2022 年 47 巻 9 号 p. 375-380
2022 年 47 巻 9 号 p. 375-380
Methyl vinyl ketone (MVK) is an environmental hazardous substrate which is mainly present in cigarette smoke, industrial waste, and exhaust gas. Despite many chances to be exposed to MVK, the cellular toxicity of MVK is largely unknown. Neurons are the main component of the brain, which is one the most vital organs to human beings. Nevertheless, the influence of MVK to neurons has not been investigated. Here, we determined whether MVK treatment negatively affects neuronal survival and axonal morphogenesis using primary hippocampal neuronal cultures. We treated hippocampal neurons with 0.1 μM to 3.0 μM MVK and observed a concentration-dependent increase of neuronal death rate. We also demonstrated that the treatment with a low concentration of MVK 0.1 μM or 0.3 μM inhibited axonal branching specifically without affecting axon outgrowth. Our results suggest that MVK is highly toxic to neurons.
Methyl vinyl ketone (MVK) is the simplest enone, a type of α,β-unsaturated carbonyl compound, which is toxic being an electrophile and alkylating agent (Siegel and Eggersdorfer, 2000) (Fig. 1A). Human beings can be exposed to MVK through many ways. One of the major routes for MVK exposure is through smoking tobacco use and secondhand smoking since the urinal concentration of MVK metabolites is increased in smokers (Chen et al., 2020). Exposure to cigarette smoke is linked to more than seven million deaths annually by causing cancers, vascular diseases, respiratory diseases, and neurological diseases (Reitsma et al., 2021; Jha et al., 2013; Sundström et al., 2008; Ohshima et al., 2018; Liu et al., 2020). MVK may play a role in the detrimental effect of cigarette smoke on health. Other than cigarette smoke, MVK is used commercially in the production of perfumes, steroids, vitamin A and plastics and is released to the environment as industrial wastes (Giersch and Schulte-Elte, 1990; Nakayama et al., 1985; Siegel and Eggersdorfer, 2000; Papa and Sherman, 1981). Furthermore, it is present in the automobile exhaust (Biesenthal and Shepson, 1997). Therefore, it is unlikely to avoid MVK exposure completely.

Methyl vinyl ketone (MVK) induces neuronal cell death in a concentration-dependent manner. (A) The chemical structure of MVK. (B) Schematic diagram of this experiment. Hippocampal neurons were cultured for 6 day and treated with MVK for 24 hr. The cultures were incubated with propidium iodide (PI) for 1 hr, which labeled the dead cells. (C) Representative images of cultured neurons labeled with Hoechst (green) and PI (red). The cells were also immunostained for NeuN (blue). Scale bar = 200 μm. (D) Pie graphs showing the average proportions of living neurons, dead neurons, living non-neuronal cells, and dead non-neuronal cells in each treatment group (n = 5 cultures). The cell counts were 4028, 4492, 5433, 4660, and 5445 cells for the control, MVK 0.1 μM, MVK 0.3 μM, MVK 1.0 μM, and MVK 3.0 μM groups, respectively. (E) Death rate of the cultured neurons (n = 5 cultures). *p < 0.05 and **p < 0.01 between the indicated groups, as assessed by the Siegel–Tukey test after the Kruskal–Wallis one-way analysis of variance-of-ranks test. The data are presented as the mean ± standard error of the mean. The circles represent individual data points.
Certain toxicity of MVK has been revealed by previous studies. Inhalation of MVK has been shown to cause respiratory and olfactory problems (Morgan et al., 2000). Acute exposure to MVK is also known to irritate the skin and eyes (New Jersey Department of Health and Senior Services, 2000). In vitro studies have shown that MVK can cause cellular death in mouse melanoma cells and GT1-7 cells (Horiyama et al., 2014; Sathishkumar et al., 2007). However, the toxicity of MVK to various types of cells, including neurons, is largely unknown.
In this study, we focused on the toxicity of MVK to neuronal cells since the brain is one of the most vital organs and damage to neuronal cells is usually irreversible. Specifically, we attempted to determine whether MVK exposure has a negative effect on neuronal survival and axonal morphogenesis because these are the basic requirements for the development of the nervous system (Kalil and Dent, 2014). We observed that MVK caused neuronal death in a concentration-dependent manner. We also observed that the exposure to low concentrations of MVK resulted in a reduction in axonal branching. Therefore, we demonstrated that MVK disrupts neuronal survival and development.
All experimental protocols used in the present study were approved by the Animal Care and Use Committee of Hokkaido University (21-0092) and were carried out in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Primary hippocampal culturePrimary hippocampal cultures were used to assess the effect of MVK on neurons. Primary hippocampal cultures were prepared from neonatal ICR mice (SLC, Shizuoka, Japan) as previously described with minor modifications (Okada et al., 2021). Briefly, 5–6 postnatal day 0 (P0) mouse pups from either sex were anesthetized, and their brains were removed. The hippocampi were dissected in ice-cold glucose-enriched Hank’s balanced salt solution with 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) at 4°C, minced, and subsequently incubated with 0.25% trypsin (Nacalai Tesque, Kyoto, Japan) and 0.01% DNase I (Roche, Basel, Switzerland) at 37°C for 30 min. After trypsinization was performed, the cells were centrifuged at 1200 rpm for 5 min at room temperature, and the supernatants were discarded. The remaining cells were plated on poly-D-lysine coated coverslips (Neuvitro, WA, USA) in plating medium and cultured in a humidified incubator at 37°C with 5% CO2. Plating density was 25,000 cells/cm2 for survival assay and 2,500 cells/cm2 for morphology assay. Low density was used in morphology assay to avoid interactions of neurites. The plating medium used was neurobasal medium (Thermo Fisher, MA, USA) containing 2% B27 supplement (Gibco, Thermo Fisher), 1% penicillin/streptomycin solution (Nacalai Tesque), 0.5 mM glutamine, 1 mM sodium pyruvate, 25 μM glutamate, 1 mM HEPES, 10% horse serum (Gibco, Thermo Fisher), and 5 μM cytosine arabinoside (Sigma-Aldrich, MI, USA). At day 1 in vitro (DIV 1), the plating medium was changed to culture medium, which was composed of neurobasal medium containing 2% B27 supplement, 1% penicillin/streptomycin solution, 1 mM glutamine, 1 mM sodium pyruvate, 1 mM HEPES, and 1 μM cytosine arabinoside. Every 3 day, 50% of the culture medium was changed.
ImmunocytochemistryWe combined immunocytochemistry with other staining methods to label and visualize the neurons, as previously described (Zhou et al., 2016). The cultured neurons were fixed with 4% paraformaldehyde at 37°C for 30 min. The cells were then incubated with blocking solution (5% goat serum (Funakoshi, Tokyo, Japan) and 0.1% Triton-X 100 in phosphate-buffered saline (PBS)) at room temperature for 1 hr. The cultures were subsequently incubated with primary antibodies at 4°C overnight. The cultures were then washed with PBS and incubated with secondary antibodies, with or without Phalloidin-iFluor 594 (1:1000; ab176757; Abcam, Cambridge, UK), at 4°C for 6 hr. The nuclei were labeled with Hoechst (1:1000; Invitrogen, MA, USA) before mounting was performed. The following antibodies were used: mouse anti-Tau1 (1:1000; MAB3420; Millipore, MA, USA), guinea pig anti-neuronal nuclear antigen (anti-NeuN) (1:1000; 266004; Synaptic Systems, Coventry, UK), Alexa 488-labeled anti-mouse IgG (1:500; A11029; Invitrogen), and Alexa 594-labeled anti-guinea-pig IgG (1:500; A11076; Invitrogen). Propidium iodide (PI, 20 mg/mL; Nacalai Tesque) was used to label the dead cells before fixation was performed.
Data acquisition and analysisData were collected from at least 5 independent experiments. For the data analysis, images of the neuronal cultures were captured using an FV1000 confocal microscope (Olympus, Tokyo, Japan) and processed using FIJI/Image J software. For the calculation of the cell death rate in each culture, five areas (635.9 μm × 635.9 μm) distant from each other were observed and analyzed. For the analysis of axonal morphology, we excluded neurons with condensed nucleus from the data set. Protrusions shorter than 5 μm are not counted as axonal branches. The details of the statistical analyses are described in the figure legends.
First, we examined the effect of MVK on neuronal survival using primary hippocampal neuron cultures. At day 6 in vitro (DIV 6), the cultures were treated with 0.1, 0.3, 1.0, or 3.0 μM MVK for 24 hr. Cultures without MVK treatment are referred as the control group. The cultures were then incubated with PI for 1 hr to label the dead cells (Fig. 1B). We labeled the nuclei of the primary cells with Hoechst and immunostained the cells for NeuN to identify the neurons. We observed few PI-positive cells in the control group. However, in the 0.3 μM MVK group and 3.0 μM MVK group, a large portion of neurons (NeuN+) and non-neuronal cells (NeuN-) were labeled with PI (Fig. 1C).
We classified all the observed cells (approximately 25,000 cells) in each group into four classes: living neurons, dead neurons, living non-neuronal cells, and dead non-neuronal cells. The cells labeled with both NeuN and Hoechst but not PI were counted as living neurons, whereas the cells labeled with NeuN, Hoechst, and PI were counted as dead neurons. For the NeuN-, the cells labeled with Hoechst only were counted as living non-neuronal cells, and the cells labeled with both Hoechst and PI were counted as dead non-neuronal cells (Fig. 1C). We calculated the average proportion of each class of cell in each group (Fig. 1D). The primary hippocampal culture was consistently composed of 75–80% neurons. In the control group, only 2.22 ± 0.79% of the cells were counted as dead neurons. The proportion of dead neurons increased as the MVK concentration increased. At the highest MVK concentration, the dead neurons accounted for nearly half of the cells (48.02 ± 7.44%, Fig. 1D). The death rate of the neurons increased linearly for the high concentrations of MVK, and the treatment with 1.0 μM MVK and that with 3.0 μM MVK significantly increased the death rate of the neurons (Fig. 1E).
For the non-neuronal cells, a higher basal death rate was observed in the control group, which is likely because cytosine arabinoside was added to kill the non-neuronal cells that underwent mitosis. Similarly, a drastic increase in the death rate of the non-neuronal cells was observed in the high-concentration MVK group (Fig. 1D).
Low concentration of MVK treatment inhibits axonal branching specificallyAs the proper axonal morphogenesis is essential for the formation of neural circuits, we also examined the changes in the axonal morphology induced by MVK treatment. The primary hippocampal cultures were fixed or treated with 0.1, 0.3 or 1.0 μM MVK for 72 hr on DIV 4. After the treatment was administered, the cultures in the control and MVK groups were fixed on DIV 7 (Fig. 2A). The axons were labeled via immunostaining against Tau1, and the dendrites were labeled with Phalloidin-iFluor 594 dye (Fig. 2B). We found that the axons from the low-dose MVK groups (0.1 μM and 0.3 μM) possessed fewer branches than those from the control group on DIV 7 but that the lengths of the axons were similar between these two groups. The neurons treated with 1 μM MVK exhibited shorter and fewer branched axons (Fig. 2B).
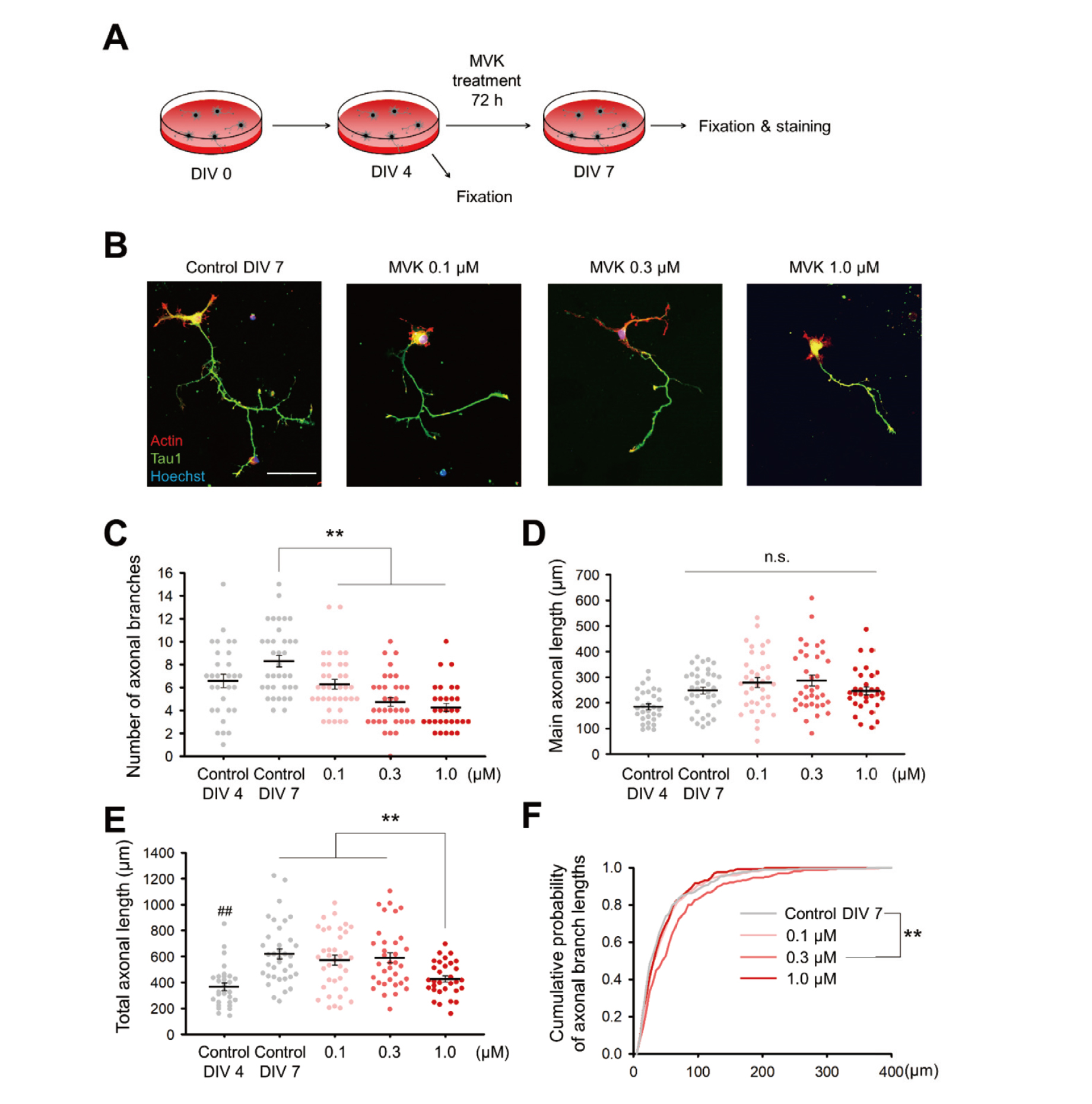
Low-concentration MVK treatment inhibits axonal branching but not axonal outgrowth. (A) Schematic diagram of this experiment. Hippocampal neurons were cultured for 4 day and treated with MVK for 72 hr. The cultures were fixed on day in vitro (DIV) 4 (i.e., before the MVK treatment) and on DIV 7 (i.e., after the MVK treatment). (B) Representative images of the cultured neurons immunostained for Tau1 to visualize the axons. Actin was labeled with Phalloidin-iFluor 594 (red), and the nuclei were labeled with Hoechst (blue). Scale bar = 50 μm. (C) Number of axonal branches. **p < 0.01 between the indicated groups by Tukey’s test after one-way analysis of variance (ANOVA). (D) Main axonal length. n.s. = no significant different among the indicated groups. (E) Total axonal length. **p < 0.01 between the indicated groups by Tukey’s test after one-way ANOVA. ##p < 0.01 vs. the control group at DIV 7 by Tukey’s test after one-way ANOVA. For the quantification of (C), (D), and (E), there were n = 30, 36, 36, 35, and 31 neurons from the control DIV 4 group, control DIV 7 group, MVK 0.1 μM group, MVK 0.3 μM group, and MVK 1.0 μM group, respectively. The data are presented as the mean ± standard error of the mean. The circles represent individual data points. (F) Cumulative probability of the axonal branch lengths. **p < 0.01 between the indicated groups by the Kolmogorov–Smirnov test. There were n = 302, 226, 167, and 132 axonal branches from the control DIV 7 group, MVK 0.1 μM group, MVK 0.3 μM group, and MVK 1.0 μM group, respectively.
We quantified the number of axonal branches, main axonal length, and total axonal length in each group (Fig. 2C–E; neurons with condensed nucleus were not included). In the control group, the neurons at DIV 7 exhibited longer axons compared with the neurons at DIV 4 (Fig. 2E), and the number of branches and main axonal length also showed a tendency to increase (p = 0.06 and p = 0.052, respectively, by Tukey’s test after analysis of variance (ANOVA); Fig. 2C–D), indicating the proper morphogenesis. However, in all of the MVK treatment groups, the number of axonal branches was significantly lower than that in the control group at DIV 7. In the 0.3 and 1.0 μM MVK treatment groups, there were significantly fewer axonal branches than in the control group at DIV 4 (p < 0.05, Tukey’s test after ANOVA; Fig. 2C). This suggests that MVK treatment not only inhibits the formation of new axonal branches but also destabilizes the existing branches. On the other hand, axonal outgrowth was not inhibited at the low MVK concentrations, since the main axonal length and the total axonal length in the 0.1 and 0.3 μM MVK groups were comparable to those in the control group at DIV 7 (Fig. 2D–E).
We also plotted the cumulative probability according to the axonal branch length (Fig. 2F). At 0.3 μM MVK, the distribution of the axonal branch length shifted to the right, indicating the development of longer axonal branches. This may be caused by the lack of newly formed branches since neurons in the 0.3 μM MVK group had much fewer branches than the neurons in the control group (Fig. 2C). The neurons treated with 1.0 μM MVK exhibited a distribution of the axonal branch length similar to the neurons in the control group, despite the axonal branch number decreasing, which suggested that the axonal outgrowth of branches was also inhibited (Fig. 2F). Along with inducing a decrease in the total axonal length (Fig. 2E), our data suggest that 1.0 μM MVK treatment also inhibits the outgrowth of axons. In conclusion, we demonstrated that low concentrations of MVK inhibit the branching of axons and that high concentrations of MVK inhibit both the branching and outgrowth of axons.
In this study, we have shown that MVK treatment to cultured hippocampal neurons can cause cellular death in a concentration-dependent manner. Furthermore, MVK specifically inhibited axon branching at low concentration. Our results indicate that MVK is harmful to neuronal survival and development. We have provided a new target molecule to understand the influence of smoking tobacco use and industrial pollution on brain function and development.
As MVK is a highly reactive electrophile, it can interact with proteins via cysteine modification and therefore alter the functions of influenced proteins (Vinogradova et al., 2020). Previous studies have shown that MVK causes cell death in cell lines via the generation of reactive oxygen species and depletion of glutathione (Horiyama et al., 2014; Sathishkumar et al., 2007). It is possible that the same mechanism underlies the cell death observed in the primary neurons in our study. In addition, we found that the non-neuronal cells in our culture, presumably meningeal cells, endothelial cells, and glial cells, were also susceptible to cell death from MVK exposure. It would be interesting to determine the effect of MVK on glial cells, given that they are a major component of the brain.
A cigarette contains approximately 0.13 mg of MVK (Kusama et al., 1978). Since adult humans have a total blood volume of approximately 5 L, one can be exposed to 0.37 μM MVK from a single cigarette if MVK is completely absorbed. Therefore, it is both surprising and alarming for us to have found out that MVK treatment, even at a concentration of 0.1 μM or 0.3 μM, can significantly reduce the number of axonal branches (Fig. 2C). Axonal branching is a process that requires on-site energy support and the coordination of multiple proteins by mitochondria (Spillane et al., 2013; Tao et al., 2014). We suspect that the oxidative stress imposed by MVK disrupts the mitochondrial function and subsequently inhibits the axonal branching of neuronal cells (Guo et al., 2013). In this study, we did not analyze the morphology of dendrites because dendrites were underdeveloped in our culture, which was probably a result of the low cell density of the culture.
In this study, we showed that MVK reduced neuronal survival and axonal morphogenesis. Since we can be exposed to MVK in many ways, it would be worthwhile to investigate whether other basic neuronal functions, such as synaptogenesis and neuronal activity, are also susceptible to negative effects from exposure to MVK. Future studies focusing on the relationship between MVK and cognitive function or neurological diseases should be performed.
This study was supported by the Pharmacological Research Foundation (H. N.), Mochida Memorial Foundation (H. N.), and Suzuken Memorial Foundation (Z. Z.).
Conflict of interestThe authors declare that there is no conflict of interest.