2023 年 48 巻 1 号 p. 1-14
2023 年 48 巻 1 号 p. 1-14
Although microsampling of blood is recommended to promote the 3Rs in toxicokinetic (TK) evaluation, there are few reports applying microsampling in actual toxicity evaluation. Here, we assessed the effects of microsampling on toxicological evaluation of methapyrilene hydrochloride, a hepatotoxic substance. Female SD rats received methapyrilene hydrochloride orally at dose levels of 0 (vehicle), 10, and 30 mg/kg BW, once daily for 4 weeks. Each dose level included a microsampling group and a non-microsampling group (n = 5). In the microsampling groups, blood sampling (50 µL/time point) was performed at 6 time points on day 1 of administration and 7 time points on day 27–28; all the animals underwent necropsy on day 29. Toxicity studies and TK analysis were performed, and through these studies in 2 organizations, cross-organization validation of the effect on toxicity evaluation was conducted. In one organization, microsampling obscured changes in some parameters in hematology due to the administration of methapyrilene hydrochloride. In the other organization, although the relationship between the developing pattern of histopathological findings in the liver and the blood sampling was suspected, it was associated with poor reproducibility; this was considered as a change within a variation range of biological reactions. Each of these phenomena was observed in only one organization without consistency. In both organizations, no effect of blood microsampling was observed in other endpoints. In conclusion, microsampling is considered to be a technique applicable to safety studies of drugs showing hepatotoxicity, as it did not show a marked influence on the toxicological evaluation of methapyrilene hydrochloride.
Toxicokinetic (TK) assessment in non-clinical safety studies is important for providing information on the relationship between systemic exposure and the toxicity of candidate drugs for development (ICH, 1994). In TK evaluation, blood sampling from experimental animals after the administration of candidate drugs is usually performed over time, and exposure analysis based on the results of drug concentration analysis is widely used.
As the volume of circulating blood is limited in rodents generally used in toxicity studies, historically, instead of using toxicity study groups, blood samples have been collected from satellite groups for TK evaluation at a volume of approximately 200 μL per time point, performed over time and utilized for exposure analysis. However, due to improvements in physicochemical analytical technology, measurement of drug concentration using very small amounts of blood with high reliability has become feasible, and microsampling, which defined as a method of sampling a small amount of blood (50 µL or less) (ICH, 2017), has been recommended to toxicity study groups (or even satellite groups) for TK evaluation (ICH, 2017). With microsampling, in addition to contributing to the reduction of distress of animals by decreasing total blood sampling amount per individual animal and decreasing the number of animals used by reduction or abolition of animals included for TK studies, there is an expectation for an advantage in toxicological evaluation, as the relationship between safety data and exposure to the test substance can be evaluated in the same animal (ICH, 2017), when applied to the toxicity study groups.
However, at present, there is limited information on the influence of blood sampling from the main study groups on toxicity and TK evaluation, and it has become a critical factor that prevents the common use of microsampling in toxicity studies. Therefore, our team has performed several evaluation studies for the effects of microsampling on toxicological parameters or TK parameters using the rat model. It has been demonstrated that microsampling from the jugular vein and tail vein shows low concern for preventing evaluation in typical toxicity studies, that there is no marked influence on toxicity parameters between blood sampling sites; this is a practicable technique in a wide range of study organizations (Yokoyama et al., 2020; Hattori et al., 2020). However, one challenge is that the data of our previous reports stated above were obtained from animals maintaining normal homeostasis, and there is a limited information for estimating the microsampling effect on toxicity parameters in animals exhibiting adverse reactions due to drug exposure.
Therefore, we then focused on assessing the effects of microsampling on toxicity evaluation under conditions after the administration of toxic drugs. To the best of our knowledge, previous reports investigating such an influence are limited to studies evaluating the influence on neurobehavioral clinical observation, respiratory parameters and some parameters in blood chemistry as well as the influence of a hematotoxic drug phenacetin or immunotoxic drug azathioprine (Li et al., 2020; Prior et al., 2015; Ohtsuka et al., 2022; Tochitani et al., 2022). In particular, we exclusively targeted the liver, an organ to which drugs pass first after absorption at the digestive tract. Thus, liver is regarded as an important target organ for toxicity development, and we aimed to assess the effects of microsampling on the evaluation of hepatotoxicity.
Methapyrilene is a known hepatotoxicity-inducing substance that causes histopathological changes in the liver, including hypertrophy of hepatocytes, single-cell necrosis, and increased mitotic figures of hepatocytes, as indicated by repeated administration to rats (Igarashi et al., 2015; Slopianka et al., 2017). In this study, methapyrilene hydrochloride was administered to rats by repeated oral administration for 4 weeks at a selected dose level near the reported LOAEL for hepatotoxicity, and the potential effects of microsampling on toxicity parameters were evaluated. Two organizations participated in the study, and a total of three studies were conducted, including a study scrutinizing any areas showing inconsistencies between the organizations in the first investigational results. In addition, we discuss whether microsampling is a technique applicable to toxicity studies for the detection of hepatotoxicity.
The animal studies were conducted in two organizations (A and B), and all studies were approved by the animal experiment ethics committee of each organization. Plasma drug concentrations were measured at the National Institute of Health Sciences. Female rats (Crl:CD (SD)) at 5 weeks of age were purchased from Charles River Laboratories Japan Inc. and used in this study. We selected female rats that are susceptible to blood sampling due to low circulating volumes. The animals were allowed free access to the diet for CRF-LPF (Oriental Yeast Co., Ltd., Tokyo, Japan) (organization A) or CRF-1 (Oriental Yeast Co., Ltd.) (organization B) and drinking water (tap water) in both organizations. The animal care conditions consisted of a temperature range of 20–26°C, 30%–70% relative humidity, 10–20 times/hr of fresh ventilation, and 12 hr/day of illumination cycle in organization A, and a temperature range of 20–26°C, 30%–70% relative humidity, 10–15 times/hr of fresh ventilation, and 12 hr/day of illumination cycle in organization B.
Study ProtocolThree 4-week repeated oral dose studies were performed. Among them, two studies were designed to examine typical toxicity study items in reference to the general toxicity study guidelines (ICH S4) and TK evaluation guidelines (ICH S3A) and were conducted in organizations A and B, independently (Experiment 1; Exp 1). An additional study (Experiment 2; Exp 2) was conducted in organization B for the purpose of validating the influence of microsampling detected in Exp 1, regarding histopathology of the liver as the test item. After approximately 1 week of quarantine/acclimation period, the animals were assigned to each group (n = 5) by stratified randomization based on body weight at grouping (6 weeks of age at the start of administration).
AdministrationFor Exp 1, the dose levels of methapyrilene hydrochloride, 10 and 30 mg/kg, were selected as they are close to the no observed adverse effect level (NOAEL) and the lowest observed adverse effect level (LOAEL), respectively, for the reported hepatotoxicity (Igarashi et al., 2015). Methapyrilene hydrochloride (Sigma-Aldrich, St. Louis, MO, USA) was suspended in 0.5% methylcellulose solution (MC; organization A: Shin-Etsu Chemical Co., Ltd., Tokyo, Japan; organization B: Wako Pure Chemical Industry Co., Ltd., Osaka, Japan) to prepare suspensions at concentrations of 2 and 6 mg/mL both at organizations A and B. The solution was administered to the rats by oral gavage once daily for 4 weeks at a dose volume of 5 mL/kg. Exp 2 was conducted at the selected dose level of 10 mg/kg only, at which the influence of microsampling was suspected in Exp 1 at organization B.
Observation and examinationsExp 1 included methapyrilene hydrochloride dose groups (10 and 30 mg/kg) and a vehicle control group, each consisting of a non-blood sampling group and a blood sampling group, with a total of six groups. Exp 2 included two groups in total: a non-blood sampling group and a blood-sampling group at a methapyrilene hydrochloride dose level of 10 mg/kg. In each study, the following observations and examinations were performed:
Clinical observation, body weight, food and water consumptionClinical observation was performed daily (Exp 1 and Exp 2), and body weight (Exp 1 and Exp 2) and food consumption (Exp 1 only) were assessed at least once weekly. Water consumption was measured in week 4, when urinalysis was performed (Exp 1 only).
Ophthalmology and clinical pathology/chemistryOphthalmology and clinical pathology examinations were performed in Exp 1 only. Ophthalmology was performed in all animals before the start of administration and at week four of administration.
Urinalysis was performed using fresh urine and 24-hr accumulated urine collected in week 4 of administration. Examination parameters included urine volume, specific gravity (organization B only), pH, glucose, protein, occult blood, ketone bodies, bilirubin, urobilinogen, electrolytes (Na, K, and Cl), urine sediment (organization A only), and urine osmotic pressure (organization A only). Examinations were performed using an automatic urine analyzer (Clinitek Advantus, Siemens Healthcare Diagnostics, K.K., Tokyo, Japan), osmotic pressure analyzer (Osmostation OM-6060, Arkray Inc., Kyoto, Japan), clinical chemistry autoanalyzer (TBA-120FR, Canon Medical Systems Corporation, Tochigi, Japan), Multistix test paper (Siemens Healthcare Diagnostics, K.K., Tokyo, Japan) at organization A, or automatic urine analyzer (Clinitek Advantus, Siemens Healthcare Diagnostics, K.K.), fully automatic electrolyte analyzer (EA07, A&T Corporation, Kanagawa, Japan), digital urine specific gravity refractometer (UG-D, Atago Co., Ltd., Tokyo, Japan), and Multistix test paper (Siemens Healthcare Diagnostics, K.K.) at organization B.
Hematology was performed on the day of necropsy, using blood and plasma collected from the abdominal aorta (organization A) or from the inferior vena cava (organization B) under anesthesia with isoflurane, after overnight fasting of the animals (for approximately 18 hr). Hematological parameters included the red blood cell (RBC) count, hematocrit (Hct), hemoglobin (Hgb), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet count, white blood cell count, neutrophils (ratio and count), lymphocytes (ratio and count), monocytes (ratio and count), basophils (ratio and count), eosinophils (ratio and count), large unstained cells (ratio and count) (organization B only), reticulocytes (ratio and count), fibrinogen, prothrombin time (PT), and activated partial thromboplastin time (APTT). The examinations were performed using a comprehensive hematology analyzer (ADVIA2120, Siemens Healthcare Diagnostics, K.K.) and a fully automatic blood coagulation analyzer (CS-2400, Sysmex Corporation, Hyogo, Japan) at organization A, or a comprehensive hematology analyzer (ADVIA2120) and a fully automatic blood coagulation analyzer (CS2000i, Sysmex Corporation) at organization B.
The blood chemistry parameters included aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), creatine phosphokinase (CPK), glucose, total cholesterol, neutral fat, blood urea nitrogen (BUN), creatinine, total bilirubin, phospholipid (organization B only), total protein, albumin, calcium, inorganic phosphorus, Na, K, Cl, A/G ratio (albumin/globulin ratio), glutamate dehydrogenase (GLDH) (organization A only), sorbitol dehydrogenase (SDH) (organization A only), and guanase (GU) (organization A only). The examinations were performed using a clinical chemistry autoanalyzer (TBA-120FR, Canon Medical Systems Corporation) at both organizations, in addition to a fully automatic electrophoresis analyzer (Epalyzer 2 Junior, Helena Laboratories Corporation, Saitama, Japan) only at organization B.
Organ weight, necropsy and histopathologyOn the day after the last administration (day 29; the day after the last blood sampling), animals were anesthetized with isoflurane and euthanized by exsanguination, followed by macroscopic examination of various organs throughout the body. In Exp 1, the weights of the brain, pituitary gland, submandibular gland, thymus, heart, liver, spleen, kidneys, adrenal glands, ovaries, lungs (organization A only), pancreas (organization A only), uterus (organization A only), and thyroid gland (organization A only) were measured, and the body weight ratio was calculated based on the body weight on the day of necropsy. Notably, the bilateral organs, such as the kidneys, were measured by combining both sides. The liver was fixed in 10% neutral buffered formalin solution, followed by slide preparation using hematoxylin-eosin (HE) staining for histopathological examination under a light microscope (Exp 1 and 2). We conducted a mutual review of histopathological evaluation of liver at both organizations, because effects of blood microsampling on evaluation of hepatotoxicity were suspected in the results of Exp1 at organization B. We confirmed that the histopathological grading results of Exp1 at one organization were not different from the evaluation by the pathologists at the other organization.
Blood sampling for assumed TKsOn the first day (day 1) and week 4 (day 27) of administration, 50 µL of blood was collected at each time point from the jugular vein without anesthesia using heparin Na-treated BD Lo-Dose 3/10 mL, 29G (Nippon Becton Dickinson Company, Ltd., Tokyo, Japan). Blood sampling was performed at 6 time points (0.5, 1, 2, 4, 8, and 24 hr post-dose) on the first day of administration, and at 7 time points (before administration and 0.5, 1, 2, 4, 8, and 24 hr post-dose) in week 4 of administration.
TKThe plasma concentrations of methapyrilene were determined using a reverse-phase liquid chromatography/mass spectrometry (LC/MS) system (Ultimate 3000 RSLC and TSQ Quantiva; Thermo Fisher Scientific, Waltham, MA, USA). The range of calibration standards was set from 10 pg/mL to 10 ng/mL, with seven points. Twenty μL of plasma was mixed with 280 μL of H2O/acetonitrile (ACN) (1:4 [vol/vol]) with 1% formic acid containing methapyrilene-d6 fumarate salt (Sigma-Aldrich Japan K.K.) as an internal standard. Subseuently mixed samples were centrifuged by 15,000 g for 10 min at 4°C, and the resulting supernatant was subsequently filtered with mono-spin Phospholipid (GL Science, Tokyo, Japan) to remove proteins and phospholipids. The resulting solution was evaporated and reconstituted in 60 μL of 60% methanol for LC/MS analysis. An InertSustain AQ-C18 column (3 μm, 2.1 x 150 mm; GL Science) was used to separate methapyrilene, and the column oven temperature was set at 50°C. The solvent composition of the mobile phase was water with 0.1% formic acid for solvent A and ACN with 0.1% formic acid for solvent B, and the mobile phase was pumped through at a flow rate of 400 μL/min with ramp gradient (from 0 to 100% of B for 4 min, 1 min hold at 100% of B, and equilibrate with 0% of B for 1 min). MS was operated in the heated ESI positive ion mode, and the detection of methapyrilene and methapyrilene-d6 was conducted using SRM at transitions 262.37 > 217.05 for methapyrilene and 268.2 > 217.05 for methapyrilene-d6. The peak areas of methapyrilene and methapyrilene-d6 were quantified using TraceFinder software (version 4.0; Thermo Fisher Scientific), and the plasma methapyrilene concentration was calculated.
The maximum concentration at the blood sampling time point in individual animals was determined from the plasma drug concentration measurement values. The mean value was regarded as the maximum drug concentration (Cmax), and the mean value of the blood sampling time point showing the maximum concentration was regarded as the time to the maximum plasma concentration (Tmax). From the mean values determined as described above, the area under the concentration-time curve (AUC0–24hr) was calculated using the trapezoidal method.
Statistics and data analysesFor quantitative values obtained from the vehicle control group and the methapyrilene hydrochloride dose groups, organization A calculated the mean values and standard deviations for each group, and utilized Bartlett’s test for homogeneity of variance (two-tailed test, P < 0.01) for body weight, hematology (except for differential white blood cell (ratio and count) and reticulocyte (ratio and count), blood biochemistry, and organ weight. In cases of homogeneous variance, one-way analysis of variance (two-tailed test, P < 0.05) was performed. When a significant difference was observed, the results of Dunnett’s test (two-tailed test, P < 0.05) were adopted, and when a significant difference was not observed, results of one-way analysis of variance were adopted. In addition, in cases of heterogeneous variance in Bartlett’s test for homogeneity of variance, logarithmic transformation and Bartlett’s test for homogeneity of variance were performed again. When heterogeneous variance was obtained again, the Kruskal–Wallis rank sum test (two-tailed test, P < 0.05) was performed. When a significant difference was observed, the results of Steel’s test (two-tailed test, P < 0.05) were adopted, and when a significant difference was not observed, the result of the Kruskal-Wallis rank sum test was adopted. For food consumption, the differential white blood cell (ratio and count), reticulocyte (ratio and count), Kruskal-Wallis rank sum test (two-tailed test, P < 0.05) were performed. When a significant difference was observed, Steel’s test (two-tailed test, judged to be significant if P < 0.05) was adopted. When a significant difference was not observed, the result of the Kruskal-Wallis rank sum test was adopted. Organization B calculated the mean values and standard deviations for each group and performed Bartlett’s test for homogeneity of variance (two-tailed test, P < 0.01). In cases of homogenous variance, one-way analysis of variance and then Dunnett’s multiple comparison test (two-tailed test, P < 0.05) was performed. In cases of heterogeneous variance, Steel’s multiple comparison test (two-tailed test, P < 0.05) was performed.
No notable clinical signs were observed during the administration period. The body weight and food consumption data are presented in Table 1. Body weight showed a normal increase in all groups, with no significant changes observed in any dose groups in comparison to the vehicle control group, regardless of whether microsampling was performed. In addition, no changes in food or water consumption were observed (data not shown for water consumption).
 Ophthalmology and clinical pathology
Ophthalmology and clinical pathology
No notable changes were observed in ophthalmology.
For urinalysis, in organization A, although squamous epithelial cells and crystals in urinary sediment were observed in the 30 mg/kg group when microsampling was performed, this was considered to be an accidental change because these changes are often observed even in non-treated animals. In organization B, no changes in comparison with the vehicle control group were observed in any of the dose groups.
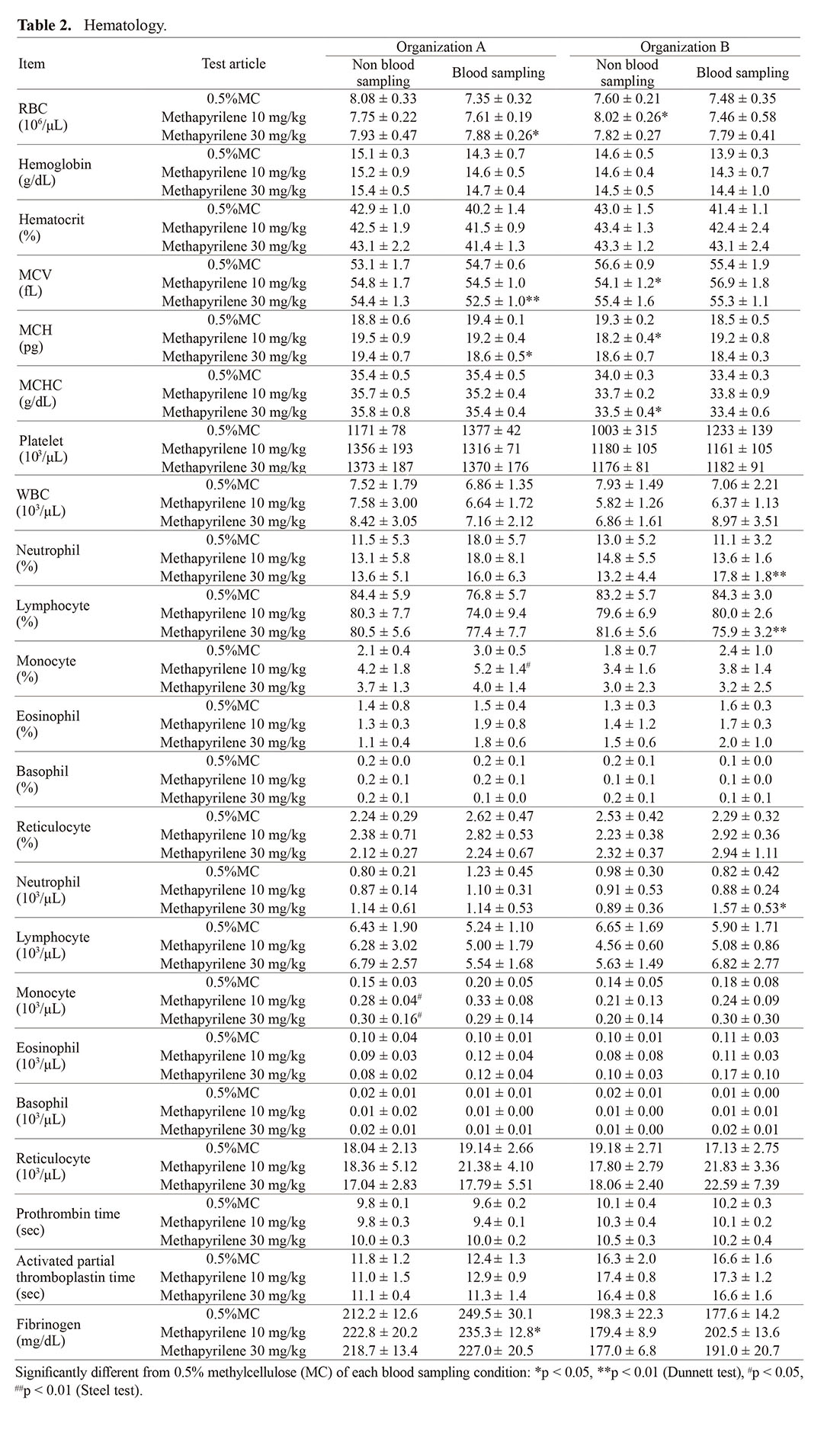
For hematology, statistical differences were sporadically observed as methapyrilene administration, but no common changes between the groups with and without microsampling were found in both organizations. In organization A, a slight decreasing trend in RBC, Hgb, and Hct and an increasing in the ratio and count of monocytes and neutrophils were observed with microsampling compared with no microsampling. When microsampling was not performed, an increase in the ratio and count of monocytes in the 10 mg/kg and higher dose groups, as well as an increasing trend of neutrophil count in the 30 mg/kg group, were observed in comparison to the control group. On the other hand, when microsampling was performed, although an increasing trend in monocyte count was observed in the 10 mg/kg and higher dose groups, no statistical significance was observed. In addition, an increase in neutrophil count was not observed in the 30 mg/kg group. In organization B, no effects on RBC parameters were observed, but when microsampling was performed, an increase in the ratio and count of neutrophils, as well as a decrease in lymphocyte percentage, were observed in the 30 mg/kg group (Table 2). However, there were no changes in the absolute lymphocyte count, indicating that the significant difference detected in percentage was associated with the changes in neutrophils, and this change in lymphocyte count was not considered to be significant. Significant differences detected in other parameters showed minimal changes with no relation to the dose levels of methapyrilene hydrochloride or within the background data range (mean ± 2SD) of normal animals, which corresponding to the physiological variation range, although some showed statistically significant changes.
For blood chemistry, in organization A, an increase in GLDH in the 10 mg/kg and higher dose groups and an increase in glucose in the 30 mg/kg group were observed, regardless of whether microsampling was performed (Table 3). Although no statistical significance was observed in the dose groups with no microsampling performed, the degree of change was comparable, showing no effect of microsampling. Although an increase in the A/G ratio was observed in the 10 mg/kg and higher dose groups with microsampling, it was a slight change within the background data range (mean ± 2SD) of normal animals. In organization B, no significant changes in clinical chemistry parameters were observed by methapyrilene treatment in the groups with or without microsampling.
 Organ weight, necropsy and histopathology
Organ weight, necropsy and histopathology
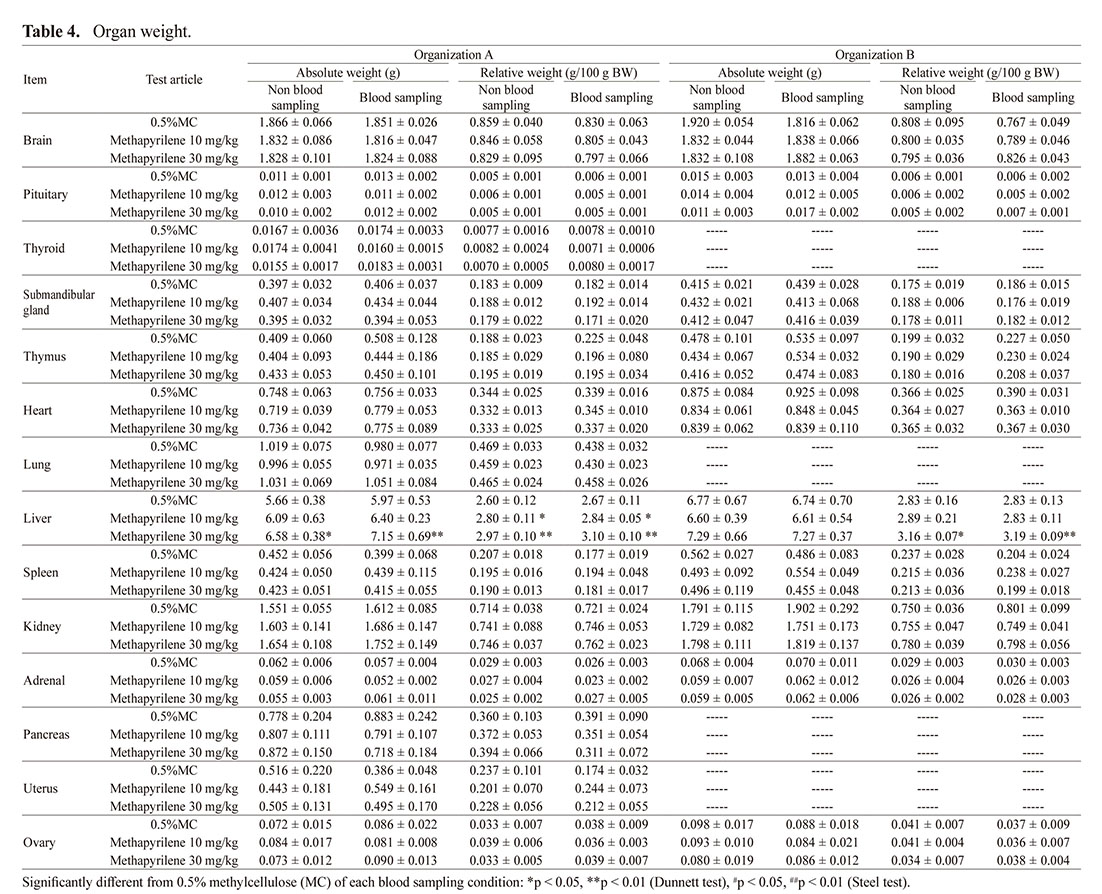
In both organizations, significant increases or increasing trend in liver weight or liver-to-body weight ratio was observed, regardless of whether microsampling was performed (Table 4). In addition, no other differences in the data were observed, regardless of whether microsampling was performed.
In necropsy, in organization A, discoloration of the liver was observed in the 30 mg/kg group regardless of whether microsampling was performed (Table 5). In organization B, a subcutaneous trace of blood (bleeding trace) was observed on the neck, which was the blood-sampling site, in all groups in which microsampling was performed. Other findings observed were background findings in rats and were interpreted not to be an effect of methapyrilene hydrochloride administration or microsampling (data not shown).

In histopathology, abnormal findings in the liver associated with single-cell necrosis, increased sizes of nucleus/nucleolus of hepatocytes, hepatocyte vacuolation or periportal mononuclear cell infiltration were observed in both organizations (Table 6). The gross finding observed in organization A (discoloration of the liver) was considered as related to hepatocyte vacuolation. In organization A, regardless of whether microsampling was performed, the findings occurred at comparable incidence and grade in 10 mg/kg and higher dose groups. On the other hand, in organization B, the findings were expressed at the comparable incidence and grade in the 30 mg/kg group regardless of the presence or absence of microsampling, however, increased sizes of nucleus/nucleolus of hepatocytes, single-cell necrosis of hepatocytes and periportal mononuclear cell infiltration in the 10 mg/kg group were observed only in which microsampling was performed (Exp 1 of Table 6), which could suggest a potential relationship between the occurrence of histopathological changes and blood microsampling. Therefore, an additional study to confirm the reproducibility of the event was conducted only in the 10 mg/kg group. The results showed that the occurrence of histopathological findings in the group in which microsampling was not performed was also consistent with those in the group in which microsampling was performed, and there was no influence of blood microsampling on the incidence or grade (Exp 2 of Table 6).
 TK
TK
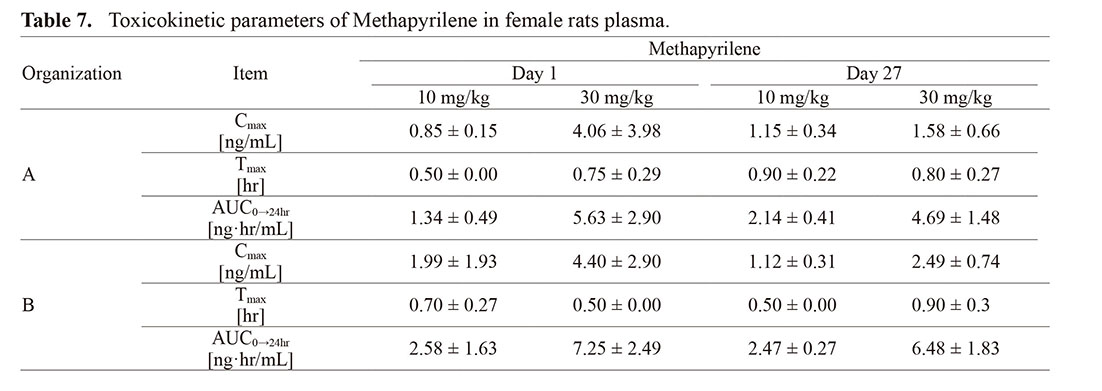
Dose-dependent increases in Cmax and AUC0–24hr for plasma methapyrilene concentration were observed, with no accumulation of methapyrilene in the blood due to repeated administration, and Tmax within 1 hr post-dose was observed in both organizations, indicating similar behavior of drug concentration in the blood in both organizations (Table 7).
In this study, we exclusively targeted the liver, a representative toxicity target organ, and conducted a total of three studies with methapyrilene hydrochloride, a known hepatotoxic substance, in two organizations by repeated administration to rats for 4 weeks under identical conditions. In these studies, we scrutinized the influence of microsampling on animals that showed adverse reactions after exposure to a hepatotoxic drug.
In all studies, during the administration period, no animals showed mortality or serious clinical signs, and only minimal influence of blood microsampling were observed in typical evaluation items including body weight, food consumption, ophthalmology, urinalysis and blood chemistry. These results indicate that we could confirm that microsampling does not affect evaluation of these parameters.
Concerning hepatotoxicity, the most important evaluation items in this study to identify the influence of microsampling, increase in liver weight, and abnormal histopathological findings in the liver were detected following methapyrilene hydrochloride administration in both organizations. There was no difference in liver weight between the cases with or without microsampling, indicating that microsampling did not affect the detection of this change by methapyrilene. For histopathology, the findings suggesting hepatotoxicity (increased sizes of nucleus/nucleolus of hepatocytes, single cell necrosis of hepatocytes, periportal mononuclear cell infiltration) at 10 mg/kg were observed only in the groups in which microsampling was performed in organization B (Exp 1), suggesting that finding development behavior at the same dose (10 mg/kg) was related to microsampling. However, in the additional study conducted under identical conditions (Exp 2), almost identical development of pathological changes at 10 mg/kg was observed between the groups with or without microsampling. The dose level of 10 mg/kg was close to the NOAEL at which hepatotoxicity has not been previously reported (Igarashi et al., 2015). In addition, these findings were not observed in any of the animals, but in only a part of the animals, both in Exp 1 and Exp 2. Considering the above results, the absence of these findings in the groups without microsampling in Exp 1 at organization B is a phenomenon encompassed within variation of biological reactions typically observed (difference between organizations, difference between studies), and the influence of microsampling on this finding and on study evaluation were considered to be minimal, if any.
In organization A, as an effect of microsampling, the possibility exists that changes in some parameters by methapyrilene may be obscured due to the effects of blood sampling. While a statistically significant increase in monocyte count was observed in the 10 mg/kg and higher dose groups without microsampling, no statistically significant difference was observed when microsampling was performed. In addition, although an increasing trend in neutrophil count was observed at 30 mg/kg without microsampling, the same change was not observed when microsampling was performed. As the trend of increase in monocyte and neutrophil counts was observed only without microsampling, there seemed to be a possibility that the increase was obscured by microsampling. However, increasing trends in monocyte counts were observed both groups with or without microsampling in organization B. In addition, the reverse trends were detected in neutrophil counts between the groups with or without microsampling in organization B: i.e., an increase in neutrophil count was observed only in the methapyrilene hydrochloride dose group with microsampling. As no change in neutrophil count was observed only with methapyrilene hydrochloride administration or with blood sampling, there seemed to be a possibility of an increase in neutrophil count due to methapyrilene hydrochloride in combination with blood sampling. The slight changes in monocyte and neutrophil counts observed in organizations A and B were considered to be due to inflammatory reactions associated with the local invasion caused by blood sampling. The difference in route of blood collection (organization A: abdominal aorta, organization B: inferior vena cava) may be one of the reasons for the slight differences of changes in hematology parameters between organizations, although the precise reason was not clear. As stated above, this phenomenon was not consistently observed between organizations, and thus in the future, it is necessary to confirm reproducibility and validate the extent of influence by conducting validation experiments using drugs affecting the immune system.
Furthermore, in addition to the above-mentioned changes, as an inevitable effect when microsampling is performed in a similar way to previous investigations (on the evaluation of toxicity studies), hematology showed a slight decrease in RBC, Hgb, and Hct in organization A (Yokoyama et al., 2020). Although the influence of these changes on toxicity evaluation is considered to be minimal because they were minimal changes within the range of background data range (mean ± 2SD) of normal animals, which corresponding to the physiological variation range, these parameters must be evaluated carefully. In addition, it is also necessary to pay attention to subcutaneous blood traces (red points, bleeding trace) at the blood-sampling site (neck) during necropsy. However, it can be clearly said that this change does not affect the conclusions of toxicity studies, as this is obviously due to blood sampling operations.
For TK measurements, the results showed generally consistent behavior of TK parameters between organizations.
In summary, microsampling did not show qualitative effects on the toxicity evaluation of the hepatotoxic substance, methapyrilene hydrochloride. Although the potential for driving the development of some findings was found quantitatively, there was poor consistency between studies or organizations, and these findings were interpreted as changes of degree within a range of physiological variation typically observed in biological reactions. The current experimental validation was performed using female rats in this study, because it is known that the influence of microsampling on the living body is generally observed in females more strongly than in males (Yokoyama et al., 2020), at least partly due to lower circulating blood volume in females. Therefore, the results of the present study suggest that microsampling can be utilized in general toxicity studies using male and female animals without giving deep concern for the evaluation of hepatotoxic substances represented by methapyrilene hydrochloride.
This work was supported in part by AMED under Grant Numbers JP19ak0101073j0003 and JP20ak0101073j0004.
Conflict of interestThe authors declare that there is no conflict of interest.