2023 年 48 巻 1 号 p. 47-56
2023 年 48 巻 1 号 p. 47-56
Drug-induced liver injury (DILI) is a major cause of market withdrawal or drug-development discontinuation because of safety concerns. In this study, we focused on drug-induced cholestasis (DIC) to establish an in vitro cytotoxicity test system and analyze its sensitivity using two-dimensional (2-D) cultured HepaRG cells and 12 types of bile acids (BAs) present in the human serum. First, to detect the cytotoxicity associated with cholestasis effectively, non-toxic BA concentrations were investigated and determined to be 100-fold the human serum value (455 μM total BAs). Next, the cytotoxicity of 31 compounds that can inhibit the bile acid export pump (BSEP) and were categorized as no-DILI-concern, less-DILI-concern, and most-DILI-concern was examined. None of the no-DILI-concern compounds yielded cytotoxicity, whereas almost all less-DILI-concern compounds (with the exception of simvastatin) and most-DILI-concern compounds (with the exception of bosentan) exhibited cytotoxicity. An investigation of the cause of cytotoxicity using 3H-taurocholic acid revealed that most-DILI-concern and less-DILI-concern compounds, but not no-DILI-concern compounds, triggered the accumulation of radioactivity in the cell lysates. Thus, the onset of cytotoxicity seemed to be associated with cholestasis. The established HepaRG cytotoxicity assessment system (sensitivity of 89%, specificity of 100%, and accuracy of 97%) was mostly superior to the Css/BSEP IC50 (> 0.1) assessment system (sensitivity of 83%, specificity of 100%, and accuracy of 72%). Therefore, the assay method using 2-D cultured HepaRG cells and 12 BAs established here can be widely applicable as a model for the in vitro potential assessment of DIC.
Drug-induced liver injury (DILI) is a major cause of market withdrawal or drug-development discontinuation because of safety concerns from the end of the twentieth century to this day (Lee, 2013; Nathwani and Kaplowitz, 2006; Van den Hof et al., 2015). Drug-induced cholestasis (DIC) constitutes a major subgroup of DILI accounting for as much as 50% of all clinical cases of DILI (Oorts et al., 2016). The patient mortality associated with DIC is estimated to be up to 10% (Sundaram and Björnsson, 2017). Multiple triggering factors or molecular initiating events are likely to lead to DIC, including transporter changes (namely, direct inhibition, internalization, and/or reduced expression), hepatocellular changes, and the interference between drugs and bile canaliculi dynamics by inducing constriction or dilatation (Gijbels et al., 2019).
Bile acid (BA) synthesis occurs in liver cells that synthesize primary BAs (cholic acid and chenodeoxycholic acid in humans) via the cytochrome P450 (CYP)-mediated oxidation of cholesterol. In the liver, BAs are usually conjugated with taurine or glycine. About 90% of the excreted BAs are reabsorbed from the ileum and recycled by the “enterohepatic circulation.” As surfactants or detergents, BAs are potentially toxic to cells, and their concentrations are tightly regulated. The disruption of the normal process of bile secretion results in cholestasis. Previously, the presence of quantified BAs in the human serum was reported (Scherer et al., 2009), which 12 BAs of them have been applied in sandwich-cultured test systems for evaluating DIC using rat (Ogimura et al., 2011; Susukida et al., 2015; Oizumi et al., 2017) and human (Ogimura et al., 2017; Sakai et al., 2019a; Kawaguchi et al., 2020) hepatocytes, various cell lines including HepG2 (Sakai et al., 2019b; Kawaguchi et al., 2020) and HepaRG (Susukida et al., 2016). Several human hepatoma-derived cell lines, in particular HepaRG cells, can differentiate from an epithelial phenotype to both canalicular- and hepatocyte-like cells under two-dimensional (2-D) culture condition (Parent et al., 2004), and are widely applied for testing enzyme induction (Turpeinen et al., 2009), transporter (Le et al., 2006), toxicology (Guillouzo et al., 2007; Sharanek et al., 2017), etc. In this study, we focused on cholestasis stemming from the stasis of endogenous BAs induced by drugs, in which the cause of DIC is the inhibition of BA transport via disruption of the bile acid export pump (BSEP).
This study aimed to establish an in vitro cytotoxicity test system for the assessment of DIC using simply 2-D cultures of HepaRG cells and the 12 BAs and to evaluate its superiority vs. previous DIC risk assessment methods. First, to mimic the BAs in the human liver, the conditions for concentrated exposure to the 12 BAs were determined. Second, under the obtained optimal conditions, HepaRG cells were exposed to the 31 DILI-risk compounds for 48 hr in the presence or absence of the BAs. Subsequently, cytotoxicity as an index was evaluated. In addition, to evaluate the onset of the toxicity associated with cholestasis, the concentration of intracellular radioactivity was assessed using 3H-labeled taurocholic acid (3H-TCA). Finally, we performed a sensitivity analysis, followed by the comparison of the established HepaRG cytotoxicity test system with the Css/BSEP IC50 (> 0.1) index (Morgan et al., 2013).
HepaRG cells and Medium-670TM were purchased from KAC Co., Ltd. (Kyoto, Japan).
Chenodeoxycholic acid, glycocholic acid hydrate, taurochenodeoxycholic acid sodium salt, and taurolithocholic acid sodium salt were purchased from Sigma-Aldrich Japan KK (Tokyo, Japan). Cholic acid, deoxycholic acid, glycochenodeoxycholic acid, lithocholic acid, taurocholic acid sodium salt, and tauroursodeoxycholic acid sodium were purchased from Nacalai Tesque Inc. (Kyoto, Japan). Glycodeoxycholic acid and ursodeoxycholic acid were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Beclomethazone propionate, benzbromarone, cyclosporin A,econazole nitrate, flutamide, indomethacin, midazolam, nicardipine hydrochloride, nimodipine, pioglitazone hydrochloride, rosiglitazone, and troglitazone were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Amiodarone hydrochloride, itraconazole, ketoconazole, oxybutynin chloride, reserpine, rifampicin, sitaxentan sodium salt, and sulfinpyrazone were purchased from Sigma-Aldrich Japan KK (Tokyo, Japan). Bosentan, olmesartan medoxomil, and ritonavir were purchased from Toronto Research Chemicals, Inc. (Toronto, ON, Canada). Fluvastatin, imatinib mesylate, and lapatinib ditosylate were purchased from Funakoshi Co., Ltd. (Tokyo, Japan). Clobetasol 17-propionate and simvastatin were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Nefazodone hydrochloride was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Telmisartan was purchased from LKT laboratories, Inc. (St. Paul, MN, USA). Tolcapone was purchased from Nacalai Tesque Inc. (Kyoto, Japan).
Dimethyl sulfoxide (DMSO), phosphate-buffered saline (PBS), 1 M hydrochloride, and 0.5 M sodium hydroxide were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). 3H-taurocholic acid and Insta-Gel plus scintillator were purchased from PerkinElmer Co., Ltd. (Boston, MA, USA). Bradford UltraTM was purchased from Funakoshi Co., Ltd. (Tokyo, Japan). Trypsin-EDTA solution was purchased from Sigma-Aldrich Japan KK (Tokyo, Japan). The Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Laboratories (Kumamoto, Japan). Cell Titer-Glo® was purchased from Promega Corporation (Madison, WI, USA). Ninety-six-well plates (Corning® Biocoat™ 96-well plate white clear bottom, collagen I coated, with lid) were purchased from Corning Life Science (Tewksbury, MA, USA). All other chemicals and solvents were of analytical grade or the highest grade commercially available.
HepaRG cell pre-culturesThe culture of HepaRG cells was prepared from vials of the frozen cells, which were pre-incubated in a 96-well plate with Medium-670TM for 1 week at 37°C in an atmosphere of 5% CO2. Medium exchange was carried out every 1–3 days.
BA composition of human serum and test compoundsTwelve different BAs (Table 1) were selected in reference to the standard BA constituents of the human serum (Ogimura et al., 2011; Scherer et al., 2009). The test compounds were chosen based on information pertaining to their DILI-concern level and various parameters, including the 50% inhibitory concentration (IC50) for BSEP and the human maximum exposure concentration (Cmax) and the human steady-state exposure concentration (Css)/BSEP IC50 values (Aleo et al., 2014; Morgan et al., 2013) (Table 2). The test compounds were selected so that the BSEP IC50 would vary in the same way for each DILI-concern level. All of the test compounds were dissolved in DMSO and the treatment concentrations were basically set at around 100-fold the human Cmax values, with the exception of cases of drug-based cytotoxicity or precipitation. The test compounds or 12 BAs were added to Medium-670TM. Stock solutions of the test compounds and BAs were originally prepared in DMSO. The final concentration of DMSO was 0.3%.
| Bile acid | Concentration (μM) |
|---|---|
| Cholic acid | 0.200 |
| Chenodeoxycholic acid | 0.340 |
| Glycochenodeoxycholic acid | 1.710 |
| Deoxycholic acid | 0.734 |
| Lithocholic acid | 0.030 |
| Ursodeoxycholic acid | 0.110 |
| Glycocholic acid | 0.410 |
| Glycodeoxycholic acid | 0.380 |
| Taurocholic acid | 0.048 |
| Taurochenodeoxycholic acid | 0.210 |
| Taurolithocholic acid | 0.087 |
| Tauroursodeoxycholic acid | 0.287 |
| Total bile acid (TBA) | 4.546 |
Note: This information was reported by Ogimura et al. (2011) and Scherer et al. (2009).
| No. | Compound | BSEP IC50 (μM) |
Human Cmax (μM) |
Treatment (μM) |
Human Css/BSEP IC50 |
|
|---|---|---|---|---|---|---|
| [No-DILI-concern] | ||||||
| N-1 | Olmesartan | 4.7 | 0.3810 | 100 | 0.0810 | |
| N-2 | Nicardipine | 7.9 | 0.0810 | 30 | 0.0040 | |
| N-3 | Reserpine | 8.4 | 0.0020 | 10 | 0.0001 | |
| N-4 | Clobetasol | 8.5 | 0.0430 | 10 | - | |
| N-5 | Nimodipine | 10.1 | 0.1950 | 20 | - | |
| N-6 | Beclomethazone | 10.3 | 0.0002 | 0.2 | - | |
| N-7 | Sulfinpyrazone | 11.0 | 44.2500 | 300 | - | |
| N-8 | Econazole | 13.4 | 0.0010 | 1 | - | |
| N-9 | Telmisartan | 16.2 | 0.1460 | 20 | 0.0090 | |
| N-10 | Oxybutynin | 27.4 | 0.0220 | 10 | 0.0001 | |
| N-11 | Midazolam | 41.7 | 0.0160 | 30 | 0.0004 | |
| [Less-DILI-concern] | ||||||
| L-1 | Cyclosporin A | 0.4 | 0.7700 | 100 | 0.4060 | |
| L-2 | Pioglitazone | 0.4 | 2.9460 | 300 | 2.3380 | |
| L-3 | Lapatinib | 6.5 | 2.6000 | 30 | 0.4000 | |
| L-4 | Itraconazole | 18.0 | 0.3600 | 100 | 0.0200 | |
| L-5 | Simvastatin | 24.7 | 0.0240 | 3 | 0.0004 | |
| L-6 | Rifampicin | 25.3 | 3.7190 | 100 | 0.1420 | |
| L-7 | Tolcapone | 34.5 | 21.0800 | 100 | 0.6110 | |
| L-8 | Fluvastatin | 36.1 | 0.6630 | 100 | 0.0020 | |
| L-9 | Indomethacin | 42.0 | 1.1340 | 100 | 0.0270 | |
| [Most-DILI-concern] | ||||||
| M-1 | Ritonavir | 2.2 | 19.6900 | 100 | 19.7960 | |
| M-2 | Ketoconazole | 3.4 | 12.5100 | 100 | 0.3810 | |
| M-3 | Rosiglitazone | 3.8 | 1.0440 | 200 | 0.1190 | |
| M-4 | Troglitazone | 5.9 | 6.3870 | 50 | 0.3130 | |
| M-5 | Nefazodone | 7.0 | 6.8000 | 30 | 0.0990 | |
| M-6 | Sitaxentan | 12.6 | 1.9900 | 100 | 0.1580 | |
| M-7 | Benzbromarone | 15.9 | 4.3390 | 100 | 0.1070 | |
| M-8 | Bosentan | 23.0 | 1.3570 | 100 | 0.0590 | |
| M-9 | Imatinib | 25.1 | 7.0900 | 30 | 0.1760 | |
| M-10 | Amiodarone | 43.0 | 1.3760 | 30 | 0.0320 | |
| M-11 | Flutamide | 68.0 | 0.3620 | 30 | - | |
Note: The information of each categorized DILI-concern, BSEP IC50, Huan Cmax, and Human Css/BSEP IC50 was obtained from Aleo et al. (2014) or Morgan et al. (2013). -: No information was reported by Aleo et al. (2014).
In order to sensitively assess the cytotoxicity of test compounds during cholestasis, we examined the optimized concentration of 12 BAs under 48 hr exposure, which is relatively longer than the 24 hr reported by Susukida et al. (2016). HepaRG cells (6 × 104 cells/well) were exposed to mixtures of the 12 BAs (0-, 50-, 100-, 150-, 200-fold concentration in human serum) at 37°C for 48 hr. Subsequently, the CCK-8 reagent was added and the ultraviolet (UV) absorbance of WST-8 formazan at 450 nm was measured according to the manufacturer’s instructions. The following equation was employed:
Cell viability (%) = (UVsample+ – UVsample−) / (UV DMSO+ – UVDMSO−) × 100.
UV sample+ : Absorbance of each concentration of 12 BAs treated HepaRG cells
UV sample− : Absorbance of each concentration of 12 BAs treated Medium-670TM
UV DMSO+ : Absorbance of DMSO treated HepaRG cells
UV DMSO− : Absorbance of DMSO treated Medium-670TM
Assessment of the cytotoxicity of DILI-concern compoundsAfter the optimal BA treatment condition was determined, cytotoxicity was evaluated among no-DILI-concern, less-DILI-concern, and most-DILI-concern compounds. HepaRG cells (6 × 104 cells/well) were exposed to each DILI compound in the presence or absence of the 12 BAs at the optimized concentration for 48 hr at 37°C. Lin and Will (2012) demonstrated that the prediction of hepatotoxicity can be significantly improved when human Cmax values are incorporated in the cell-based assay. Additionally, a dose-response study found that the 100-fold Cmax scaling factor represented a reasonable threshold to differentiate safe vs. toxic drugs using human primary hepatocytes (Xu et al., 2008) and HepaRG cells (Tomida et al., 2017). Thus, the treatment concentrations of the compounds were basically 100-fold the human Cmax value, and if the cytotoxicity was strong or insolubility was observed, the treatment concentration was lowered. After 48 hr of incubation, the Cell Titer-Glo® (ATP assay) reagent was added and the luminescence of the generated oxyluciferin was measured at an excitation of 338 nm and emission of 458 nm at room temperature. The following equation was employed:
Cell viability (%) = (Lumisample+ – Lumi sample−) / (Lumi DMSO+ – LumiDMSO−) × 100.
Lumisample+ : Luminescence of each test compound treated HepaRG cells
Lumisample− : Luminescence of each test compound treated Medium-670TM
LumiDMSO+ : Luminescence of DMSO treated HepaRG cells
LumiDMSO− : Luminescence of DMSO treated Medium-670TM
Accumulated 3H-TCA radioactivity in HepaRG cellsTo confirm whether the cytotoxicity of the BSEP inhibitors was derived from cholestasis, radioactivity in the cells was determined using 3H-TCA. HepaRG cells (6 × 104 cells/well) were exposed to tritium-labeled TCA (2 μM), the 12 BAs at the optimized concentration, and DILI compounds and cultured at 37°C for 48 hr. The medium was then removed from the culture plate and the cells were washed twice with PBS, treated with trypsin, treated with 0.5 M sodium hydroxide, and neutralized with 1 M hydrochloride. A fraction of the neutralized cell suspension was collected for protein quantification using Bradford UltraTM, whereas another fraction was mixed with Insta-Gel plus scintillator to measure radioactivity, followed by the calculation of the radioactivity per protein (dpm/mg protein). The following equation was employed:
Percentage of Control (%) = Sample (each compound) radioactivity / Control (DMSO) radioactivity × 100.
Sensitivity analysesTo evaluate the superiority of the established HepaRG cytotoxicity assessment for DIC, we compared this system with the previously proposed DIC risk index (i.e., the Css/BSEP IC50 > 0.1). Sensitivity analyses were performed for each assessment, and the sensitivity, specificity, positive and negative predictive values, and percentage missed were calculated. Table 3 provides the formula used.
| Test | Formula |
|---|---|
| Accuracy | (True Positive + True Negative) / Total × 100 |
| Sensitivity | True Positive / (True Positive + False Negative) × 100 |
| Specificity | True Negative / (True Negative + False Positive) × 100 |
Data are expressed as the mean ± standard deviation (S.D.) of three independent determinations. The statistical significance of the cytotoxicity data was determined using two-tailed Student’s t-test (Microsoft Office Excel 2010 and 2013). Significance was set at P < 0.05.
First, we investigated the maximum non-toxic concentrations of the 12 BAs in HepaRG cells. The concentration-dependent cytotoxicity was assessed after the BAs were added to the cultured medium under 48-hr treatment conditions (Fig. 1). Cytotoxicity was observed at a treatment concentration of BAs of 150-fold or higher. Therefore, it was suggested that a BA concentration of 100-fold the human serum value (455 μM in total) was optimal as the maximum concentration that did not cause cytotoxicity.
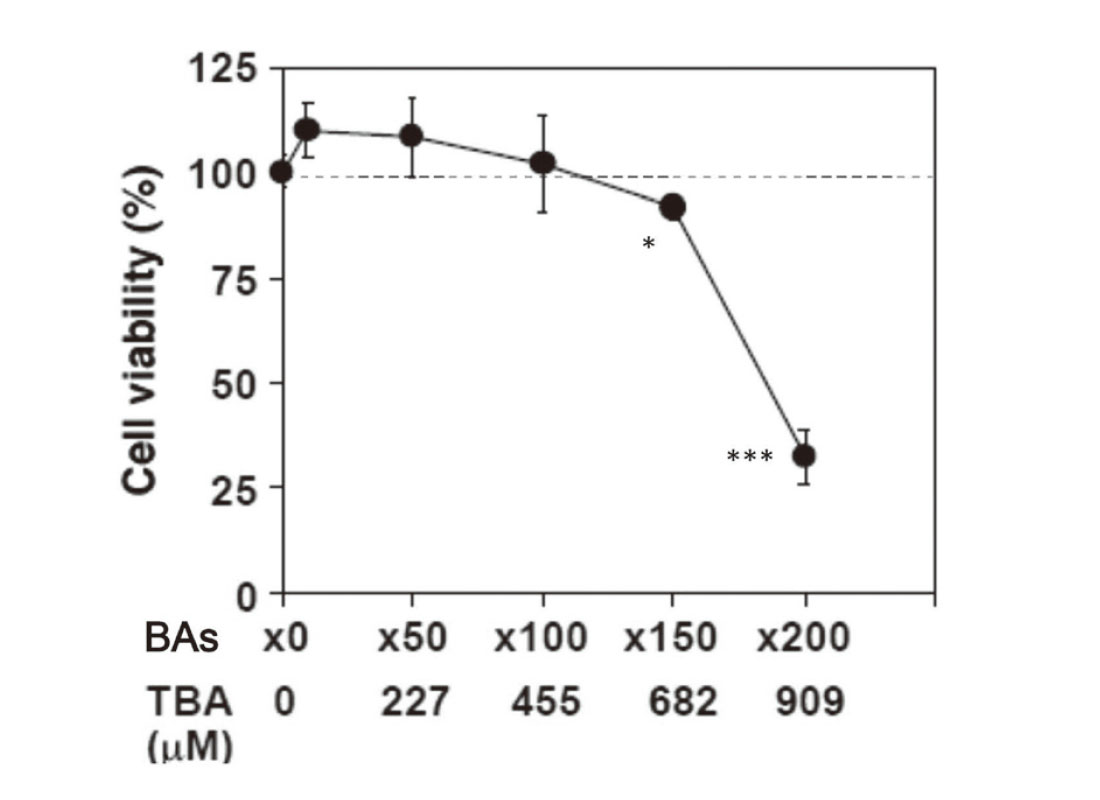
Cytotoxicity in HepaRG cells exposed to several concentrations of the 12 Bas. TBA, total bile acid. Each point represents the mean ± S.D. of triplicate determinations. *P < 0.05 and ***P < 0.001 compared with the control treatment (TBA = 0 μM).
We then evaluated the cytotoxicity of several DILI-concern compounds with BSEP-inhibition capability in the presence or absence of the 12 BAs at 100-fold concentration in the human serum. For the evaluation of cytotoxicity, Cell-Titer Glo® using the luminescence method, which seems to be more sensitive and discriminative than CCK-8 using the UV method, was employed. This is because when evaluating dye-containing compounds such as rifampicin (reddish-orange color), UV measurements are interfered with when compared to luminescence measurements. Although the treatment concentration of sulfinpyrazone was 300 μM because of the occurrence of insoluble precipitation, cytotoxicity was not observed for any of the no-DILI-concern compounds. In contrast, almost all less-DILI-concern compounds (with the exception of simvastatin) and most-DILI-concern compounds (with the exception of bosentan) exhibited significant cytotoxicity (Fig. 2 and Table 4).

Cytotoxicity in HepaRG cells exposed to several DILI-concern compounds in the presence or absence of 12 the Bas. N-1: Olmesartan, N-2: Nicardipine, N-3: Reserpine, N-4: Clobetasol, N-5: Nimodipine, N-6: Beclomethasone, N-7: Sulfinpyrazone, N-8: Econazole, N-9: Telmisartan, N-10: Oxybutynin, N-11: Midazolam; L-1: Cyclosporin A, L-2: Pioglitazone, L-3: Lapatinib, L-4: Itraconazole, L-5: Simvastatin, L-6: Rifampicin, L-7: Tolcapone, L-8: Fluvastatin, L-9: Indomethacin, M-1: Ritonavir, M-2: Ketoconazole, M-3: Rosiglitazone, M-4: Troglitazone, M-5: Nefazodone, M-6: Sitaxentan, M-7: Benzbromarone, M-8: Bosentan, M-9: Imatinib, M-10: Amiodarone, M-11: Flutamide. The white bar indicates “absent 12 BAs (0 μM TBA)” and the black bar indicates “present 12 BAs (455 μM TBA).” Each bar represents the mean ± S.D. of triplicate determinations compared with the control treatment (DMSO). *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the 0 μM TBA treatment.
| No. | Compound | Cytotoxicity | No. | Compound | Cytotoxicity | No. | Compound | Cytotoxicity | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [No-DILI-concern] | [Less-DILI-concern] | [Most-DILI-concern] | ||||||||||
| N-1 | Olmesartan | - | L-1 | Cyclosporin A | + | *** | M-1 | Ritonavir | + | *** | ||
| N-2 | Nicardipine | - | L-2 | Pioglitazone | + | * | M-2 | Ketoconazole | + | *** | ||
| N-3 | Reserpine | - | L-3 | Lapatinib | + | *** | M-3 | Rosiglitazone | + | * | ||
| N-4 | Clobetasol | - | L-4 | Itraconazole | + | *** | M-4 | Troglitazone | + | ** | ||
| N-5 | Nimodipine | - | L-5 | Simvastatin | - | M-5 | Nefazodone | + | * | |||
| N-6 | Beclomethazone | - | L-6 | Rifampicin | + | * | M-6 | Sitaxentan | + | * | ||
| N-7 | Sulfinpyrazone | - | L-7 | Tolcapone | + | *** | M-7 | Benzbromarone | + | *** | ||
| N-8 | Econazole | - | L-8 | Fluvastatin | + | * | M-8 | Bosentan | - | |||
| N-9 | Telmisartan | - | L-9 | Indomethacin | + | *** | M-9 | Imatinib | + | ** | ||
| N-10 | Oxybutynin | - | M-10 | Amiodarone | + | * | ||||||
| N-11 | Midazolam | - | M-11 | Flutamide | + | ** | ||||||
-: Negative, +: Positive.
*P < 0.05,**P < 0.01, and ***P < 0.001 compared with the 0 μM TBA treatment.
We then evaluated whether the onset of cytotoxicity was associated with cholestasis using 3H-TCA. Most-DILI-concern (ritonavir and troglitazone) and less-DILI-concern (cyclosporin A and pioglitazone) compounds led to the accumulation of radioactivity in the cell lysates (Fig. 3). Conversely, the no-DILI-concern (nicardipine and telmisartan) compounds did not induce the accumulation of radioactivity in the cell lysates. These results suggest that the cytotoxicity reported above was derived from the cholestasis situation. Interestingly, bosentan (most-DILI-concern compound) did not trigger the accumulation of radioactivity, in agreement with the cytotoxicity result.

Radioactivity of HepaRG cell lysates after exposure to several DILI-concern compounds with 12 BAs (455 μM TBA) and 3H-TCA (2 μM). Most-DILI-concern: ritonavir, troglitazone, bosentan; less-DILI-concern: cyclosporin A, pioglitazone; non-DILI-concern: nicardipine, telmisartan. Each bar represents the percentage of control radioactivity (dpm/mg protein) and the mean ± S.D. of triplicate determinations. *P < 0.05 and ***P < 0.001 compared with the control treatment (DMSO).
Finally, we carried out a sensitivity analysis of DIC risk evaluation for both the HepaRG cytotoxicity assessment and the Css/BSEP IC50 (> 0.1) assessment systems. In this study, the cytotoxicity of 31 compounds was evaluated, whereas 25 compounds for which human Css was previously reported by Morgan et al. (2013) were selected and analyzed. The HepaRG cytotoxicity assessment system exhibited an accuracy of 97%, a sensitivity of 89%, and a specificity of 100% (Table 5). In turn, the Css/BSEP IC50 (> 0.1) assessment system had an accuracy of 72%, a sensitivity of 63%, and a specificity of 100%. These results suggest that the HepaRG cytotoxicity assessment system is superior for DIC risk evaluation compared with the Css/BSEP IC50 (> 0.1) assessment system.
| Assessment | True positive |
True negative |
False positive |
False negative |
Accuracy (%) |
Sensitivity (%) |
Specificity (%) |
|---|---|---|---|---|---|---|---|
| HepaRG cytotoxicity | 17 | 6 | 0 | 2 | 97 | 89 | 100 |
| Human Css/BSEP IC50 | 12 | 6 | 0 | 7 | 72 | 63 | 100 |
| (> 0.1) |
Note: The sensitivity analysis between the HepaRG cytotoxicity assessment and human Css/BSEP IC50 (> 0.1) assessment systems was carried out using 25 compounds (no-DILI-concern, 6; less-DILI-concern, 9; most-DILI-concern, 10.
DIC is a major cause of attrition during drug development and post-marketing withdrawal (Navarro and Senior, 2006). The underlying mechanisms of DIC development are complex and involve multiple transporters and nuclear receptors (Deferm et al., 2019). To date, comprehensive in vitro models, such as sandwich-cultured human hepatocytes, have been more suited because of the presence of metabolizing enzymes and transporters relevant to bile salt disposition (De Bruyn et al., 2013). However, in the case of human hepatocytes, there are individual differences in metabolic activity and transporter expression level, and a stable and reproducible DIC evaluation test system is required. Furthermore, when considering a versatile and simple 2-D culture, it has been reported that human liver cancer-derived cell lines, such as HepG2 (Ramaiahgari et al., 2014) and Huh-7 (Molina-Jimenez et al., 2012) do not form bile ducts. Therefore, we focused on a DIC risk assessment model using the human liver-derived HepaRG cell line, which can be used to easily construct a highly reproducible test system by forming bile ducts in 2-D culture.
As surfactants or detergents, bile acids are potentially toxic to cells, and their concentrations are tightly regulated. The disruption of the normal process of bile secretion results in cholestasis. In turn, cholestasis is associated with an increase in serum BA content, which can reach 60-fold or more of the normal values, as well as with major changes in BA profiles (Humbert et al., 2012; Trottier et al., 2012). Because bile acid components are concentrated in the liver in the state of cholestasis, we added artificially concentrated bile acids in this test system.
Before establishing an in vitro test system using 2-D cultured HepaRG cells, the concentration of BAs to be added during the 48-hr treatment was examined, and the optimal concentration of BAs observed in the human serum was 100-fold (Fig. 1). As a result of a similar investigation, it has been reported that the treatment time and bile acidity are different in the studies using HepaRG cells, but the addition of a concentration of 30- to 60-fold was applied (Sharanek et al., 2017).
The commercially available compounds that have the ability to inhibit the BSEP largely showed cytotoxicity in the less-DILI-concern and most-DILI-concern compound categories (BSEP IC50: 0.4–68.0 μM) (Fig. 2 and Table 4). Conversely, no-DILI-concern compounds (BSEP IC50: 4.7–41.7μM), which have some inhibitory potency for BSEP and have been rarely reported to cause DILI, did not exhibit cytotoxicity. These results were consistent with reports of cholestasis (including jaundice) with less-DILI and most-DILI-concern compounds (LiverTox. Database, 2022; Lee et al., 2011). Thus, based on the results of this study and considering the human exposure concentration (Cmax) of the test compounds, it was confirmed that the risk of cholestasis cannot be attributed only to the inhibitory ability of BSEP.
Meanwhile, despite exposure to a concentration of 100-fold the human Cmax in this study, the cytotoxicity of simvastatin and bosentan among the less/most-DILI-concern compounds could not be detected. Simvastatin is metabolized by CYP3A4 and has been reported to contribute to DILI (hepatocellular injury type) because of the increase in alanine aminotransferase (ALT), which was likely to be associated with the production of reactive metabolites (LiverTox. Database, 2022). In the present study, Medium-670TM (containing low DMSO concentration) was used as the culture medium for HepaRG cells purchased from a supplier, but if Medium-620TM (containing high DMSO concentration), which activates CYP metabolic activity, especially CYP3A4 (Kanebratt and Andersson, 2008) was used, it was possible to evaluate the effects of metabolism. Furthermore, exposure of less-DILI-concern and most-DIL-concern compounds to HepaRG cells at the concentrations 100-fold the human Cmax without the 12 BAs showed little cytotoxicity. The reason for this may be that RM was not generated due to the use of Medium-670 TM. Conversely, bosentan was found to cause at least an elevation of liver aminotransferases that was threefold the upper limit of the normal value in about 16.8% of patients, which was accompanied by elevated bilirubin in a small number of cases during clinical studies (Fattinger et al., 2001). In addition, bosentan strongly inhibits not only BSEP but also Na+-dependent taurocholate cotransporting polypeptide (NTCP) transporters, and no accumulation of 3H-TCA was observed in this work (Fig. 3). This result was consistent with the absence of cytotoxicity for bosentan. It has been postulated that the dynamic movements of bile canaliculi (BC) could be controlled by pericanalicular myosin activity (Sharanek et al., 2016). Treatment with bosentan induced BC dilatation, which was reported to show a decrease in myosin light chain kinase 2 (MLC2) phosphorylation with time. Moreover, bosentan causes toxicity in primary human hepatocytes; it is a strong PXR activator (Weiss et al., 2013) and is mainly metabolized by CYP3A4 and CYP2C9 (Gatfield et al., 2012) into four metabolites. Of note, an association between bosentan and the direct RhoA//Rho-kinase (ROCK) inhibitor fasudil has been reported. In fact, fasudil has been successfully used to reverse pulmonary arterial hypertension (PAH) findings in animal models (Liu et al., 2011). The bosentan-induced BC dilatation was strongly enhanced by co-addition of fasudil to HepaRG cells, suggesting that the combination of the two compounds results in enhanced cholestatic effects (Burbank et al., 2017). As mentioned above, to increase the positive rate of DILI risk in this test system, it seems necessary to revise the test conditions change to Medium-620TM as the metabolism medium or specific to individual mechanisms (e.g., in the case of bosentan, fasudil is used in combination with a trapping assay for reactive metabolites) (Kakutani et al., 2019).
By using 3H-TCA as a marker of BA, it was clarified that the cytotoxicity of the test compounds were caused by intracellular cholestasis. In general, there are reports that highly hydrophobic BAs are involved in liver injury (Sharanek et al., 2017). Among the 12 BAs used in the present test system, unconjugated secondary hydrophobic BAs, such as deoxycholic acid, and lithocholic acid may have contributed to cytotoxicity.
It was suggested that the established in vitro test system could be superior for DIC risk evaluation compared with the Css/BSEP IC50 (> 0.1) assessment system (Table 5). Alternatively, high-level studies have been conducted on the risk assessment of cholestasis DILI using rats (Susukida et al., 2015; Susukida et al., 2015; Oizumi et al., 2017) and human (Ogimura et al., 2017; Sakai et al., 2019a; Kawaguchi et al., 2020) hepatocytes, and various cell lines, including HepG2 (Sakai et al., 2019b; Kawaguchi et al., 2020) and HepaRG (Susukida et al., 2016), mainly in sandwich-culture. Although these bio-mimicking 3-D test systems cannot be directly compared with the present test system, the prediction accuracy of the present test system is considered to be comparable to that of reported risk predictions in spite of simply 2-D culture.
Recent studies on DILI have proposed various biomarkers (apolipoprotein E, miR-122(-5p), miR-129, miR-382-5p, miR-4463, pre-miR-4270, glutamate dehydrogenase (GLDH), γ-Glu-citrulline, genome-wide association studies (GWAS) for genetic susceptibility, high-mobility group box 1 (HMGB 1), interleukin (IL)-9, IL-17, integrin subunit beta 3 (ITGB 3), keratin 18 (K18), metabolomic classification model (P-cresol sulfate vs. phenylalanine and inosine vs. bilirubin), platelet-derived growth factor (PDGF)-bb, regulated on activation, normal T expressed and secreted (RANTES) serum metabolites (BAs)), which are expected to reduce not only DIC but also DILI risk (Weber and Gerbes, 2022). In the future, it is expected that these biomarkers will be taken into account in DILI risk assessment.
In conclusion, we constructed an in vitro cholestasis risk test system using 2-D cultured HepaRG cells and 12 BAs of 100-fold the human serum value and evaluated its usefulness. This test system can be a prospective predictive method for DIC.
We would like to thank Dr. Hiroyuki Sasabe for scientific advice, and MARUZEN-YUSHODO Co., Ltd. (https://kw.maruzen.co.jp/kousei-honyaku/) for the English language editing.
Conflict of interestThe authors declare that there is no conflict of interest.