2023 年 48 巻 10 号 p. 527-534
2023 年 48 巻 10 号 p. 527-534
We investigated the usefulness of circulating miR-216a-5p and miR-217-5p that are pancreas-enriched micro RNAs (miRNAs) as biomarkers of acute pancreatic damage, and compared them with conventional pancreatic biomarkers in L-arginine-induced acute pancreatitis mouse model. As the results, amylase and lipase levels apparently increased and peaked on Day 3 when acute pancreatitis including acinar cell degeneration/necrosis and inflammatory cell infiltration reached its peak. In contrast, miR-216a-5p and miR-217-5p increased from Day 1 when histopathological findings in the acinar cells were limited to decreased zymogen granules, and the increases in ratios were much higher than those of amylase and lipase. The miRNAs remained at high levels until Day 5 when the pseudo-tubular complex and replacement of inflammatory cells and fibrotic cells were apparent instead of necrosis, whereas amylase and lipase levels decreased to the control levels. Furthermore, we examined the relationship between biomarker levels and histopathological degeneration/necrosis scores in the acinar cells. miR-216a-5p and miR-217-5p levels increased depending on the score of degeneration/necrosis, and all individual miRNAs exceeded the control levels from a score of 2 (focal necrosis), whereas all individual amylase and lipase levels exceeded the control levels at scores of 4 (lobular necrosis) and 3 (sublobular necrosis), respectively. In conclusion, we demonstrated that circulating miR-216a-5p and miR-217-5p could detect pancreatic damage earlier with greater magnitude, and the sensitivity to detect acinar cell degeneration/necrosis was superior to that of conventional biomarkers in the L-arginine-induced acute pancreatitis mouse model.
Acute pancreatitis is an inflammatory disorder of the pancreas that is associated with substantial morbidity and mortality, and its well-known etiology is gallstones, excessive alcohol consumption, and various drugs (Jones et al., 2015; Lee and Papachristou, 2019). Despite disparate causes, premature digestive enzymes are activated within acinar cells, resulting in cell death and multiple parallel events, including activation of the inflammatory cascade, endoplasmic reticulum stress, autophagy, and mitochondrial dysfunction in acinar cells are accompanied (Saluja et al., 2019). The diagnosis of acute pancreatitis is generally based on subjective clinical assessment and the enzyme activities of serum amylase and lipase (Greenberg et al., 2016; Jones et al., 2015). Conventional biomarkers are useful when sufficiently elevated, but are not sensitive for diagnosis (Goodwin et al., 2014), and their specificity is also insufficient because the pancreas is not the only source of amylase and lipase; they could be released from other organs, including the liver and gastrointestinal tract (Whitten et al., 1988; Yadav et al., 2002).
MicroRNAs (miRNAs) are a family of small non-coding RNAs consisting of 21–25 nucleotides that regulate gene expression at the post-transcriptional level and are associated with developmental, physiological, and pathological processes (He and Hannon, 2004). Some miRNAs show tissue- and cell-restricted expression, and have been demonstrated as potential biomarkers of tissue injury (Harrill et al., 2016; Schofield et al., 2021) as the miRNAs would leak into circulation from damaged cells and exist in highly stable forms resistant to ribonuclease digestion. For example, the plasma levels of miRNAs abundant and/or specific to the liver (miR-122, miR-192, miR-103a, and miR-885), kidney (miR-21, miR-155, miR-18a, and miR-30a), cardiac muscle (miR-1, miR-133a, miR-208a/b, and miR-499), skeletal muscle (miR-1, miR-133a, miR-133b, and miR-206), and brain (miR-124, miR-9, miR-384, and miR-922) were elevated in drug-induced damage or pathological animal models (Bailey et al., 2019; Laterza et al., 2009; Schofield et al., 2021).
miR-216a and miR-217 are abundant in the pancreas and are localized only in the acinar cells (Endo et al., 2013; Erdos et al., 2020; Wang et al., 2017). Although the physiological function of these miRNAs in the pancreas is not clear, the miR-216a or miR-217 knockout (KO) aggravated acinar injury and increased ductal formation in caerulein-induced pancreatitis model mice, and the recovery was delayed (Sutaria et al., 2019), suggesting that these miRNAs may protect acinar cells against several stimulations. Circulating miR-216a and miR-217 outperform amylase and lipase for the detection of acute pancreatic damage in drug-induced and pancreatic duct ligation-induced acute pancreatic injury, which has been demonstrated mainly in rats (Endo et al., 2013; Erdos et al., 2020; Kong et al., 2010; Wang et al., 2017). There is little literature on other species, but Goodwin et al. demonstrated the usefulness of the miR-216a and miR-217 in mice, where they could detect pancreatic injury as well as amylase or lipase in the caerulein-induced and pancreatic duct ligation-induced models, but not in the arginine-induced model (Goodwin et al., 2014). Additionally, plasma or serum miR-216a levels increase with pancreatitis severity in patients with acute pancreatitis (Kuśnierz-Cabala et al., 2015; Zhang et al., 2017).
To date, many rodent models of acute pancreatitis have been developed and utilized to investigate the mechanisms and pathogenesis of the disease, including cerulein-induced, alcohol-induced, diet-induced, immune-mediated, and gene knockout models as non-invasive models (Su et al., 2006). Cerulein-induced models are widely and commonly used as conventional models, but the model has several disadvantages in that the induced lesions are relatively mild and variable at the maximum doses of cerulein. The L-arginine-induced acute pancreatitis model is also a noninvasive model, and a single intraperitoneal administration of a large dose of L-arginine, an essential amino acid, induces acute necrotizing pancreatitis in animals. The model possesses several advantages, including stability, higher reproducibility of induced changes, and similarity of histopathological characteristics in humans (Kui et al., 2014; Su et al., 2006). The exact mechanism is not yet fully understood, but may involve excessive nitric oxide production, lipid peroxidation, and/or metabolic acidosis (Kui et al., 2014; Yildiz and Hamaloglu, 2010).
In this study, we investigated the usefulness of plasma miR-216a and miR-217 as biomarkers of exocrine pancreatic damage by comparing them with conventional biomarkers using the L-arginine-induced acute pancreatitis mouse model.
Male C57BL/6JJcl mice (CLEA Japan Inc., Tokyo, Japan) at 8 weeks of age were used, because this strain and gender were well used in the L-arginine-induced pancreatitis models (Dawra et al., 2007; Ohkawara et al., 2017). A total of 36 mice were used, and 6 mice were allocated to the control or each time point. The mice were housed in a room maintained under controlled temperature (20–26°C), relative humidity (25–75%), and a 12-hr light/dark cycle (7:00 to 19:00). A standard commercial diet (CRF1; Oriental Yeast Co. Ltd., Tokyo, Japan) and tap water were provided ad libitum.
L-arginine hydrochloride (L-arginine) (Sigma-Aldrich, Tokyo, Japan) in physiological saline (pH 7.0) was administered intraperitoneally once to mice at a dose of 6 g/kg (Day 0). The dose of L-arginine was determined based on previous reports (Ohkawara et al., 2017) and a preliminary investigation. Under isoflurane anesthesia, blood was collected from the heart into EDTA-2K-containing tubes for miRNA measurements and coagulation promoter-containing tubes for biomarker measurements on Days 1 (24 hr after the administration), 2, 3, 5, and 7. As a control, physiological saline was administered, and blood collection and euthanization were conducted on Day 7. After blood collection, the mice were euthanized by exsanguination under anesthesia and the pancreas was removed.
All experiments were approved by the animal experiment ethics committee chair, based on the animal welfare bylaws of the Research Institute, EA Pharma Co., Ltd.
Histopathological examinationThe excised pancreas was fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned. Paraffin sections were processed for hematoxylin and eosin (HE) and immunohistochemical staining using an anti-cleaved caspase-3 rabbit polyclonal antibody (Cell Signaling Technology, Tokyo, Japan).
Histopathological features of pancreatitis were graded according to a modified version of a previous report (Van Laethem et al., 1995), and the slides were read in a blinded manner. Acinar cell degeneration/necrosis was defined as loss of the acinar cell structure, loss of basal basophilic/apical acidophilic staining of the cytoplasm and pyknotic nucleus, and rupture of the cytoplasmic membrane. It was scored on a scale of 0 (normal), 1 (decreased zymogen granules), 2 (focal necrosis, < 5% of the total area of parenchyma), 3 (sublobular necrosis, 5–20% of the total area of parenchyma), and 4 (lobular necrosis, ≥ 20% of the total area of parenchyma). Edema, inflammatory cell infiltration, and replacement of inflammatory and fibrotic cells were scored on a scale of 0 (normal) to 3 (severe). In addition, the number of caspase-3-positive acinar cells in each specimen was counted and the number of positive cells per unit area was calculated.
Measurement of biomarkersSerum amylase and lipase levels were measured using Fujidrychem slides AMYL-PIII and v-LIP-P (Fujifilm, Tokyo, Japan).
Plasma miR-216a-5p and miR-217-5p levels were measured by digital polymerase chain reaction (PCR). Briefly, total RNA was extracted from 240 µL of plasma using the miRNeasy Serum/Plasma Kit (QIAGEN, Tokyo, Japan). cDNA was synthesized from total RNA using a TaqMan microRNA Reverse Transcription kit (Thermo Fisher, Tokyo, Japan). After preparing the PCR reaction solution with 2 × ddPCR SuperMix for Probe (Bio-Rad, Tokyo, Japan), PCR was conducted using a ProFlexTM PCR System (Thermo Fisher, Tokyo, Japan), and the fluorescence intensity was read using a QX200 Droplet Reader (Bio-Rad, Tokyo, Japan) and QuantaSoft software (Bio-Rad).
Statistical analysisFor amylase, lipase, miR-216a-5p, and miR-217-5p, the mean values and standard deviation (S.D.) were calculated for each group. In addition, Dunnett’s test or Dunn’s test (two-tailed, significance level: p < 0.05) was performed for multiple comparisons between the control group and each L-arginine group, when the variance was homogeneous or heterogeneous by Bartlett’s test, respectively.
In the histopathological examination of the L-arginine-administered mice, zymogen granules decreased on Day 1, acinar cell degeneration/necrosis and inflammatory cell infiltration appeared on Day 2 and peaked on Day 3. Instead of necrosis, a pseudo-tubular complex and replacement of inflammatory and fibrotic cells were apparent on Day 5, and pancreatic damage almost disappeared on Day 7 (Fig. 1). The total score of edema, inflammatory cell infiltration, and acinar cell degeneration/necrosis increased on Day 1, peaked on Day 3, and then almost recovered. In immunohistochemistry for caspase-3, the number of positive acinar cells increased on Day 1, peaked on Day 3, and returned to control levels on Days 5 and 7 (Table 1).

Histopathological features of the pancreas in mice following a single administration of physiological saline (A) or L-arginine on Days 1, 2, 3, 5 and 7 (B, C, D, E and F, respectively). Each bar shows 50 μm.
| Histopathological score | Control | Day 1 | Day 2 | Day 3 | Day 5 | Day 7 |
|---|---|---|---|---|---|---|
| Findings | ||||||
| Edema | 0.0 ± 0.0 | 1.2 ± 0.8 | 0.7 ± 0.5 | 1.5 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Acinar cell degeneration/necrosis | 0.0 ± 0.0 | 0.8 ± 0.4 | 2.8 ± 0.8 | 3.3 ± 0.5 | 1.0 ± 1.1 | 0.7 ± 0.5 |
| Inflammatory cell infiltration | 0.0 ± 0.0 | 0.3 ± 0.5 | 1.2 ± 0.4 | 1.7 ± 0.8 | 0.7 ± 0.5 | 0.3 ± 0.5 |
| Replacement of inflammatory cells and fibrotic cells | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.2 ± 0.8 | 0.8 ± 0.4 |
| Immunohistochemistry | ||||||
| No. of caspase-3 positive acinar cells/cm2 | 9.6 ± 12 | 380 ± 430 | 1700 ± 600 | 4900 ± 2800 | 7.8 ± 11 | 46 ± 29 |
Mean ± S.D.
miR-216a-5p and miR-217-5p were measured in L-arginine-administered mice on Days 1, 2, 3, 5 and 7, and compared to the conventional biomarkers for pancreatic damage. Plasma miR-216a-5p and miR-217-5p levels increased on Day 1 (8.3- and 10-fold, respectively), peaked on Day 3, and remained high (29- and 18-fold, respectively) until Day 5. Serum amylase and lipase, conventional biomarkers, apparently increased on Day 3, although the lipase level of 3.3-fold on Day 2 was statistically significant. The increase in ratios of the miRNAs and conventional biomarkers on the peak day (miR-216a-5p and miR-217-5p were 700- and 920-fold, respectively), were much higher than those of amylase and lipase (4.5- and 11-fold, respectively) (Fig. 2, Table 2).
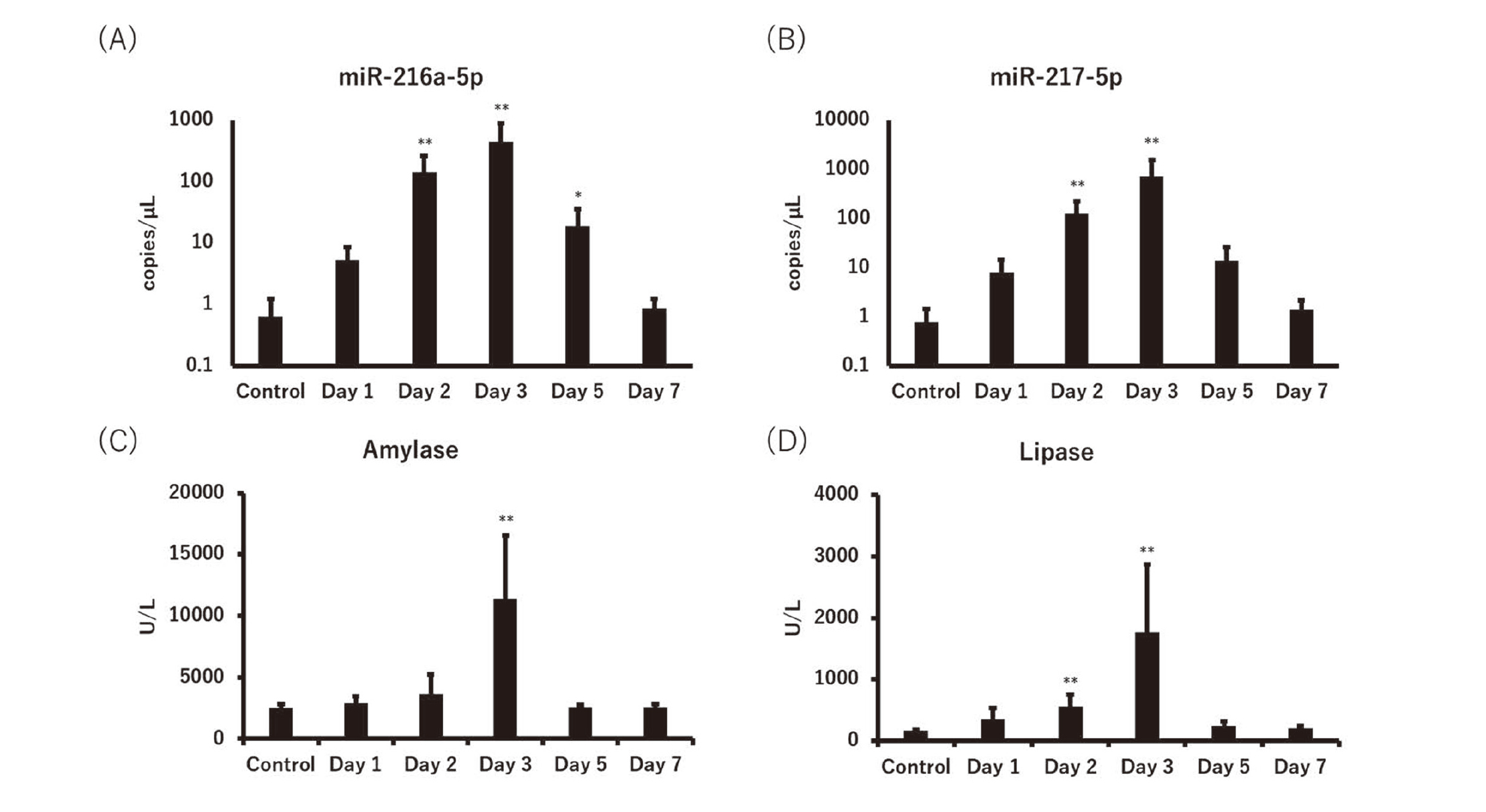
Micro RNAs (miRNAs) and conventional biomarkers for pancreatic injury in the L-arginine-administered mice. (A) miR-216a-5p, (B) miR-217-5p, (C) amylase, and (D) lipase levels. Data represent mean + standard deviation (S.D.) *p < 0.05 and **p < 0.01 (vs. control).
| Biomarker | Day 1 | Day 2 | Day 3 | Day 5 | Day 7 |
|---|---|---|---|---|---|
| miR-216a-5p | 8.3 | 220 | 700 | 29 | 1.4 |
| miR-217-5p | 10 | 160 | 920 | 18 | 1.8 |
| Amylase | 1.1 | 1.4 | 4.5 | 1.0 | 1.0 |
| Lipase | 2.1 | 3.3 | 11 | 1.5 | 1.2 |
Mean of the increase ratio to the control group
The correlation between biomarker levels and histopathological scores for acinar cell degeneration/necrosis was analyzed. miR-216a-5p and miR-217-5p levels increased depending on the acinar cell degeneration/necrosis score, and all individual miRNA levels exceeded the maximum value of the control from a score of 2. Amylase and lipase also increased depending on the acinar cell degeneration/necrosis score, but all individual values of amylase and lipase exceeded the maximum value of the control at scores of 4 and 3, respectively (Fig. 3, Table 3).
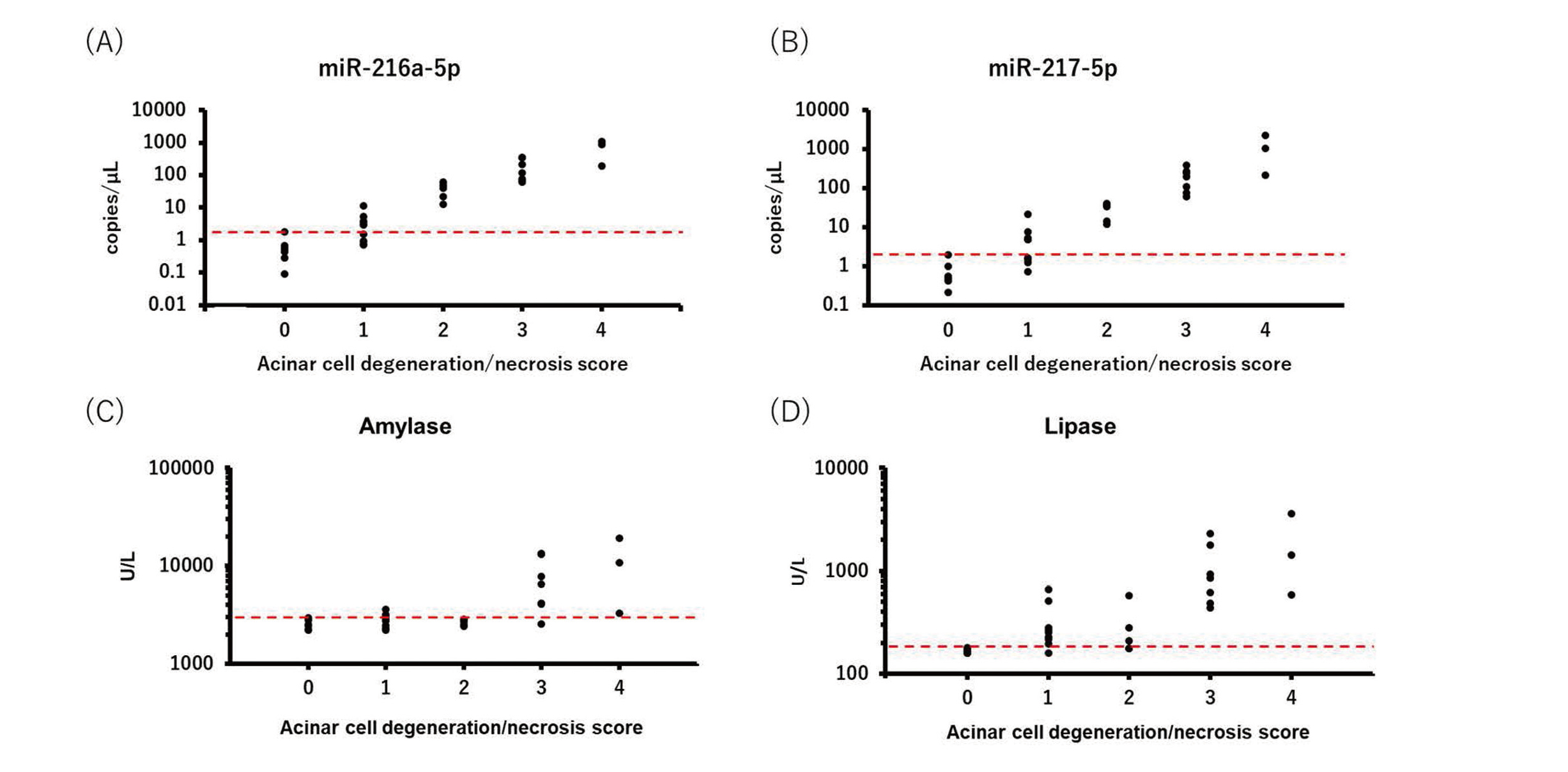
Correlation between the biomarker levels and acinar cell degeneration/necrosis scores. Individual biomarker levels were classified by the acinar cell degeneration/necrosis score. (A) miR-216a-5p, (B) miR-217-5p, (C) amylase, and (D) lipase levels. A red dotted line represents the maximum level at score 0 in the control mice.
| Biomarker | Acinar cell degeneration/necrosis | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| miR-216a-5p | 5.4 | 59 | 280 | 1100 |
| miR-217-5p | 7.1 | 36 | 250 | 1500 |
| Amylase | 1.1 | 1.0 | 2.9 | 4.4 |
| Lipase | 1.8 | 1.7 | 6.4 | 11 |
Mean of the increase ratio to the control group
After a single intraperitoneal administration of L-arginine to mice, edema, inflammatory cell infiltration, and acinar cell degeneration/necrosis were observed. The histopathological changes peaked on Day 3 and mostly recovered on Day 7, which was consistent with previous studies (Dawra et al., 2007; Kui et al., 2014). On macroscopic examination, there were no abnormal findings in the other organs, although slight hemorrhage was sporadically observed in the lungs (data not shown); it was reported that lung injury occurred in an L-arginine-induced acute pancreatitis mouse model (Huang et al., 2012). Therefore, acute pancreatitis was demonstrated to be induced by L-arginine in the mouse model.
Plasma miR-216a-5p and miR-217-5p levels increased from Day 1 (8.3 and 10-fold, respectively) when the acinar cell change was limited to decreased zymogen granules, and no changes were noted in serum amylase and lipase levels. Both the miRNAs and conventional biomarkers peaked on Day 3, but the increase in ratios of miR-216a-5p and miR-217-5p (700- and 920-fold, respectively) were much higher than those of amylase and lipase (4.5- and 11-fold, respectively). These results indicate that miR-216a-5p and miR-217-5p are useful biomarkers for the early detection of acute pancreatitis with a great increased ratio, although the time point of statistically significant increases in miRNAs and lipase were on the same Day 2. In previous studies, the potential of miR-216a and miR-217 as biomarkers for acute pancreatitis was revealed mainly in rats, and a few were reported in mice and dogs. miR-216a-5p and miR-217-5p could detect tissue injury more apparently than amylase and lipase in several types of chemical-induced pancreatitis model rats (Erdos et al., 2020); however, their superiority to the conventional biomarkers is considered higher in mice (miR-216a: 700-fold, miR-217: 920-fold, amylase: 4.5-fold, lipase: 11-fold) than in rats when referring to the literature (miR-216a: approximately 10 to 100-fold, miR-217: 100 to 1500-fold, amylase: approximately 2.5–7.5-fold, lipase: approximately 8 –93 fold) (Calvano et al., 2016; Erdos et al., 2020; Smith et al., 2016). miR-216a and miR-217 are localized in pancreatic acinar cells and released into the bloodstream when the cellular membrane is disrupted (Erdos et al., 2020), and their expression is more abundant in mice than in rats (Wang et al., 2017). Therefore, in this study, we attribute the excellent increase in the plasma levels of miR-216a and miR-217 to their abundance in pancreatic acinar cells.
miR-216a-5p and miR-217-5p remained at high levels until Day 5 (29- and 18-fold, respectively), whereas amylase and lipase levels decreased to the control levels. Since degeneration/necrosis and caspase-3-positive findings in acinar cells were hardly observed, it was unlikely that the miRNAs leaked from the injured acinar cells at this point. Consistent with our results, it was reported that increased levels of miR-216a and miR-217 persisted longer than amylase and lipase in the cerulein- or L-arginine-induced acute pancreatitis rat models (Goodwin et al., 2014; Smith et al., 2016; Wang et al., 2017), which would suggest that miR-216a-5p and miR-217-5p in circulating blood would be relatively stable. Plasma level of miR-216a and miR-217 could not reflect the initial of recovery phase; however, they could detect pancreatic damage even if the timing of measurement was not optimal.
We examined the relationship between biomarker levels and histopathological degeneration and necrosis scores in acinar cells. The levels of miRNAs and conventional biomarkers increased depending on the degeneration/necrosis score. All individual values of miR-216a-5p and miR-217-5p exceeded the control level by a score of 2. However, all individual values of amylase and lipase exceeded the control level at scores of 4 and 3, respectively. The results indicated that the sensitivity of miR-216a-5p and miR-217-5p in detecting acinar cell degeneration/necrosis was superior to that of amylase and lipase. In addition, plasma miR-216a-5p and miR-217-5p could detect the pharmacological effects of the anti-pancreatitis drug in the cerulein-induced acute pancreatitis mouse model (data not shown), demonstrating its usefulness as a pharmacodynamic marker. Contradictory to our study, Goodwin et al. reported that the circulating levels of miR-216a and miR-217 did not change in the L-arginine-administered mice (Goodwin et al., 2014). In their study, the dose of L-arginine was lower compared with ours and no histopathological change was observed in pancreas; therefore, we consider that the potentials of miR-216a and miR-217 could not be evaluated appropriately. To our best knowledge, we clarified the usefulness of miR-216a and miR-217 as pancreatic biomarkers in the L-arginine-induced acute pancreatitis mouse model for the first time.
Chronic pancreatitis (CP) can be diagnosed based on imaging criteria and clinical features, including abnormal amylase or lipase concentrations (Beyer et al., 2020; The Japanese Society of Gastroenterology, 2015). Unlike acute pancreatitis, serum amylase and lipase levels do not increase, but rather decrease in patients with CP, because enzyme synthesis and entry into the blood circulation would be reduced (Kwon et al., 2016; The Japanese Society of Gastroenterology, 2015). Circulating miR-579-3p and miR-30a-3p decreased, and 13 miRNAs, including miR-27a-3p, miR-194-5p, and miR-361-5p, increased in patients with CP compared to those in healthy participants (Chhatriya et al., 2020; Desai et al., 2021), and several miRNAs (miR-21, miR-34a, miR-198, miR-210-3p, and miR-217) discriminate CP from pancreatic ductal adenocarcinoma (PDAC) (Guz et al., 2021; Vychytilova-Faltejskova et al., 2015). miR-216a and miR-217 are expressed exclusively in pancreatic acinar cell in normal conditions, and their expression is presumably suppressed with the loss and atrophy of acinar cells in CP. Therefore, we expect that miR-216a and miR-217 might be potential biomarkers for CP, although further studies are required.
In conclusion, we demonstrated that circulating miR-216a-5p and miR-217-5p could detect pancreatic damage earlier with greater magnitude, and the sensitivity to detect acinar cell degeneration/necrosis was superior to that of conventional biomarkers in the L-arginine-induced acute pancreatitis mouse model.
Conflict of interestThe authors declare that there is no conflict of interest.