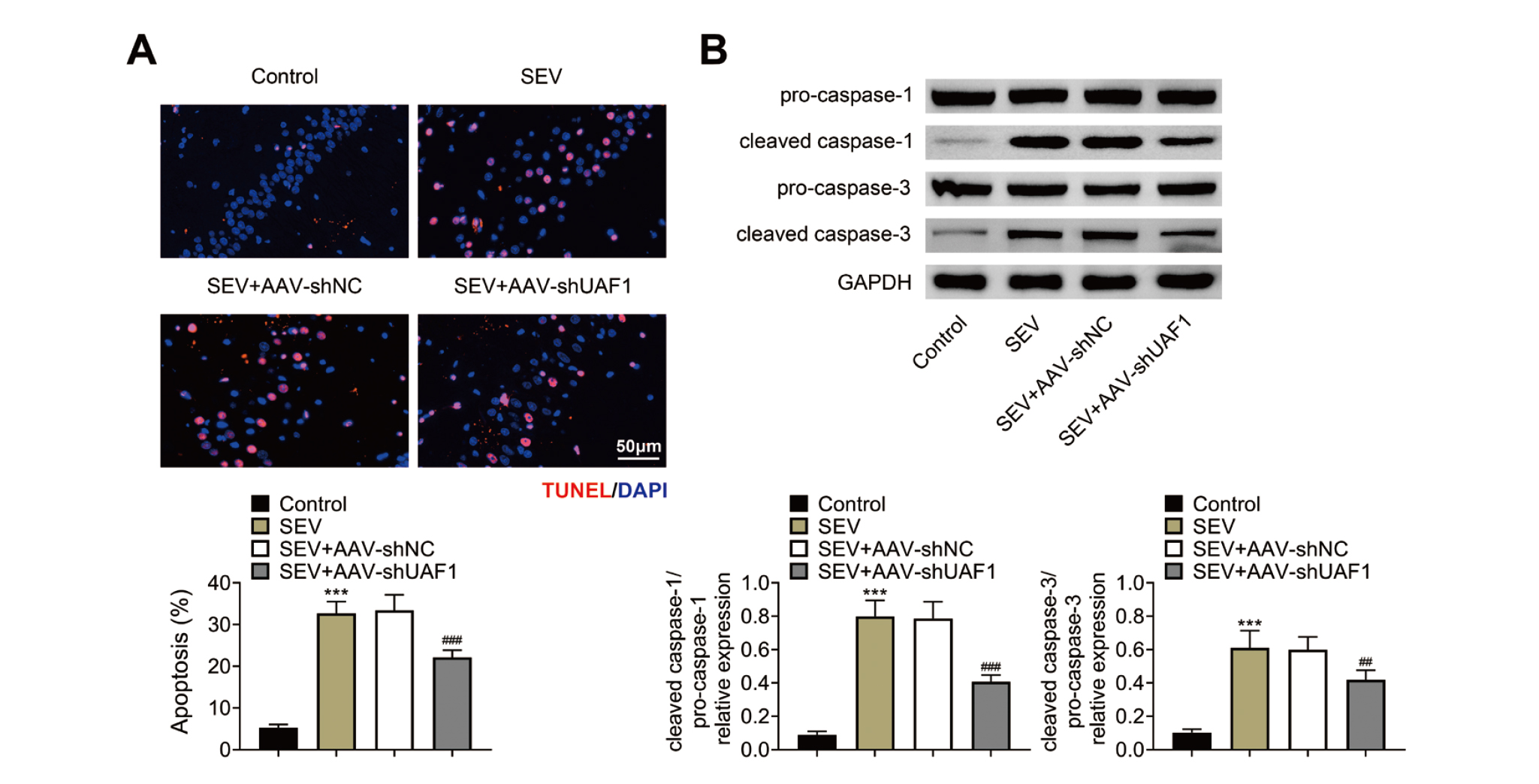
Downregulation of UAF1 alleviates SEV-mediated pyroptosis and apoptosis. (A) TUNEL staining was used to assess cell death in the hippocampus of rats. (B) Western blotting was used to quantify the protein levels of pro-caspase-1, cleaved caspase-1, pro-caspase-3 and cleaved caspase-3 of in the hippocampus. Five rats per group and each experiment was repeated three times. (***P < 0.001, vs. Control group; ##P < 0.01, ###P < 0.001, vs. SEV+AAV-shNC group).
Moreover, we further explored the role of UAF1 in regulating the activation of NLRP3 inflammasome. The expression of p-IκBα, p-p65 and NLRP3 was significantly increased in the hippocampal tissues of rats with SEV treatment, and this was reversed by UAF1 downregulation (Fig. 5A, 5B). This result indicated that knockdown of UAF1 could inhibit SEV-mediated activation of NLRP3 inflammasome.
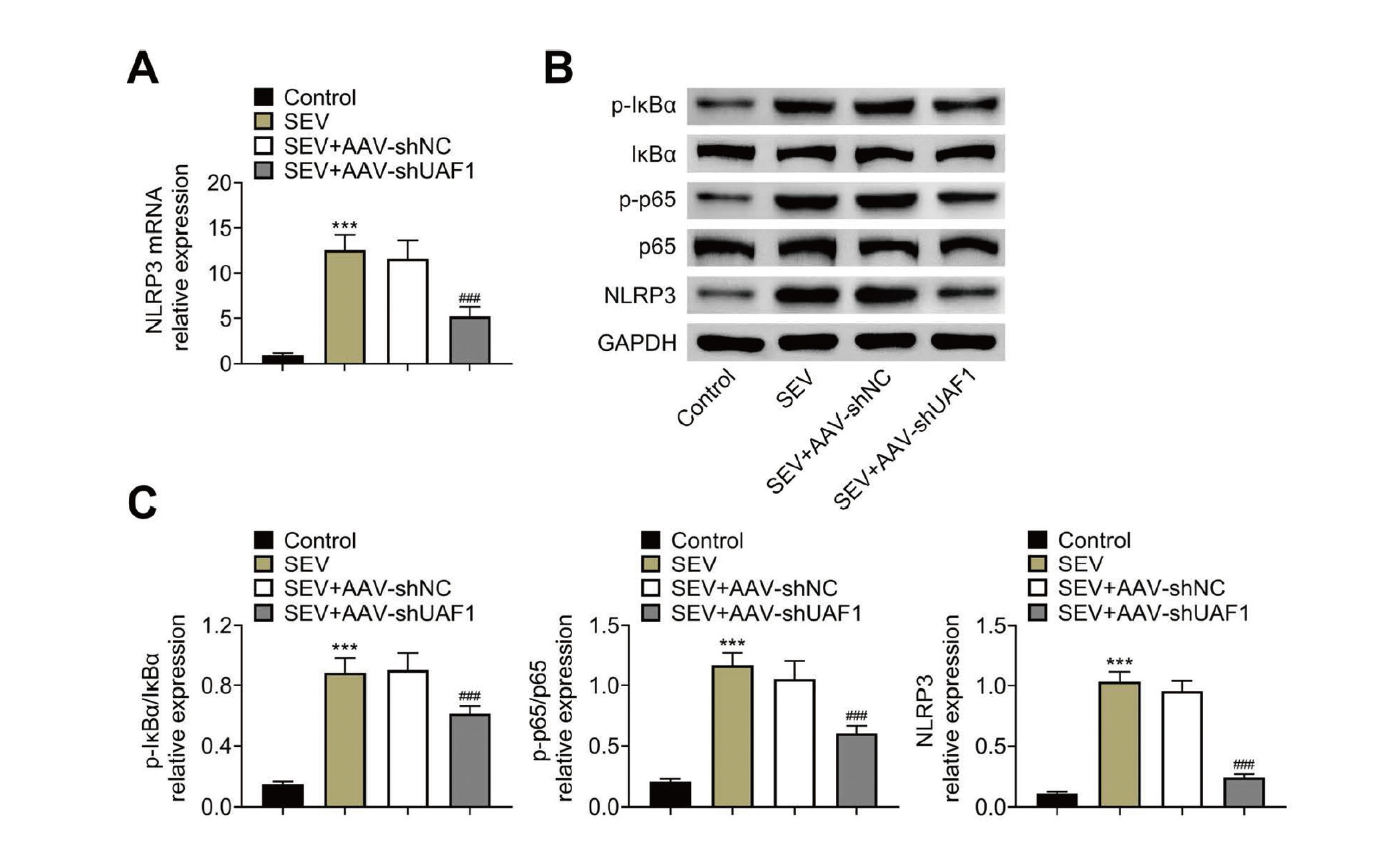
Downregulation of UAF1 inhibits SEV-mediated NLRP3 inflammasome activation. (A) qRT-PCR was used to quantify the mRNA levels of NLRP3 in the hippocampus of rats. (B, C) Western blotting was performed to assess the protein levels of p-IκBα, IκBα, p-p65, p65 and NLRP3 in the hippocampus of rats. Five rats per group and each experiment was repeated three times. (***P < 0.001, vs. Control group; ###P < 0.001, vs. SEC+AAV-shNC group).
SEV is the most commonly used anesthetic in patients of all ages in modern anesthesiology. However, evidence has demonstrated that SEV may cause cognitive impairment, which is correlated to the dosage, duration and number of anesthetic exposures (Xu et al., 2018a). Therefore, prevention and treatment of SEV-mediated cognitive impairment is highly important. In this study, we assessed the role of UAF1 in SEV-mediated cognitive impairment. The results demonstrated for the first time, that knockdown of UAF1 with AAV injection into the dorsal hippocampus significantly alleviated SEV-mediated cognitive impairment, cell death, inflammatory responses and excessive oxidative stress.
The MWM test is widely used in the study of neurocognitive disorders to assess spatial learning and memory ability in animals (Del Rosario et al., 2012). Here, we used the MWM test to assess SEV-mediated cognitive impairment, and the results demonstrated that SEV treatment significantly increased the platform-crossing time and escape latency, which were consistent with previous reports (Huang et al., 2020; Sun et al., 2021; Yu et al., 2020). In addition, we observed that SEV exposure led to an increase in the expression of UAF1 in hippocampus, indicating that UAF1 might be involved in SEV-mediated cognitive impairment. This was further validated by the MWM test, which showed that downregulation of UAF1 with AAV treatment significantly rescued SEV-mediated increase in the platform-crossing time and escape latency. Altogether, our findings indicate that UAF1 depletion exerts protective effects against SEV-mediated cognitive impairment.
In addition, anesthetics have been reported to trigger apoptosis (Shi et al., 2020; Wang et al., 2021). In agreement with this, we found that the number of apoptotic cells in the hippocampal tissues was significantly increased after SEV treatment and this was rescued by UAF1 downregulation. In addition, downregulation of UAF1 significantly restored SEV-induced increase in cleaved caspase-1 (a pyroptosis marker) and cleaved caspase-3 (an apoptosis marker) levels, suggesting that UAF1 depletion attenuates SEV-mediated pyroptotic and apoptotic cell death. A previous study has reported that UAF1 expression is increased in prostate cancer, and silencing of UAF1 causes increase in cell apoptosis and decrease in cell colony forming ability in pancreatic cancer (McClurg et al., 2015), suggesting that UAF1 may have an anti-apoptotic role in pancreatic cancer. It is possible that UAF1 may have different roles in different cell or tissue contexts.
SEV exposure translocates NF-κB from the cell cytoplasm to the nucleus, resulting in the activation of NF-κB signaling and the expression of pro-inflammatory factors, such as IL-1β, IL-6, IL-18 and TNF-α (Tian et al., 2022). In addition, SEV exposure causes excessive oxidative stress, leading to long-term injury (Gao et al., 2020). Moreover, UAF1 deubiquitinase complex also targets p65, thereby increasing its expression (Song et al., 2020). Here, we found that downregulation of UAF1 significantly neutralized increase in IL-1β, IL-18, TNF-α, MAD, p-IκBα/IκBα and p-p65/p65 levels, and decrease in SOD and CAT levels, demonstrating that UAF1 silencing could rescue SEV-mediated excessive inflammation and oxidative stress.
Growing evidence has demonstrated that NLRP3 inflammasome and its downstream caspase 1-mediated generation of IL-1β and IL-18 play an important role in neurodegeneration, neuroinflammation, and oxidative stress (Li et al., 2021). Blocking of NLRP3/caspase-1 pathway is a promising method to alleviate SEV-induced neuroinflammation and cognitive impairment (Shao et al., 2020; Wang et al., 2019). Song et al. (2020) reported that the UAF1-USP1 deubiquitinase complex could inhibit the ubiquitination-mediated degradation of NLRP3, as well as facilitate the transcription of NLRP3 through NF-κB signaling, leading to the accumulation of cellular NLRP3 and the subsequent NLRP3 inflammasome activation. Consistently, we found that downregulation of UAF1 decreased NLRP3 expression at both mRNA and protein levels. We speculate that UAF1 regulates the transcription and translation of NLRP3 through regulating NF-κB signaling and the protein ubiquitination of NLRP3, respectively. These results suggested that downregulation of UAF1 might be serve as a NLPR3 inhibitor to assuage SEV-induced neuroinflammation and cognitive impairment. In addition, it has been demonstrated that NLRP3 inhibition could inhibit oxidative stress in brain (Joaquim et al., 2021; Xu et al., 2019). Thus, we conjecture that UFA1 knockdown increased the levels of antioxidative enzymes, likely through downregulating NLRP3 expression.
SEV has been shown to regulate the expression of non-coding RNAs, such as microRNAs and long non-coding RNAs (lncRNAs) (Shen et al., 2021; Zhao et al., 2021; Jiang et al., 2021; Bao et al., 2021) which play a vital role in the regulation of RNA transcription (Ratti et al., 2020). Thus, we speculate that SEV may upregulate UAF1 transcript and protein levels through regulating the expression of non-coding RNAs. Further studies will be carried out to elucidate this.
In conclusion, this study reveals that knockdown of UAF1 alleviates SEV-induced cognitive impairment and neurotoxicity in rats by inhibiting pro-inflammatory signaling and oxidative stress. This study may provide a therapeutic method for POCD.
Conflict of interestThe authors declare that there is no conflict of interest.