2023 年 48 巻 11 号 p. 571-583
2023 年 48 巻 11 号 p. 571-583
Various chemicals, including pesticides, heavy metals, and metabolites of tobacco, have been detected in fetal environment. Fetuses are exposed to these chemicals at relatively low concentrations; however, their risk of developing neurological and behavioral disorders increases after birth. We aimed to evaluate the effects of five chemicals (diethylphosphate, cotinine, octachlorodipropyl ether, mercury, and selenium) detected in the serum of pregnant mothers on neural development using human neurospheres (NSphs) differentiated from induced pluripotent stem cells. Exposure to each chemical at serum concentrations revealed no effects on NSph development. However, combined exposure to the five chemicals caused a significant decrease in NSph size and altered gene expression and neural differentiation. Thus, we next focused on DNA methylation to investigate changes in NSph properties caused by chemical exposure. Combined exposure to chemicals had extremely small effects on the DNA methylation status of NSphs at individual gene loci. However, stochastic changes in methylation status caused by chemical exposure were significantly accumulated throughout the entire genome. These results suggest that the five chemicals acted as epimutagens that alter the epigenetic status during human neural development at the biological level. Taken together, we showed for the first time, the epimutagen-induced alterations in neural differentiation at serum concentrations using an in vitro human neuronal model.
Numerous chemicals are widely used in household, agricultural and urban environments, and various chemicals have been detected in fetal environment (Mori et al., 2003; Kortenkamp et al., 2007; Iwasaki et al., 2011). Although the concentration of chemicals detected in fetal environment is relatively low (approximately 0.1–100 ppb level) and far from the levels reported in pharmacological studies, prenatal exposure to environmental chemicals is associated with fetal growth restriction and low birth weight (Whyatt et al., 2004; Jaddoe et al., 2008; Washino et al., 2009; Zhu et al., 2010). This implies that the effects of chemical exposure during the fetal development appear after birth.
Epigenetic systems are essential for normal embryonic development through transcriptional regulation of cell type-specific gene expression. Epigenetic marks, such as DNA methylation and histone modification, dramatically change with differentiation process and cellular conditions (Shiota, 2004; Lieb et al., 2006; Golob et al., 2008; Ikegami et al., 2009). Moreover, epigenetic systems are related to cell plasticity and stability depending on the cellular environment and cell fate decisions. Considering these findings, the long-lasting effects of chemicals on abnormal phenotypes may be attributed to epigenetic alterations. Chemicals that disrupt epigenetic systems are called as epimutagens (Holliday and Ho, 2002).
We have previously performed epimutagen screening using mouse embryonic stem cells (ESCs) and human induced pluripotent stem cells (iPSCs). Of the 25 environmental chemicals detected in the blood samples of pregnant mothers (Arai et al., 2011), five chemicals [diethylphosphate (DEP), cotinine, octachlorodipropyl ether (S-421), mercury, and selenium] disturbed epigenetic systems at the serum concentrations in mouse ESCs (Arai et al., 2011) and human iPSCs (Arai et al., 2015).
Recently, there have been concerns regarding the effects of chemicals on neurological development in children. Exposure to pesticides and heavy metals during the fetal period increases the risk of developing neurological and behavioral disorders after birth (Eskenazi et al., 2007; Harari et al., 2010; Rauh et al., 2011; Llop et al., 2013). Although neurotoxicity caused by chemicals has been reported, most neurotoxic concentrations are hundreds to thousands of times the concentration range detected in human blood samples (Huang et al., 1993; Arai et al., 2011; Woehrling et al., 2011; Li et al., 2017; Sudo et al., 2019; Huang et al., 2021). Therefore, the effects of these chemicals on neuronal development during the fetal period have not yet been elucidated. In addition, differences between human and animal cells, or different cell types, display different sensitivities to chemicals (Wilson et al., 2014; Boncler et al., 2019; Chen et al., 2020). Therefore, it is better to use human neuronal cells to understand the effects of chemicals on the fetal neurogenesis. We aimed to investigate the effects of five chemicals (DEP, cotinine, S-421, Hg, and Se) on neural development at serum concentrations using iPSC-derived human neural stem cells.
MEFs were isolated from 13.5 days post coitum fetuses of pregnant ICR mice (Charles River Japan, Yokohama, Japan) and cultured in Dulbecco’s Modified Eagle’s Medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (HyClone, Thermo Fisher Scientific, Waltham, MA, USA), 1% penicillin and streptomycin (Thermo Fisher Scientific), and 55 µM 2-mercaptoethanol (Thermo Fisher Scientific). To generate MEF feeder cells, MEFs were irradiated with 30 Gy gamma rays. All animal experiments were approved by the Institutional Animal Care and Use Committee of University of Miyazaki (no. 2017-009). All animal care and experimental procedures were performed in accordance with the Japan Act on Welfare and Management of Animals, and all applicable regulations.
Human iPSC cultureHuman iPSCs (MRC-iPSCs and Edom-iPSCs) used in this study have been previously described (Nishino et al., 2011). Human iPSCs were maintained on MEF feeder cells in KnockOut Dulbecco’s Modified Eagle Medium (Thermo Fisher Scientific) supplemented with 20% KnockOut Serum Replacement (Thermo Fisher Scientific), 1% GlutaMAX (Thermo Fisher Scientific), 1% nonessential amino acids (Thermo Fisher Scientific), 1% penicillin and streptomycin (Thermo Fisher Scientific), 55 µM 2-mercaptoethanol (Thermo Fisher Scientific), and 10 ng/mL recombinant human basic fibroblast growth factor (Fujifilm Wako Pure Chemical, Osaka, Japan). Handling of human cells was in accordance with the ethical standards of the Helsinki declaration.
Neurosphere (NSph) formation and neural differentiationNSph, neural stem cell derived from human iPSC, were cultured as previously described (Matsumoto et al., 2016). Briefly, iPSCs were dissociated into single cells with TrypLE Select (Thermo Fisher Scientific), and then cultured in Neurobasal Plus Medium (Thermo Fisher Scientific) supplemented with B27 supplement (Thermo Fisher Scientific), 20 ng/mL recombinant human basic fibroblast growth factor, 10 µM Y-27632 (Fujifilm Wako Pure Chemical), and 10 ng/mL human leukemia inhibitory factor (Fujifilm Wako Pure Chemical) under 5% oxygen. NSphs were repeatedly passaged by dissociation into single cells and then cultured as described above. NSph diameter was measured using the ImageJ software provided by the National Institute of Health (http://rsb.info.nih.gov/ij/). For neural differentiation, NSphs were adhered to poly-L-ornithine (Fujifilm Wako Pure Chemical)- and fibronectin (Fujifilm Wako Pure Chemical)-coated dishes and cultured in Neurobasal Plus medium supplemented with B27 supplement, 10 ng/mL brain-derived neurotrophic factor (Fujifilm Wako Pure Chemical), 10 ng/mL glial cell-derived neurotrophic factor, 1 mM dibutyryl-cAMP (Santa Cruz Biotechnology, CA, USA), and 200 µM ascorbic acid (Fujifilm Wako Pure Chemical) for 14 days. All experiments were independently performed at least twice.
Chemical exposure of NSphsNSphs were cultured with five chemicals (DEP, cotinine, S-421, Hg, and Se) at concentrations equivalent to the serum levels determined in a previous study (Arai et al., 2011) based on the concentrations in cord blood samples and/or the serum of pregnant mothers. Hg (Kanto chemical, Tokyo, Japan) and Se (Kanto Chemical) were diluted with nitric acid (HNO3) (Fujifilm Wako Pure Chemical), and DEP (Fujifilm Wako Pure Chemical), cotinine (Sigma-Aldrich), and S-421 (Sigma-Aldrich) were diluted with ethanol (EtOH, Fujifilm Wako Pure Chemical). The final concentrations of HNO3 and EtOH were 0.0063% and 0.1%, respectively. The five-chemical mixture (5-chem) was also added to the culture medium based on the serum levels. The chemical mixture was dissolved in HNO3 or EtOH, and the final concentrations of HNO3 and EtOH in the culture medium were 0.0063% and 0.1%, respectively.
Quantitative gene expression analysisTotal NSph RNA was extracted using an RNeasy Plus Mini Kit (Qiagen, Hilden, Germany). First-strand cDNA synthesis was performed using the Superscript III First-strand Synthesis System (Thermo Fisher Scientific) with random hexamers. Quantitative real-time polymerase chain reaction (qPCR) was performed using SYBR® Green PCR master mix (Applied Biosystems, Woburn, MA, USA). Data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression levels, and relative expression levels are presented as the fold-change in expression, which was calculated using the 2(-Delta Delta C(T)) method (Livak and Schmittgen, 2001). The PCR primer sequences are listed in Supplemental Table 1. All experiments were performed in triplicate and the results are presented as mean values with standard deviations.
ImmunohistochemistryNeurally differentiated NSphs obtained by adherent culture were fixed in 4% paraformaldehyde for 10 min. After permeabilization with 0.2% Triton X-100 for 5 min, samples were treated with blocking buffer (1% bovine serum albumin and 0.01% Tween-20 in phosphate-buffered saline) for 60 min. The samples were incubated with anti-TUBB3 mouse monoclonal antibodies (MMS-435P; Covance, Princeton, NJ, USA) diluted in blocking buffer (1:200) for 60 min, followed by three washes with phosphate-buffered saline containing 0.05% Tween-20. After incubation with fluorescent secondary antibodies (Alexa Fluor 488 goat anti-mouse immunoglobulin G, Thermo Fisher Scientific) diluted in blocking buffer (1:200) for 60 min, the samples were washed again and counterstained with 0.2 μg/mL 4′,6-diamino-2-phenylindole (DAPI; Dojindo, Kumamoto, Japan). All the reactions were performed at room temperature (15-25°C). Images of TUBB3-positive neuronal fibers were acquired using a fluorescent microscope (BZ-9000; KEYENCE, Osaka, Japan), and neuronal fiber in 16–24 visual fields was quantified using the ImageJ software. Briefly, RGB images were converted to 8-bit grayscale images (0−255 gradation). Next, the intensity thresholds of TUBB3 images were determined using the automatic threshold setting of the ImageJ program (between 3−115 gradation), and neuronal fiber area was measured using the ImageJ software.
Genome-wide DNA methylation analysisGenomic DNA was purified from human NSphs using the DNeasy Blood & Tissue Kit (Qiagen), according to the manufacturer’s protocol. Genome-wide DNA methylation analysis was performed using an Illumina Infinium Human MethylationEPIC BeadChip (Illumina Inc., CA, USA). Methylated and unmethylated signals were used as a β-value, which is a quantitative score of DNA methylation levels, and the degree of methylation was represented between “0” (completely unmethylated) and “1” (completely methylated). All DNA methylation data were submitted to the Gene Expression Omnibus database under accession number GSE235146.
Statistical analysisAll statistical analyses were performed using the GraphPad Prism software (version 8.0; San Diego, CA, USA). Statistical comparisons of the sphere diameter and neuronal fiber area detected by anti-TUBB3 antibodies were performed using the Kruskal-Wallis test, and those of genome-wide DNA methylation and marker gene expression data were performed using Student’s t-test and Tukey test, respectively.
To investigate the effects of the five chemicals (DEP, cotinine, S-421, Hg, and Se) on human neural development, we exposed human neural stem cells, NSphs; MRC-NSph and Edom-NSph to each chemical at serum concentrations reported in a previous study (Arai et al., 2011) (Table 1) for two weeks (Fig. 1A). Differentiation of iPSCs to NSphs was confirmed by the expression of PAX6 and SOX2, the marker genes for neural stem cells (Supplemental Fig. 1). The effects of the chemicals on NSphs were evaluated based on the sphere diameter, as previously reported (Toyoshima et al., 2016).
| Chemical | Category | Exposure concentration (ppb)a | Diluting solvent |
|---|---|---|---|
| DEP | pesticides | 0.1 | EtOH |
| Cotinine | tobacco | 100 | EtOH |
| S-421 | pesticides | 0.01 | EtOH |
| Hg | heavy metals | 1.0 | HNO3 |
| Se | heavy metals | 100 | HNO3 |
| 5-chemb | mixture | 0.01–100c | EtOH + HNO3 |
a Serum level of pregnant mothers based on a previous study (Arai et al., 2011).
b Mixture of five chemicals (DEP, cotinine, S-421, Hg, and Se).
c Five chemicals mixed at the respective exposure concentration.
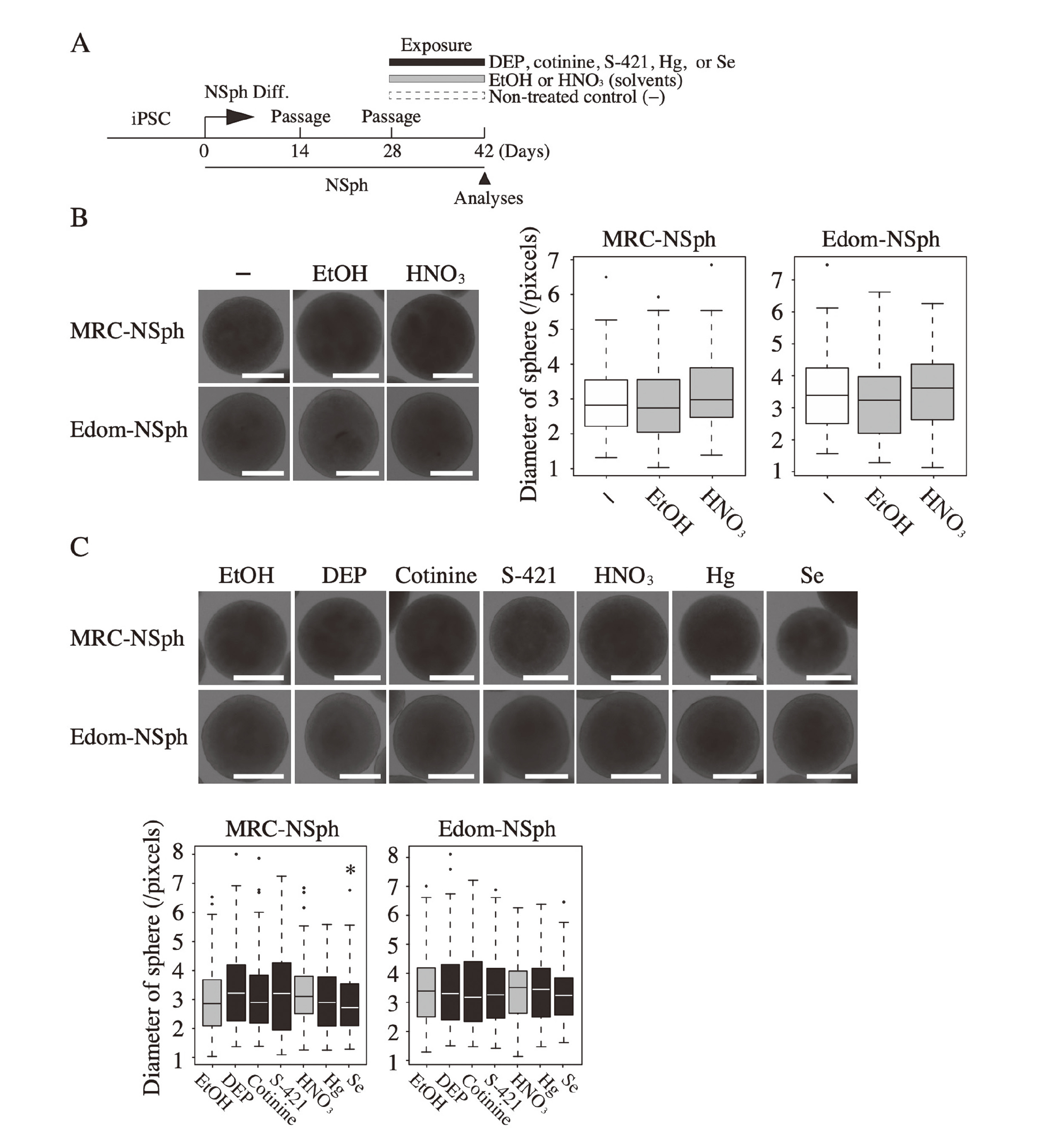
Effects of a single chemical on NSph formation. (A) Schematic diagram of NSph culture (42 days). MRC-NSphs and Edom-NSphs were exposed for two weeks (days 28–42) to five chemicals (DEP, cotinine, S-421, Hg, or Se) based on maternal serum concentration or their diluting solvent (EtOH or HNO3). (B) Images of solvent- or non-treated NSphs after two weeks of exposure (left panel). NSph (n = 78−108) diameter is shown as a box plot (right panel). Scale bar = 250 µm. (C) Images of chemical-exposed NSphs (upper panel). After two weeks of exposure to each chemical, NSph (n = 126−268) diameter was measured and is shown as a box plot (lower panel). Scale bar = 250 µm. * p < 0.05. In the boxplot images, black and gray boxes represent the data of chemical- and solvent-exposed NSphs, respectively.
We first analyzed the effects of the diluting solvents (EtOH or HNO3) on NSph formation (Fig. 1B). Exposure to EtOH or HNO3 at final concentrations (0.1% or 0.0063%, respectively) did not affect the sphere size. We then exposed NSphs to each of the five chemicals; there was no significant difference in sphere size between chemical- and solvent-exposed NSphs, except for Se-exposed MRC-NSph (Fig. 1C). These results suggest that exposure to a single chemical, with the exception of Se, did not adversely affect the development of human neural stem cells at the concentrations detected in the serum of pregnant mothers. Of the 5 chemicals analyzed in this study, only Se caused significant cell death in human neural stem cells when exposed to concentrations 10 times higher than maternal serum levels (data not shown). Thus, Se is more cytotoxic than the other chemicals, which may have led to the decrease in sphere size in MRC-NSph, even when exposed to serum concentrations.
Combined exposure to five chemicals caused a decrease in NSph sizeIn the previous study, the five chemicals used in this study had no effect on heterochromatin- or embryoid body-formation in undifferentiated human iPSCs at maternal serum concentrations (Arai et al., 2015). Furthermore, mixed exposure to multiple chemicals in the same category, such as pesticides or heavy metals, also did not affect heterochromatin formation in human iPSCs. In contrast, combined exposure to the five chemicals (DEP, cotinine, S-421, Hg, and Se), which were found to affect mouse ESCs, affected heterochromatin- and embryoid body-formation in human iPSCs (Arai et al., 2015). This indicates that adverse effects on cells are more enhanced with combined exposure to chemicals than with exposure to a single chemical. In addition, there is concern about the effects of chemical mixtures, and combined exposure to chemicals during pregnancy leads to lower birth weight in children (Kalloo et al., 2020; Hu et al., 2021; van den Dries et al., 2021). Thus, we next examined the effects of combined exposure to the five chemicals (5-chem, DEP, cotinine, S-421, Hg, and Se) at serum concentrations on NSphs (Fig. 2A).

Effect of combined exposure to five chemicals on NSph formation. (A) Schematic diagram of NSph culture exposed to five-chemical mixture (5-chem; DEP, cotinine, S-421, Hg, and Se) at serum level concentrations. (B) Images of 5-chem- or diluting solvent-exposed NSphs after two weeks of exposure (left panel). The sphere diameter (n = 248−603) was measured using the ImageJ software and is shown as a box plot (right panel). Scale bar = 250 µm. * p < 0.05
After two weeks of exposure to the chemicals, the sphere diameter was significantly decreased in 5-chem-exposed NSphs compared with that in the solvent-treated control (Fig. 2B). These results indicate that combined exposure to the five chemicals disrupted NSph formation at serum concentrations. In addition, there was no significant difference in the percentage of viable cells and cell numbers between the 5-chem- and solvent-treated samples, although there was a trend toward lower cell numbers in the 5-chem-treated samples on culture day 14 (Supplemental Fig. 2). Thus, combined exposure to chemicals did not cause NSphs cytotoxicity at the levels detected in the serum of pregnant mothers.
Increased expression of neuronal-related genes in chemical-exposed NSphsBecause 5-chem affected NSph formation, we performed gene expression analysis to verify whether combined exposure to chemicals affected NSph properties (Fig. 3). The expression of paired box 6 (PAX6), nestin (NES), and SRY-box transcription factor 2 (SOX2), the marker genes for neural stem cells, did not differ between the 5-chem-, solvent-, and non-treated samples, except for MRC-NSph at PAX6 gene. However, the expression of microtubule associated protein 2 (MAP2), RNA binding fox-1 homolog 3 (RBFOX3), tubulin beta 3 class III (TUBB3), and doublecortin (DCX), neuronal marker genes, was significantly increased in 5-chem-exposed NSphs compared with that in controls. The expression of neuronal marker genes increased with neural differentiation (Supplemental Fig. 3), suggesting that an increased proportion of neurons promoted the expression of neuronal-related genes. In addition, increased expression of PAX6 gene was observed in MRC-NSph. High expression level of Pax6 in mouse NSphs is conducive to neuronal differentiation (Hack et al., 2004). Therefore, the increased expression of neural stem cell- and neuronal-related genes in NSphs following chemical exposure might reflect changes in the properties of stem cells.

Changes in gene expression profile of NSphs following combined exposure to five chemicals. Gene expression analysis of marker genes for neural stem cells and neurons in NSphs exposed to a mixture of chemicals for two weeks. For a five-chemical mixture- (5-chem; DEP, cotinine, S-421, Hg, and Se), diluting solvent-, and non-treated NSphs, the relative expression levels were measured by qPCR. The data are shown as the mean ± standard deviation based on GAPDH expression. * p < 0.05.
Increased expression of neuronal-related genes in NSphs was expected to affect subsequent neuronal differentiation. In this experiment, we differentiated NSph into neurons and examined the effect of combined exposure to the five chemicals on neural differentiation. After exposing NSphs to 5-chem or solvent for two weeks, the chemicals and solvent were removed, and NSphs were adherently cultured and differentiated into neurons (Fig. 4A). Fourteen days after the induction of neural differentiation, neuronal fibers were detected using a specific antibody against TUBB3 (Fig. 4B), and neuronal fiber area was measured using the ImageJ software based on the obtained immunofluorescence images. The analysis revealed that neuronal fiber area was significantly increased in 5-chem-exposed MRC-neurons compared with that in solvent- or non-treated controls (Fig. 4C). Similarly, there was a trend toward increased neuronal fiber area in 5-chem-exposed Edom-neurons. This suggests that exposure to the 5-chem altered neural differentiation in vitro.
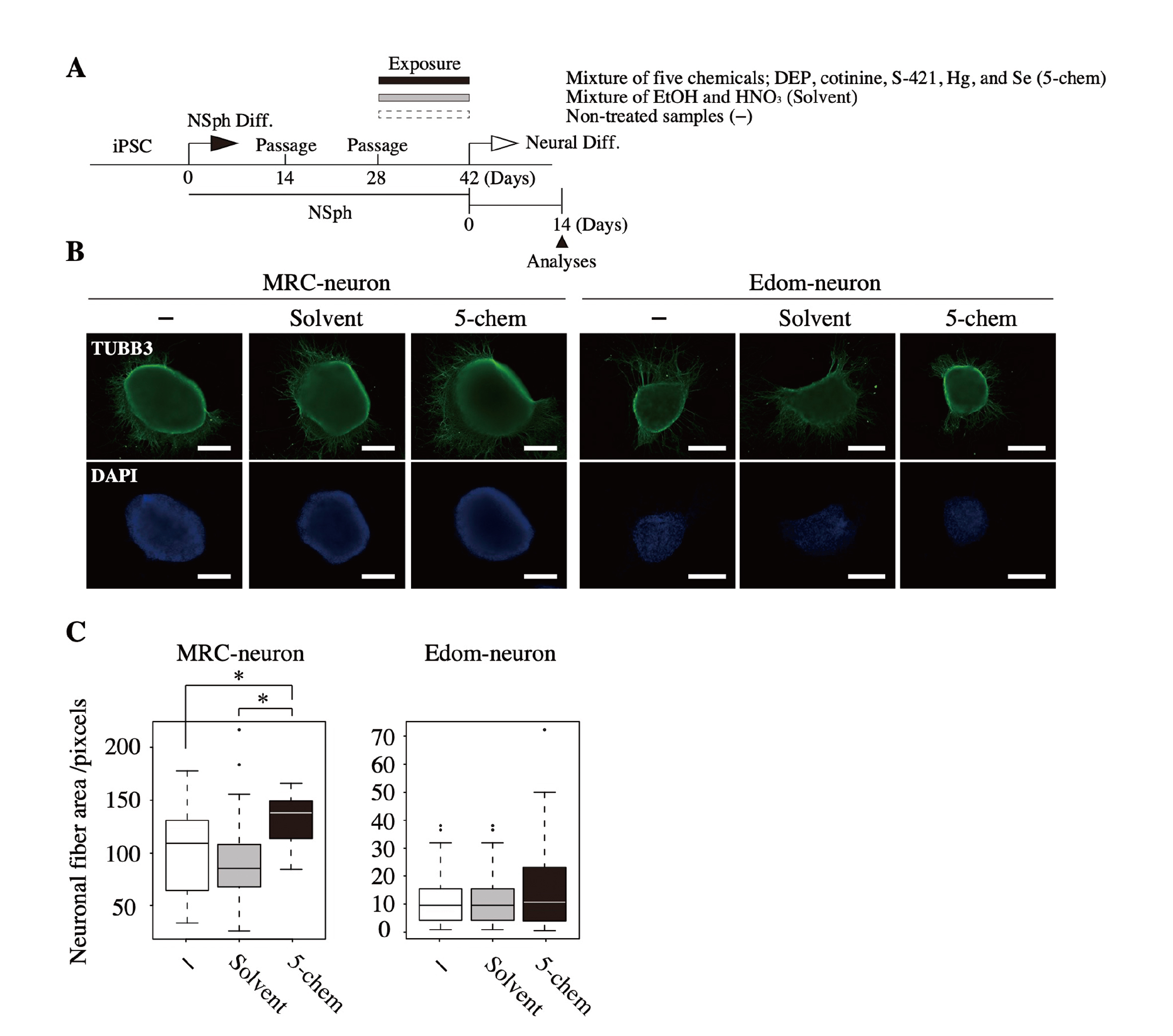
Effect of combined exposure to five chemicals on neural differentiation. (A) Schematic diagram of the differentiation of MRC-NSphs and Edom-NSphs. (B) Images of neural differentiation of NSphs. Upper and lower panels show the images of neuronal fibers stained with anti-TUBB3 antibody and nuclei stained with DAPI. MRC-neuron, MRC-NSph-derived neurons; Edom-neuron, Edom-NSph-derived neurons. Scale bar = 250 µm. (C) Measurement of neuronal fiber area of NSph-derived neurons. Based on the images obtained using the anti-TUBB3 antibody, the expanded neuron area was measured using the ImageJ software. For each sample, 16−27 spheres were analyzed and are shown as a box plot. * p < 0.05.
As shown in Fig. 4, combined exposure to chemicals during NSph development affected subsequent neural differentiation. This indicates that the effects of chemical exposure during the stem cell period persisted for a long time after differentiation. To investigate the sustained effects of exposure to the 5-chem on neuronal differentiation, we analyzed DNA methylation, which is involved in the regulation of cell fate decisions.
Genome-wide DNA methylation analysis of chemical- and solvent-exposed NSphs using Infinium Human MethylationEPIC BeadChip, with a particular focus on genic regions, was performed and DNA methylation data of 857,357 probes excluding blank values and high detection p-values (p > 0.05) were finally obtained. For the analysis, we used the values obtained by subtracting the methylation levels of non-treated samples from those of 5-chem- or solvent-treated samples in each probe. DNA methylation analysis revealed that the differences in DNA methylation levels in 5-chem- or solvent-treated samples compared with those in non-treated controls were extremely small (< 0.05) for most probes (99.97%) in both MRC- and Edom-NSphs, regardless of chemical exposure (Fig. 5A). Moreover, the methylation data of 5-chem- and solvent-treated samples calculated in comparison with those of non-treated controls were classified as hypermethylation (Hyper) or hypomethylation (Hypo) at each probe. The proportions of these probes were comparable (43–49% and 51–57%) between the 5-chem- and solvent-exposed samples, respectively (Fig. 5B). In addition, regarding each chromosome and probe position (upstream region of the first exon or gene body), the proportion of hyper- or hypo-methylated probes did not differ between the 5-chem- and solvent-exposed samples (Figs. 5C and 5D). These results suggest that chemical exposure did not cause dynamic changes in DNA methylation in specific genomic regions. Furthermore, combined exposure to chemicals did not affect DNA methylation status of the repetitive sequences Alu and LINE1 (Supplemental Fig. 4), non-genic regions that occupy 10–20% of the human genome (Cordaux and Batzer, 2009).

Genome-wide DNA methylation analysis in chemical-exposed NSphs using Infinium Human MethylationEPIC BeadChip. (A) Distribution of changes in methylation level. Methylation data for each probe of five-chemical mixture- or solvent-treated NSphs (5-chem or Solvent) were subtracted from those of non-treated control, and the distributions of the differences in methylation levels are shown in a histogram. Values close to “0” indicate no difference in methylation levels for 5-chem- or solvent-treated samples compared with those of non-treated control. Probes with difference in methylation level greater or less than 0 indicate hypermethylated (Hyper) or hypomethylated (Hypo), respectively, of chemical- or solvent-treated NSphs compared with that of non-treated control. (B) Summary of the percentage of hypermethylated (Hyper) or hypomethylated (Hypo) probes in chemical- or solvent-treated NSphs compared with those in non-treated control. Similarly, changes in methylation levels for each chromosome (C) or probe position (D) are summarized. Upstream region, probes at transcription start site, 5′-untranslated region (UTR), or the first exon; Gene body, probes at gene body or 3′-UTR. MRC, MRC-NSph; Edom, Edom-NSph. (E) DNA methylation fluctuation of chemical- and solvent-exposed NSphs. Methylation levels of chemical- and solvent-treated NSphs were subtracted from those of non-treated control in all probes and the sums of the absolute values of the differences in methylation level are plotted as “DNA methylation fluctuation” (left panel). Similarly, ten thousand probes were randomly extracted five times from all probes, and the amounts of DNA methylation fluctuations are shown as the mean ± standard deviation (right panel). * p < 0.05. (F) DNA methylation fluctuation of chemical- and solvent-exposed NSphs in hyper (Hyper-probes)- and hypo (Hypo-probes)-methylated probes. The sum of the absolute values of differences in methylation level was calculated for hyper- and hypo-methylated probes based on data in (B). (G) Ten thousand probes were randomly extracted five times from hyper- and hypo-methylated probes, and the amounts of DNA methylation fluctuations are shown as the mean ± standard deviation. * p < 0.05.
As no significant effects of chemicals on the DNA methylation status of each probe in the NSphs were observed, we aggregated all methylation information of approximately 800,000 probes. Differences in the methylation levels of each probe between chemical-/solvent- and non-treated samples were calculated, and the sum of their absolute values was taken as “DNA methylation fluctuation”. Interestingly, 5-chem-exposed NSphs tended to have a greater amount of DNA methylation fluctuation than solvent-treated controls (Fig. 5E, left panel). Furthermore, by randomly extracting 10,000 probes from all probes, the amount of DNA methylation fluctuation in 5-chem-exposed NSphs was significantly larger than that in the solvent-exposed sample (Fig. 5E, right panel). The same tendency was observed when 1,000 and 100,000 probes were randomly extracted (Supplemental Fig. 5). Considering the data in Figs. 5C and 5D, regardless of the absence of dynamic changes in DNA methylation of a specific region, stochastic changes in methylation status caused by chemical exposure accumulated throughout the entire genome.
Furthermore, when hyper- and hypo-methylated probes calculated in Fig. 5B were analyzed separately, in the hypermethylated probes, differences in the amount of DNA methylation fluctuations between the chemical- and solvent-exposed samples was small (Fig. 5F). However, in the randomly extracted 10,000 probes from hypermethylated probes, the amount of DNA methylation fluctuations were significantly increased in chemical-exposed MRC-NSphs compared with those in solvent-treated control (Fig. 5G). As shown in Fig. 5B, the ratio of hypermethylated probes in MRC-NSphs was lower in 5-chem- than in solvent-treated samples (47% vs 49%). These results suggest that the amount of methylation fluctuations in individual hypermethylated probes is greater in 5-chem- than in solvent-exposed MRC-NSphs, which led to the significant difference in the amount of methylation fluctuations in randomly extracted probes. In hypomethylated probes, the amount of DNA methylation fluctuations was larger in chemical-exposed samples than in solvent-exposed samples (Fig. 5F). Moreover, in the randomly extracted 10,000 probes from hypomethylated probes, significant increases in the amount of DNA methylation fluctuations were observed in both chemical-exposed MRC- and Edom-NSphs (Fig. 5G). These results suggest that MRC-NSphs were more affected by chemical exposure and that more DNA demethylation changes occur in both the cell lines.
Neurotoxicity of various chemicals has been investigated; however, many of these studies have mainly analyzed concentrations several hundred times higher than the biological levels. In this study, we examined the effects of chemicals on neural development at concentrations detected in the serum of pregnant mothers. A human iPSC-derived neuronal model revealed that the combination of five chemicals affected neural development and differentiation and caused altered DNA methylation throughout the genome. Cancer cell lines derived from adult tissues have been commonly used to evaluate the toxicity of environmental chemicals. However, we used human iPSC-derived neural stem cells, an in vitro model for early embryonic development. Moreover, sensitivity to chemicals varies between young and adult individuals; young individuals show higher sensitivity to chemicals in animal models (Chakraborti et al., 1993). Therefore, iPSC-derived neuronal cells might be more sensitive to chemicals, and iPSCs are considered a more suitable model for the evaluation of the effect of chemicals on actual fetal neurogenesis. Taken together, we constructed an in vitro screening system to evaluate the effects of chemicals detected in the maternal environment on fetal neural development.
Exposure to each of the five chemicals used in this study did not affect NSph formation, except for Se-exposed NSph. In a previous study using undifferentiated human iPSCs, a single-chemical exposure does not alter heterochromatin formation (Arai et al., 2015). However, the combined exposure to chemicals affected the cellular development and differentiation of NSphs. This indicates that combined exposure to chemicals has a greater risk of affecting neural development than a single-chemical exposure. Previous epidemiological studies have shown that high levels of multiple chemicals were detected in the same cord blood samples (Stewart et al., 2006; Kortenkamp et al., 2007; Maekawa et al., 2017). This means that fetuses are simultaneously exposed to multiple chemicals in actual exposure situations. In addition, cotinine and Hg have been detected in cord blood serum at the same levels as maternal serum (Berlin et al., 2010; Sakamoto et al., 2021), and there is a risk that chemicals may pass through the placenta and directly expose the fetus at the concentrations used in the present study. It is not known whether the five chemicals are present in maternal serum at the same time. However, this study demonstrated the importance of assessing the effects of combined exposure to chemicals on fetal neurodevelopment under biological exposure conditions.
Of the five chemicals used in this study, single exposure to Se reduced NSph size; Se is known to be cytotoxic, especially to cancer cells (Olm et al., 2009). On the other hand, rat bone marrow stem cells have been reported to promote neuronal differentiation at low concentrations of Se exposure but to cause cell death at high concentrations (Jalali et al., 2022). These results are similar to those of combined exposure to the five chemicals in human NSphs. However, our preliminary data show that combination of the five chemicals resulted in a greater reduction in NSph size compared to single exposure to Se (data not shown). These results suggest that combined exposure to chemicals containing Se has a stronger effect on NSph formation than single Se exposure.
Combined exposure to the five chemicals resulted in a smaller NSph size than that of the controls, and subsequent changes in neural differentiation were observed. A similar decrease in NSph size was observed in iPSCs derived from patients with schizophrenia (Chen et al., 2020). Therefore, a decrease in sphere size caused by chemical exposure is a phenomenon observed in pathological models of neural stem cells, which indicates changes in NSph properties. Since neural differentiation in an in vitro culture is long, predicting the differentiation potential before differentiation might lead to a reduction in time and labor. Therefore, verification of NSph size might be a useful index of its ability for subsequent neural differentiation in vitro. Furthermore, it has been reported that subsequent neuronal differentiation is suppressed in iPSCs derived from patients with schizophrenia (Chen et al., 2020). On the other hand, combined exposure to the five chemicals resulted in further expansion of neuronal fibers. Patients with autism spectrum disorder have greater brain weight than healthy individuals, as well as an increased number and size of neurons (Courchesne et al., 2011). These results suggest that expansion of neuronal fibers observed with chemical exposure resembles the phenotype in autism spectrum disorder. Chemical exposure during fetal period has been reported to increase the incidence of autism spectrum disorder (Ornoy et al., 2015). Therefore, combined exposure to chemicals used in this study may lead to an understanding of chemical-induced abnormalities of neural differentiation in autism spectrum disorder.
Further expansion of NSph-derived neuronal fibers was observed following chemical exposure before differentiation. This suggests that the effects of chemicals on neural stem cells persist long after differentiation. In addition, chemical exposure increased the expression of neuronal-related genes in the NSph. These results indicate that chemical exposure caused altered stem cell-properties in a part of NSph cells. Furthermore, DNA methylation status is cell type-specific and reflects the properties of the cell population (Arai et al., 2013, 2016); thus, clear DNA methylation changes in chemical-exposed NSph were expected. However, no hotspots with extreme changes in DNA methylation status were observed in the chemical-exposed NSphs. In the neural differentiation process, complex gene expression is regulated by epigenetic systems, including DNA methylation and histone modifications (Yao et al., 2016), but chemical-induced changes in the capacity for neural differentiation might not be explained by alterations in DNA methylation of particular genes.
However, chemical exposure caused small but significant changes in DNA methylation of NSphs genome. These results suggest that the five chemicals examined might act as epimutagens at concentrations close to the biological exposure range, and that “epigenetic mutations” caused by chemical exposure were accumulated in the entire genome. DNA methylation and histone modification affect chromatin structure and are involved in gene expression, cell proliferation, and cell differentiation (Shiota, 2004; Lieb et al., 2006; Golob et al., 2008; Ikegami et al., 2009). A previous study reported that combined exposure to the five chemicals used in this study caused altered heterochromatin formation in human iPSCs (Arai et al., 2015). Therefore, it is possible that epigenetic mutations due to genome-wide DNA methylation changes observed after chemical exposure affected the chromatin structure in NSphs. Changes in chromatin structure due to abnormal DNA methylation have been implicated in various diseases (Robertson, 2005; Spielmann et al., 2018; Nacev et al., 2020; Vogel-Ciernia and Wood, 2014), and abnormal neural development is associated with chromatin dysplasia (Kramer and van Bokhoven, 2009; Fujita and Yamashita, 2018). Another study on neurological disorders emphasizes the search for causative gene(s), and genetic mutations in hundreds of disease-related genes are associated with abnormal neural differentiation at disease onset, for example autism (Xu et al., 2012). Thus, abnormal neural differentiation are difficult to explain by particular genes. It is unclear whether chemical-induced DNA methylation changes in the entire genome are directly linked to the capacity of neural differentiation. However, this study implies that whole-genome epigenetic information, including DNA methylation, might provide a new clue for elucidating chemical-induced abnormal neural differentiation.
In conclusion, combined exposure to the five epimutagens at maternal serum levels affected the development of human iPSC-derived neural stem cells and subsequent neural differentiation. Neurological disorder in children are currently serious health problems. Although extensive research has been carried out worldwide, the mechanisms of disease onsets are still unknown. Combining the knowledge of conventional genetic mutations with that of epigenetic mutations caused by environmental factors, including chemicals, might lead to a better understanding of the pathogenesis of neurological disorders.
Financial support for this study was provided by Grant-in-Aid for Scientific Research (18K11679 and 21K12277) (to YA). YA thanks Dr Ken Takasawa for his kind cooperation with this project.
Conflict of interestThe authors declare that there is no conflict of interest.