2023 年 48 巻 1 号 p. 37-45
2023 年 48 巻 1 号 p. 37-45
Transient Receptor Potential Ankyrin 1 (TRPA1), which is expressed in the airways, has causative and exacerbating roles in respiratory diseases. TRPA1 is known as a target of sick building syndrome-related air pollutants, such as formaldehyde. Thus, an in vitro TRPA1 activation assay would be useful for predicting the potential risk of air pollution. In this study, we used human TRPA1 (hTRPA1)- and mouse TRPA1 (mTRPA1)-expressing cell lines to measure TRPA1 activation by the emerging indoor air pollutants 2-ethyl-1-hexanol (2-EH), a mixture of 2,2,4-trimethyl-1,3-pentanediol 1- and 3-monoisobutyrate (Texanol), and 2,2,4-trimethyl-1,3-pentanediol diisobutyrate (TXIB). The results indicated that 2-EH activated both hTRPA1 and mTRPA1 in a concentration-dependent manner, whereas TXIB did not activate hTRPA1 or mTRPA1. Texanol also activated hTRPA1 in a concentration-dependent manner. In contrast, a bell-shaped concentration-dependent curve was observed for mouse TRPA1 activation by Texanol, indicating inhibitory effects at a higher concentration range, which was also reported for menthol, a typical TRPA1 modulator. To further elucidate the mechanism underlying the species difference in TRPA1 activation by Texanol, V875G and G878V mutations were introduced into hTRPA1 and mTRPA1, respectively, which were reported to be key mutations for the inhibitory effect of menthol. These mutations switched the inhibitory effects of Texanol; thus, hTRPA1/V875G, but not mTRPA1/G878V, was inhibited at higher concentrations of Texanol. These results indicate that Texanol shares an interaction site with menthol. Overall, these findings suggest that careful interpretation is necessary when extrapolating rodent TRPA1-dependent toxicological effects to humans, especially with respect to the risk assessment of indoor air pollutants.
Because many people spend a lot of time indoors, the quality of the indoor environment is important to their health. Volatile organic compounds (VOCs) in indoor air are believed to contribute to sick building syndrome and multiple chemical sensitivity (Win-Shwe et al., 2013; Redlich et al., 1997). They could also exacerbate asthma and chronic obstructive pulmonary disease (COPD) (Maung et al., 2022; Bentayeb et al., 2015). From 1997 through 2002, the Ministry of Health, Labour, and Welfare (MHLW) in Japan established indoor air guidelines to maintain public health for 13 VOCs and semi-volatile organic compounds, including aldehydes, alkanes, aromatic hydrocarbons, pesticides, and phthalates (MHLW, 2000a, 2000b, 2001, 2002). In a recent nationwide survey of indoor air pollutants in Japan (Takeuchi et al., 2020), three resin additive-related compounds were found at high concentrations and/or high frequency, including 2-ethyl-1-hexanol (2-EH), 2,2,4-trimethyl-1,3-pentanediol 1- and 3-monoisobutyrate (Texanol), and 2,2,4-trimethyl-1,3-pentanediol diisobutyrate (TXIB), which were selected as candidate compounds to establish indoor air guidelines (MHLW, 2017) (Fig. 1). 2-EH is derived from the alcoholic moiety of di(2-ethylhexyl)phthalate, a widely used plasticizer, and 2-ethylhexyl acrylate is used in paint and adhesives, which are readily released by alkali or enzymatic hydrolysis reactions. Texanol is a premier coalescent for latex paints, which is also produced by TXIB hydrolysis in the environment and in mammals (Nielsen et al., 1997). TXIB is used as a film-forming aid and plasticizer for polyvinyl chloride resins (Yu and Crump, 1998).
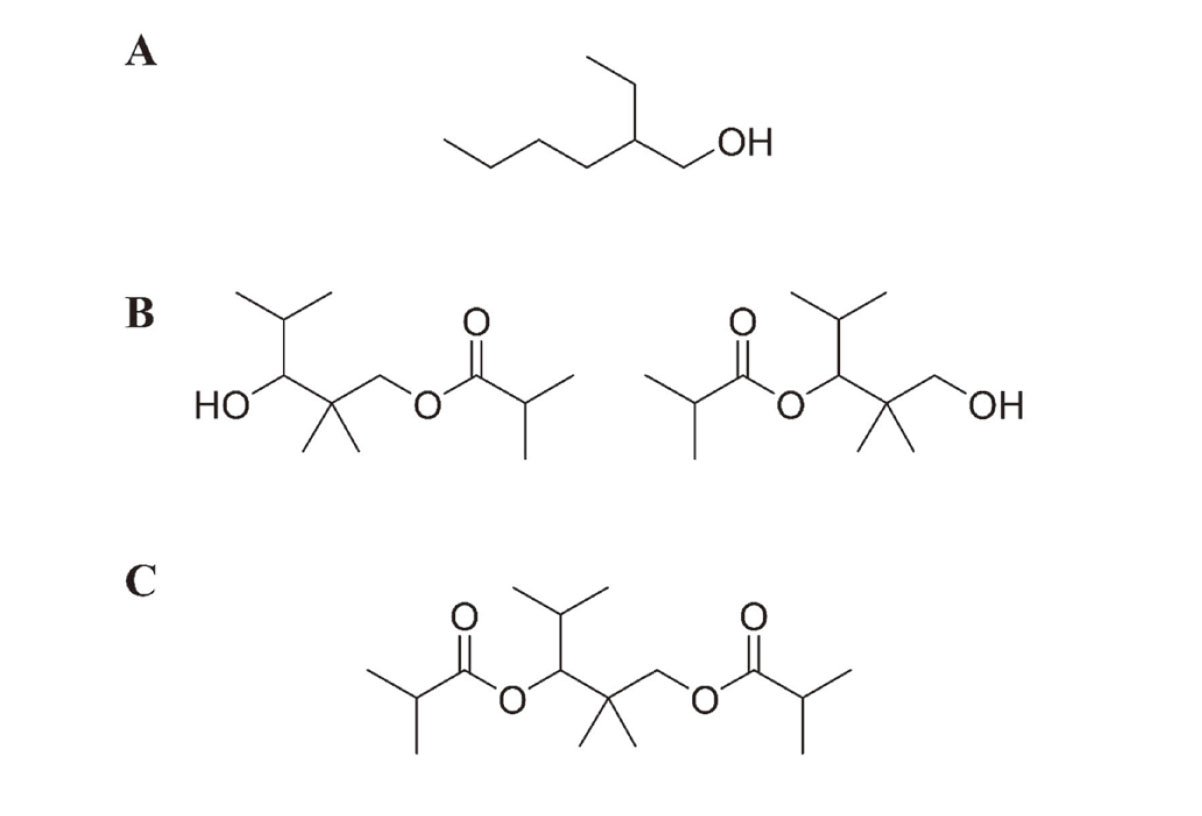
Structures of resin additive-related chemicals used in this study. A, 2-ethyl-1-hexanol (2-EH); B, 2,2,4-trimethyl-1,3-pentanediol 1- and 3-monoisobutyrate (Texanol); C, 2,2,4-trimethyl-1,3-pentanediol 1,3-diisobutyrate (TXIB).
To establish indoor air guidelines, adequate toxicological data should be provided including chronic toxicity, carcinogenicity, allergenicity, and irritancy; however, only limited data are available regarding the toxicity of these candidate compounds, except 2-EH. In a human eye irritation study, a 4-hr exposure of 2-EH at 10 ppm (equivalent to 54 mg/m3 at 25°C) or more increased the blink rate of volunteers in a concentration-dependent manner (Kiesswetter et al., 2005). Based on this study, a non-observed adverse effects concentration of 1.5 ppm (8 mg/m3) and a lowest-observed adverse effects concentration (LOAEC) of 10 ppm (54 mg/m3) were established for 2-EH. Both European Commission Employment, Social Affairs and Inclusion, and the Japan Society for Occupational Health recommended an 8-hr occupational exposure limit (a permissible limit in working environments) of 1 ppm (5.3 mg/m3) for 2-EH, based on the LOAEC value from this human eye irritation study (European Commission, 2011). The German Federal Environmental Agency set the precautionary guide value (RWI) of 0.1 mg/m3 and the health hazard guide value (RWII) of 1 mg/m3 as an indoor air guideline for 2-EH, which was derived from the LOAEC in the human eye irritation study divided by an uncertainty factor of 60 (period, 6; individual difference, 10) (Anonymous, 2013).
Texanol exhibited weak or middle potency irritation in the rabbit eye, which is also classified as a weak skin irritant by the guinea pig maximization test (O’Donoghue, 1984). TXIB had weak potency in the rabbit eye irritation OECD TG 405 test (OECD, 2021). Although TXIB is not classified as a skin irritant in the OECD TG 404 test, it caused weak skin irritation in a 24-hr guinea pig maximization test (OECD, 2015).
As outlined above, toxicity data are not sufficient for a precise risk assessment, thus the MHLW deferred the establishment of indoor air guidelines for these candidate compounds. In the present study, to gather information on airway irritation, the activation of human and mouse transient receptor potential ankyrin 1 (TRPA1) by 2-EH, Texanol, and TXIB was determined in a heterologous expression system in vitro. TRPA1 expressed in the airway plays causative and exacerbating roles in respiratory diseases, such as bronchial hyperresponsiveness and airway inflammation (Simon and Liedtke, 2008). TRPA1 is activated by many types of indoor air pollutants, such as formaldehyde, acrolein, cigarette smoke, 1-hexanol, 1-ocatanol, and phthalic acid mono(2-ethylhexyl) ester (MEHP) (Conklin et al., 2017; Jin et al., 2019; Komatsu et al., 2012; Kurhanewicz et al., 2017; Mori et al., 2022), as well as the representative TRPA1 agonists, allyl isothiocyanate (AITC) and menthol (Jordt et al., 2004; Karashima et al., 2007). Among these chemicals,1-hexanol, 1-ocatanol, MEHP, and menthol have been reported to show species-specific responses to TRPA1. Hence, in this study, we used both human TRPA1- and mouse TRPA1-expressing cell lines to screen for airway irritation caused by 2-EH, Texanol, and TXIB.
Allyl isothiocyanate (AITC, 99.0%) was purchased from the Fujifilm Wako Pure Chemical Co. (Osaka, Japan). (-)-Menthol (> 99.0%), 2-ethyl-1-hexanol (2-EH, > 99.5%), 2,2,4-trimethyl-1,3-pentanediol 1- and 3-monoisobutyrate (Texanol) (> 60%, 2,2,4-trimethyl-1,3-pentanediol 1-monoisobutyrate; ca. 40% 2,2,4-trimethyl-1,3-pentanediol 3-monoisobutyrate), and 2,2,4-trimethyl-1,3-pentanediol diisobutyrate (TXIB, > 98.5%) were obtained from the Tokyo Chemical Industry (Tokyo, Japan). Dimethyl sulfoxide (DMSO, > 99.7%; Hybri-Max) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Hank’s balanced salt buffer (+) without phenol red (HBSS) was obtained from the Nissui Pharmaceutical Co. (Tokyo, Japan). HEPES was purchased from Dojindo (Kumamoto, Japan). Other chemicals were of the highest grade commercially available.
Cell cultureThe Flp-In 293 cell lines stably expressing wild-type hTRPA1 and mTRPA1 (Flp-In 293/hTRPA1 and Flp-In 293/mTRPA1 cells) were established in our previous studies (Mori et al., 2022; Ohkawara et al., 2012). The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, high glucose; Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (Thermo Fisher Scientific), antibiotic solution (100 U/mL penicillin and 100 µg/mL streptomycin; Wako), and 2 mM GlutaMAX (Thermo Fisher Scientific). The cells were maintained in a humidified atmosphere containing 5% CO2 and 95% air at 37°C.
Site-directed mutagenesisFull-length human TRPA1 (hTRPA1; GenBank accession number NM_007332.3) and mouse TRPA1 (mTRPA1; GenBank accession number NM_177781) cDNAs were cloned as previously reported (Mori et al., 2022; Ohkawara et al., 2012). The hTRPA1 and mTRPA1 cDNAs were recombined with a pDONR207 (Thermo Fisher Scientific) entry plasmid through a BP (attB and attP) reaction using the Gateway BP clonase enzyme mix (Thermo Fisher Scientific). Using these two entry plasmids as templates, V875G and G878V-coding mutations were introduced into the hTRPA1 and mTRPA1 cDNAs by overlapping PCR (QuikChange site-directed mutagenesis kit; Agilent Technologies, Waldbronn, Germany), and the constructs were designated hTRPA1/V875G-pDONR207 and mTRPA1/G878V-pDONR207, respectively. The primers for site-directed mutagenesis are listed in Table 1. The mutations were confirmed by sequence analysis (Eurofins Genomics KK, Tokyo, Japan). hTRPA1/V875G and mTRPA1/G878V cDNAs were recombined with a pEF5/FRT/V5-DEST transfection plasmid (Thermo Fisher Scientific) through an LR (attL and attR) reaction using the Gateway LR reaction clonase enzyme mix (Thermo Fisher Scientific), and the constructs were designated hTRPA1/V875G-pEF5/FRT/V5 and mTRPA1/G878V-pEF5/FRT/V5, respectively.
| Mutation | Sense primer (5’ to 3’) | Antisense primer (5’ to 3’) |
|---|---|---|
| hTRPA1/V875G | GAAAACTTTGTTGAGGTCTACAGGTGTATTTATCTTCCTTCTTCTGG | CCAGAAGAAGGAAGATAAATACACCTGTAGACCTCAACAAAGTTTTC |
| mTRPA1/G878V | ATTGCTGAGATCGACCGTAGTGTTTATCTTCCTCC | GGAGGAAGATAAACACTACGGTCGATCTCAGCAAT |
Flp-In 293 cells (Thermo Fisher Scientific) were co-transfected with pOG44 and the hTRPA1/V875G-pEF/FRT/V5 or mTRPA1/G878V-pEF/FRT/V5 plasmids using lipofectamine 3000 (Thermo Fisher Scientific). Clones stably expressing mutant TRPA1 (Flp-In 293/hTRPA1/V875G or Flp-In 293/mTRPA1/G878V) were selected in 200 µg/mL hygromycin B. During the initial cultivation of the clones, a reduced hygromycin concentration of 50 μg/mL was used instead of the typical 200 μg/mL. The cells were maintained in a humidified atmosphere containing 5% CO2 and 95% air at 37°C.
Intracellular Ca2+ measurementCells were seeded at 80%–90% confluence in 96-well plates (poly-D-lysine black-walled, clear-bottomed plates; Greiner Bio-One, Frickenhausen, Germany) in growth medium and incubated in 5% CO2 at 37°C. After overnight culture, the medium was replaced with 100 µL of 20 mM HEPES buffered HBSS (pH 7.4) containing 1 × Calcium 6 dye (Molecular Devices, Sunnyvale, CA, USA) and the plates were incubated for 2 hr at 37°C in the dark. The fluorescence intensity was recorded using a FlexStation 3 microplate reader (Molecular Devices) at 485 nm excitation and 525 nm emission using SoftMax Pro 7.1.2 software (Molecular Devices). Background intensity was recorded for 20 sec prior to testing the chemicals. Then, 100 µL of test chemical solution was mechanically applied to the wells and fluorescence intensity was monitored for 60 sec. Test chemicals were dissolved in HBSS containing DMSO at a final concentration of less than 0.2%. Changes in cellular fluorescence are expressed as the percentage of cellular fluorescence elicited by the positive control for TRPA1, AITC (100 μM). Concentration-dependent curves were fitted using Prism 8 (GraphPad Software, San Diego, CA, USA).
Statistical analysisAll experiments were performed in triplicate and repeated twice for confirmation. All values are expressed as the mean ± SD. The statistical significance of the differences between sample mean values was assessed using a one-way analysis of variance (ANOVA) with Tukey’s post-hoc test. Data analyses were performed using Prism 8 software.
To evaluate the hTRPA1 and mTRPA1 activation effects of 2-EH, Texanol, and TXIB, the intracellular Ca2+ levels were measured using the cell lines stably expressing hTRPA1 (Flp-In 293/hTRPA1) and mTRPA1 (Flp-In 293/mTRPA1). As shown in Fig. 2A, 2-EH caused an increase in intracellular Ca2+ levels in both Flp-In 293/hTRPA1 and Flp-In 293/mTRPA1 cells in a concentration-dependent manner (EC50 values of 225 µM and 460 µM, respectively). Texanol also activated both hTRPA1 and mTRPA1 (Fig. 2B), whereas the concentration-response curves were quite different from one another. Concentration-dependent activation of hTRPA1 with an EC50 value of 180 µM was observed upon using Texanol, whereas a bell-shaped dependency was observed for the mTRPA1 activation using Texanol. Moreover, TXIB had no effects on both hTRPA1 and mTRPA1 (Fig. 2C). Fig. 2D depicts the activation of hTRPA1 and mTRPA1 by menthol, a representative TRPA1 agonist with inhibitory effects on mTRPA1 at higher concentrations (Xiao et al., 2008). Similar species differences were observed in Texanol and menthol, suggesting that high concentrations of Texanol may inhibit mTRPA1 activation but not hTRPA1.
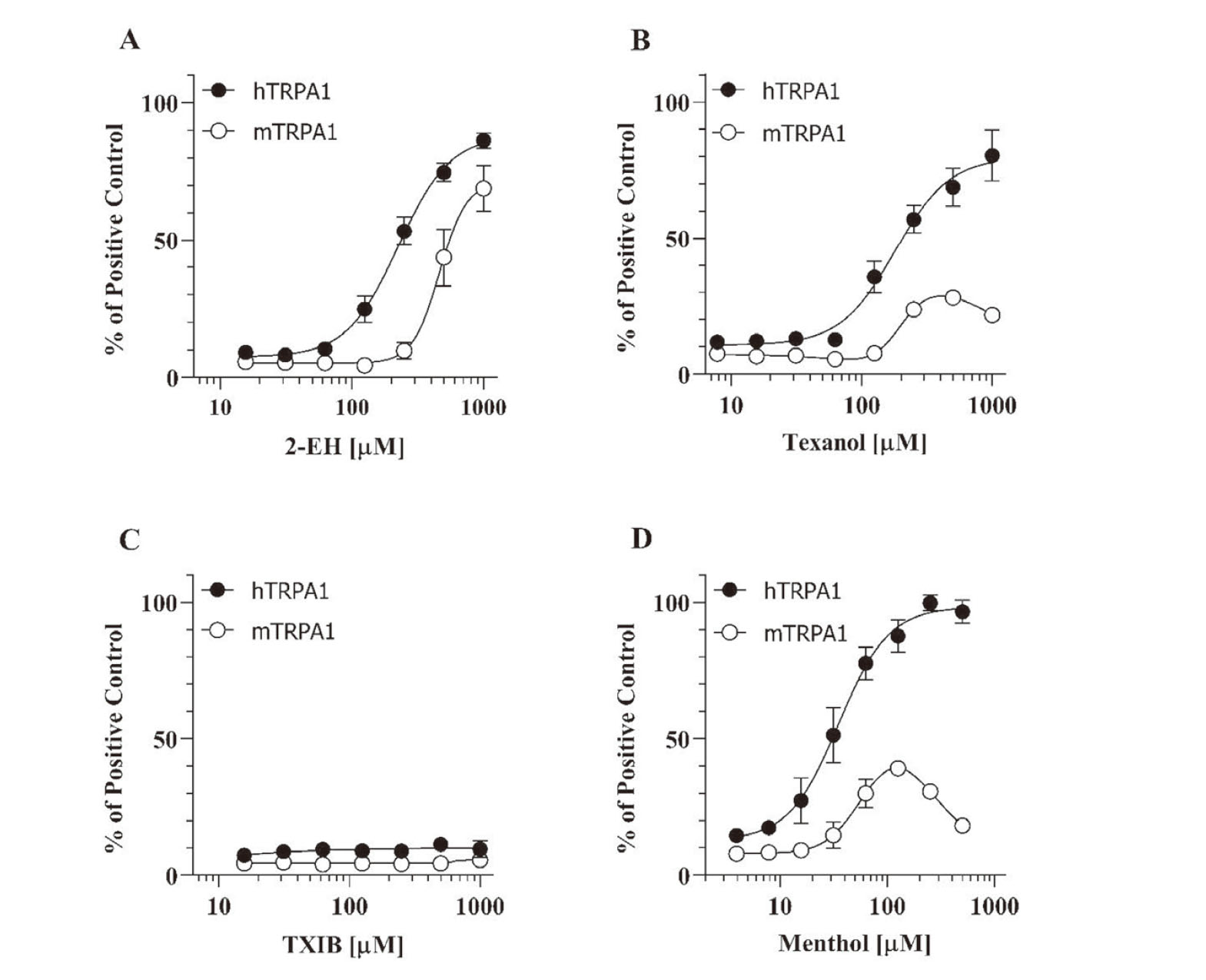
Activation of hTRPA1 and mTRPA1 by 2-EH, Texanol, TXIB, and menthol. Intracellular Ca2+ levels of Flp-In 293/hTRPA1 cells (filled circles) and Flp-In 293/mTRPA1 cells (open circles) were measured using a FlexStation 3. Test chemicals (A, 2-EH; B, Texanol; C, TXIB; D, menthol) were serially diluted 2-fold in HBSS containing DMSO at a final concentration of less than 0.2%. The data are presented as the average response of three wells, and expressed as percentage of the response observed with 100 μM AITC (positive control). Each data point represents the mean ± SD (n = 3).
To clarify the inhibitory effects on TRPA1, Flp-In 293/hTRPA1 and Flp-In 293/mTRPA1 cell lines were treated with a submaximal concentration of AITC (25 µM) in the presence of Texanol (1000 µM) or menthol (500 µM). AITC was selected as an agonist to clarify the inhibitory effects, because AITC is known to activate hTRPA1 and mTRPA1 through a mechanism different from menthol, i.e., a covalent modification of reactive cysteines (Hinman et al., 2006; Xiao et al., 2008). As shown in Fig. 3A, both Texanol and menthol exhibited no inhibitory effect on the AITC-induced activation of hTRPA1. In contrast, mTRPA1 activation by AITC was significantly inhibited by 80% and 85% in the presence of Texanol and menthol, respectively (Fig. 3B). These results distinctly demonstrated that Texanol inhibited the activation of mTRPA1 at higher concentrations, and that the inhibitory effect was species-specific, similar to menthol.
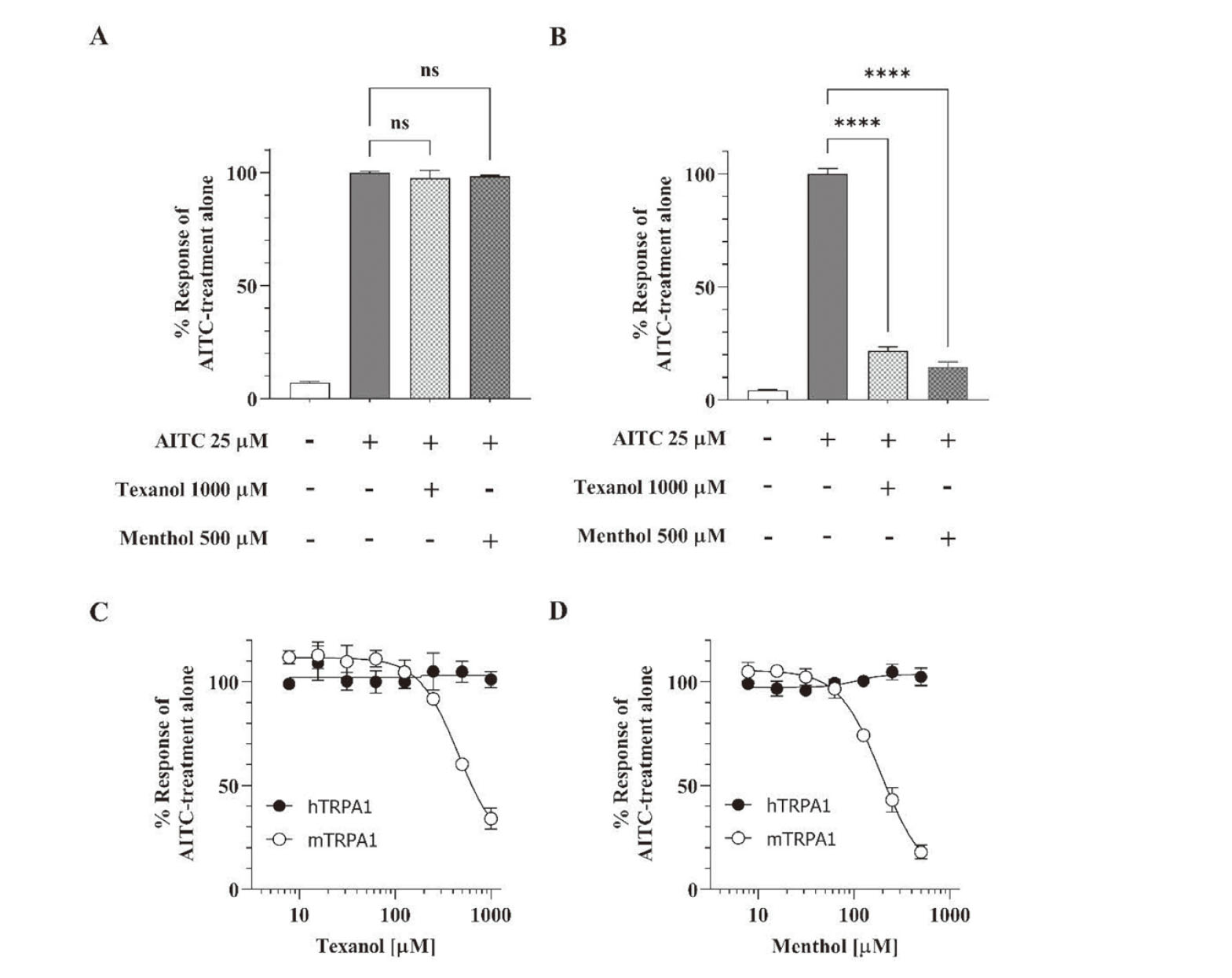
Inhibitory effect of Texanol and menthol on the AITC-induced activation of hTRPA1 and mTRPA1. The responses of AITC (25 µM, a sub-maximally effective concentration) was measured in the presence or absence of the test chemicals in Flp-In 293/hTRPA1 cells (A, filled circles in C and D) and Flp-In 293/mTRPA1 cells (B, open circles in C and D). The cells were treated with AITC and test chemicals at the same time and fluorescence intensity was immediately monitored for 60 sec. The data are presented as the average response of three wells, and expressed as percentage of the response observed with 25 µM AITC treatment alone. Each data point represents the mean ± SD (n = 3). Statistical analyses are presented as follows: ns, not significant; ****, p < 0.0001 (one-way ANOVA with Tukey’s post-hoc test).
The inhibitory effect of Texanol was further characterized by titration of the AITC-induced activation of TRPA1 with varying concentrations of Texanol. As shown in Fig. 3C and 3D, Texanol as well as menthol inhibited AITC-induced mTRPA1 activation in a concentration-dependent manner, where the IC50 values were 450 µM for Texanol and 190 µM for menthol.
Activation of hTRPA1/V875G and mTRPA1/G878V by TexanolA previous study reported that the amino acid residue, G878 in mTRPA1 (corresponding to V875 in hTRPA1), played a key role in a species-specific inhibition by menthol (Xiao et al., 2008). In their study, G878V mutation of mTRPA1 provided hTRPA1-like response to menthol. Conversely, V875G mutation of hTRPA1 resulted in mTRPA1-like response. Considering the similar behavior of Texanol and menthol in the mTRPA1 activation/ inhibition, they might share the interaction site with each other. To test this hypothesis, activation of TRPA1 mutants were studied using Flp-In 293/hTRPA1/V875G and Flp-In 293/mTRPA1/G878V cell lines. As a result, hTRPA1/V875G showed bell-shaped concentration dependence for both Texanol and menthol (Fig. 4A and Fig. 4B, respectively). Accordingly, as shown in Fig. 4, sigmoidal concentration-response curves were obtained for mTRPA1/G878V treated with Texanol and menthol. These results suggest that the G878 amino acid residue (corresponding to V875 in hTRPA1) plays a critical role in the species differences in the TRPA1 activation by Texanol as well as menthol.
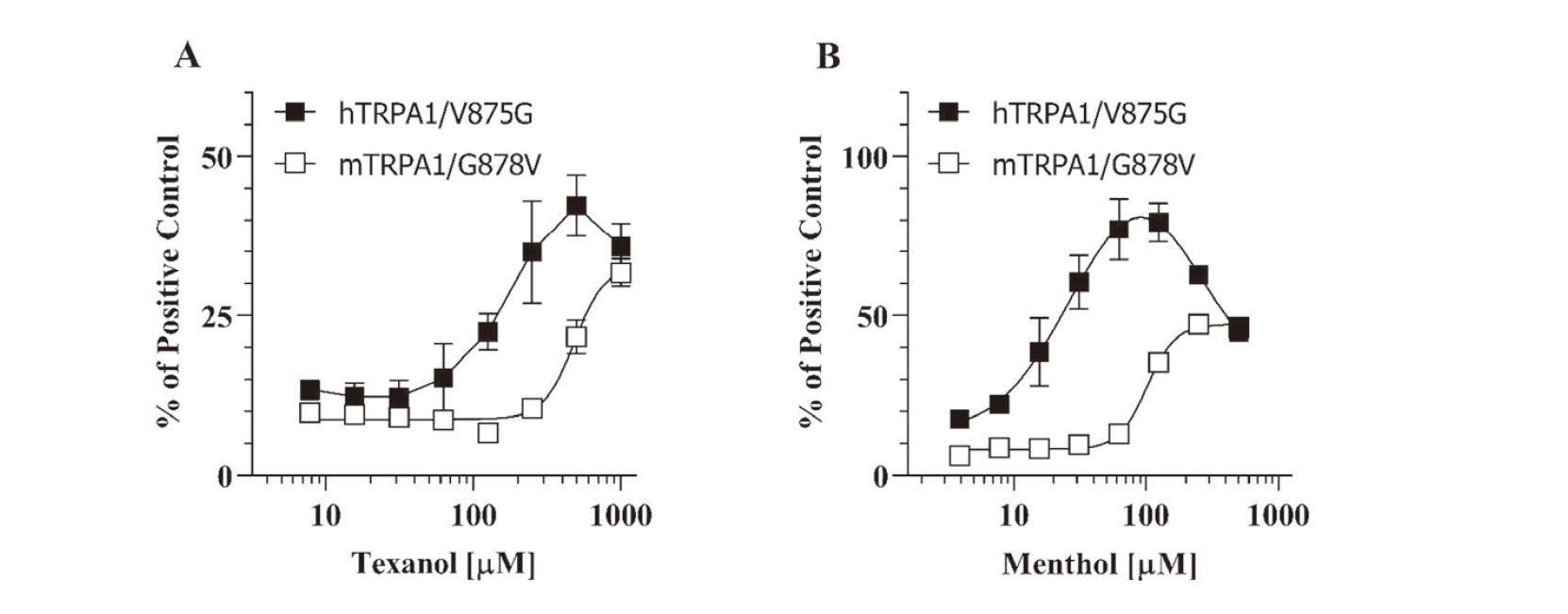
Concentration dependence of the activation of hTRPA1/V875G and mTRPA1/G878V by Texanol and menthol. Intracellular Ca2+ levels of Flp-In 293/hTRPA1/V875G cells (filled squares) and Flp-In 293/mTRPA1/G878V cells (open squares) was measured using a FlexStation 3. Test chemicals (A, Texanol; B, menthol) were serially diluted 2-fold in HBSS containing DMSO at a final concentration of less than 0.2%. The data are presented as the average response of three wells, and expressed as percentage of the response observed with 100 μM AITC (positive control). Each data point represents the mean ± SD (n = 3).
Finally, we investigated the inhibitory effect of Texanol on the AITC-induced activation of TRPA1 mutants using Flp-In 293/hTRPA1/V875G and Flp-In 293/mTRPA1/G878V cell lines. As shown in Fig. 5A, Texanol and menthol significantly inhibited the AITC-induced activation by 50% and 70%, respectively. In contrast, the introduction of the G878V mutation into mTRPA1 canceled the inhibitory effect of Texanol on the AITC-induced activation of mTRPA1 (Fig. 5B). These results demonstrated that high concentrations of Texanol and menthol inhibit mTRPA1 activation through the interaction with the G878 amino acid residue (corresponding to V875 in hTRPA1).

Critical role of G878 of mTRPA1, which corresponds to V875 of hTRPA1, on the inhibitory effects of Texanol and menthol. The response of AITC (25 µM, a sub-maximally effective concentration) was measured in the presence or absence of test chemicals in Flp-In 293/hTRPA1/V875G cells (A) and Flp-In 293/mTRPA1/G878V cells (B). The cells were treated with AITC and test chemicals simultaneously and fluorescence intensity was immediately monitored for 60 sec. The data are presented as the average response of three wells, and expressed as percentage of the response observed with 25 µM AITC treatment alone. Each data point represents the mean ± SD (n = 3). Statistical analyses are presented as follows: ns, not significant; ***, p < 0.001; ****, p < 0.0001 (one-way ANOVA with Tukey’s post-hoc test).
TRPA1 is a nociceptive ion channel that is expressed not only in primary sensory neurons (i.e., dorsal root ganglia and trigeminal ganglia), but also in non-neuronal tissues (i.e., such as the pancreas, gastrointestinal tract, bladder, and lung) (Talavera et al., 2020). TRPA1 activation by noxious cold, mechanical, and chemical stimuli elicits a painful sensation and is involved in neurogenic inflammation via the release of neuropeptides, such as calcitonin gene-related peptide and substance P (Nassini et al., 2014). Several studies have indicated that TRPA1 expressed in the airway plays a causative and exacerbating role for respiratory diseases including asthma, COPD, and chronic cough (Long et al., 2019; Wallace, 2017; Yang and Li, 2016). Thus, a direct measure of TRPA1 activation in an in vitro system may predict potential airway disorders caused by indoor air chemicals.
In the present study, we examined the activation of human and mouse TRPA1 by 2-EH, Texanol, and TXIB in an in vitro heterologous expression system. Both 2-EH and Texanol, but not TXIB, activated TRPA1, and 2-EH activated hTRPA1 and mTRPA1 in a concentration-dependent manner (Fig. 2A). The activation of TRPA1 by 2-EH has already been reported by Lehmann et al. (2017). Using an in silico analysis, Araki et al. (2020) identified three amino acid residues, hydrophobic L881 and F909 as well as hydrophilic T874, that play an important role in the interaction of hTRPA1 with 2-EH (Araki et al., 2020). Alignment of hTRPA1 and mTRPA1 (Fig. 6) revealed that the amino acid residues are conserved between the human and mouse species. This may explain why no significant difference was observed between the activation of hTRPA1 and mTRPA1 by 2-EH (Fig. 2A). In contrast, Texanol activated hTRPA1 and mTRPA1 in a different concentration-dependent manner (Fig. 2B). mTRPA1 activation by Texanol exhibited a typical bell-shaped concentration curve, indicating that high concentrations of Texanol inhibited the activation of mTRPA1 (Fig. 3B and C). Previous studies reported that several TRPA1 agonists modulate hTRPA1 and mTRPA1 differently.

Alignment of the deduced amino acid sequences of human and mouse TRPA1. The deduced amino acid sequences were obtained from the NCBI GenBank reference sequences (human TRPA1, NM_007332.3; mTRPA1, NM_177781).
Caffeine-activated mTRPA1 exerted inhibitory effects on hTRPA1 (Nagatomo et al., 2010). The substitution of M268P of mTRPA1 (corresponding site of hTRPA1/P267) inverted the agonistic effect of caffeine, which exhibited an antagonistic effect similar to that observed for hTRPA1. In another example, menthol activated hTRPA1 in a concentration-dependent manner, whereas mTRPA1 activation by menthol exhibited a bell-shaped concentration dependence (Karashima et al., 2007). Intriguingly, mutant hTRPA1 harboring an amino acid substitution of V875G (corresponding site of mTRPA1/G878) also exhibited a bell-shaped concentration dependence similar to mTRPA1 (Xiao et al., 2008). In addition, S873 and T874 of hTRPA1, which are conserved between human and mouse, are key amino acid residues that interact with menthol (Xiao et al., 2008).
In the present study, we determined the possible involvement of the V875G amino acid substitution in the species difference in activation of TRPA1 by Texanol. Consequently, activation of hTRPA1/V875G by Texanol exhibited bell-shaped concentration dependence and high concentrations of Texanol inhibited the activation of hTRPA1/V875G (Fig. 4A). Consistently, the antagonistic effect of Texanol was reversed in mTRPA1/G878V (Fig. 5B). Similar to menthol, Texanol inhibited the activation mTRPA1 by AITC, which activates TRPA1 through an electrophilic reaction with cysteine residues. These results clearly demonstrate that Texanol interacts with human and mouse TRPA1 at the same site as menthol, V875 in hTRPA1 and G878 in mTRPA1. Among the three resin additive-related chemicals examined, only TXIB neither activated hTRPA1 nor mTRPA1 (Fig. 2C). Although TXIB is hydrolyzed to Texanol in humans and mammals (Nielsen et al., 1997), TXIB, unlike Texanol, showed no effects on TRPA1 activation in an in vitro assay. These results may show the limitation of in vitro assay from the lack of metabolic competence. Further studies in vivo will be necessary to clarify the biological effect of TXIB mediated by TRPA1.
In conclusion, we evaluated human and mouse TRPA1 activation by 2-EH, Texanol, and TXIB, which are potentially hazardous indoor air pollutants. The data presented here contribute to the toxicological characterization of their airway irritancy. Although there is no information on the irritancy of Texanol to humans, comparable EC50 values of 2-EH and Texanol (225 µM and 180 µM, respectively) obtained in this study suggest that Texanol may exert irritant effects at a similar concentration observed for 2-EH (54 mg/m3). We found that 2-EH is an agonist of both human and mouse TRPA1 with a similar concentration dependence. In contrast, Texanol activated TRPA1 in a species-dependent manner similar to menthol, which is a representative agonist of TRPA1. Interestingly, Texanol and menthol share a key amino acid residue in their interaction with TRPA1. These results suggest that careful interpretation is necessary when extrapolating TRPA1-dependent toxicological effects in rodents to humans, particularly for the risk assessment of indoor air pollutants.
This study was partly supported by Grant-in-Aid for Scientific Research (C) (JSPS KAKENHI Grant number: 18K06641) and Health and Labour Sciences Research Grant from the Ministry of Health, Labour, and Welfare of Japan (Grant number: H27-Kagaku-Ippan-009 and H30-Kagaku-Shitei-002).
Conflict of interestThe authors declare that there is no conflict of interest.