2023 年 48 巻 11 号 p. 607-615
2023 年 48 巻 11 号 p. 607-615
ICH S3A Q&A focused on microsampling (MS) was published to help accelerate the use of MS and states that MS is useful because toxicokinetic (TK) evaluation with conventional blood sampling volume requires many animals for TK satellite groups; however, there are few reports of MS application in mice. We investigated the influence of MS on toxicity evaluation in mice by comparing the toxicity parameters with and without MS after a single oral administration of 1-naphthylisothiocyanate (ANIT), a hepatotoxic substance. Blood samples (50 µL/point) were collected from the tail vein of 3 mice per group at 2 or 3 time points during a 24-hr period, and toxicity was evaluated 2 days after administration. ANIT-related changes suggesting liver or gallbladder injury were noted in blood chemistry and histopathology. Some of these changes such as increases in focal hepatocyte necrosis and inflammatory cell infiltration in the liver as well as mucosal epithelium necrosis in the gallbladder were apparently influenced by MS. A tendency to anemia was noted in animals with MS but not without MS, which was also noted in the vehicle-treated controls, suggesting influence of blood loss. The current results indicate that ANIT hepatotoxicity could be evaluated in mice in which blood samples were collected by MS for most parameters; however, parameters in anemia and pathology in the liver and gallbladder were influenced by MS in this study condition with ANIT. Therefore, MS application in mice should be carefully considered.
In November 2017, ICH S3A Q&A focused on microsampling (MS) was published to accelerate the use of MS for toxicokinetic (TK) evaluation (ICH, 2017). MS is usually characterized by 50 µL or less of blood being collected at each sampling time point, which is now considered to be sufficient for TK evaluation due to advancements of measuring instruments (Cobb et al., 2019). MS can contribute to animal welfare mainly in rodents by minimizing the amount of blood drawn, thereby reducing pain and distress in animals. It is possible to eliminate TK satellite groups or reduce the number of animals required in the TK satellite groups if TK evaluation is conducted using MS in the main study group. The Q&A states that MS is useful, especially in mice, because TK evaluation with conventional blood sampling volume requires a very large number of animals for TK satellite groups. In conventional mouse toxicity studies, animals are usually euthanized after a single point of blood sampling. If MS can be applied to mouse toxicity studies and the animals can be used for other examinations such as hematology and blood chemistry after TK sampling, it can make a significant contribution to the 3Rs (Replacement, Reduction, and Refinement).
Although ICH S3A Q&A has been issued as described above, MS is not yet sufficiently widespread in the non-clinical toxicity studies of drugs. The AAPS Microsampling Discussion Group (the AAPS Bioanalytical Community Microsampling Subgroup) and the European Bioanalysis Forum (EBF) performed a survey on MS among their members (Patel et al., 2019). The survey found that studies using MS for plasma preparation are included in submissions as follows: Discovery support: 52.94%; Preclinical (non GLP) – Standard PK in rodents: 50.00%, Other studies: 46.67%; Preclinical (GLP) – Standard PK in rodents: 43.75%, Other studies: 35.71%. These results showed that there was a tendency toward a lower percentage of GLP studies applying MS.
There are several reports on the influence of MS on toxicity evaluation in rats. The influence of a 50 µL/time point blood sampling was evaluated in SD rats that received phenacetin as a hemotoxic substance in a 28-day study protocol (6- to 7-point blood samplings within 24 hr) in 2 facilities (Ohtsuka et al., 2022). There were decreases in red blood cell counts, hematocrit, and hemoglobin as hematological toxicity by phenacetin. The application of MS did not significantly deteriorate or mask the toxic influences of phenacetin, not only for hematological parameters but also for other parameters such as blood chemistry, organ weights, and histopathology.
The influence of a 50 µL/time point blood sampling was also evaluated in SD rats that received methylene blue trihydrate and azathioprine as hemotoxic substances in a 14-day study protocol (7-point blood sampling on Days 1-2 and 13-14) (Tochitani et al., 2022). Changes were noted to the same extent in groups treated with methylene blue or azathioprine both with and without MS, suggesting that the influence of MS on toxicity evaluation was limited. Based on these previous reports, it would be feasible to apply MS to toxicity studies using rats.
On the other hand, there are few reports on toxicity studies in which MS is applied to mice. There is a report on a trial of MS in 13-week-old C57BL/6 mice treated with a low molecular weight chemical (Wang et al., 2020), in which slight reduction of red blood cell count, hemoglobin, and hematocrit values were observed; however, influence of MS on toxicity evaluation was not investigated on the factors other than the above 3 red blood cell-related parameters in this trial.
In this report, we investigated the influence of MS on toxicity evaluation in mice treated with a single dose of 1-naphthylisothiocyanate (ANIT), which is a hepatotoxic substance in mice (Tang et al., 2016), to evaluate the feasibility of MS during the toxicity evaluation using mice.
Six-week-old Crl:CD1(ICR) mice were purchased from Jackson Laboratory Japan, Inc. (Kanagawa, Japan). The animals were quarantined for 5 days and acclimated from the day of receipt to the day before treatment. The animals were assigned to each group based on body weights on the day before treatment by the stratified-by-weight randomization method to get almost the same mean body weight among the groups. The animals were housed 3 animals per polycarbonate cage (180W × 285D × 127H mm) in an animal room, which was maintained at a temperature between 19°C and 25°C and at a relative humidity between 35% and 75% with a flow of 6 to 20 air changes per hour. Lighting was controlled to give a 12-hr light cycle from 7 a.m. to 7 p.m. All mice had free access to tap water through a water bottle and a pellet diet (CR-LPF, Oriental Yeast Co., Ltd., Tokyo, Japan).
Vehicle and test article1-naphthylisothiocyanate (ANIT, Lot No. CBJF0-HE, Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) was suspended in corn oil (for biochemistry, Lot No. PTQ3546, FUJIFILM Wako Pure Chemical Corporation) to make a 7.5 mg/mL dosing formulation.
Study designSix groups were set to investigate the influence of MS on toxicity evaluation in mice (see Table 1) as follows: the vehicle control group without blood sampling (Group 1), vehicle control group with 3-point blood sampling (Group 2), ANIT-treated group without blood sampling (Group 3), ANIT-treated group with 3-point blood sampling (Group 4), and ANIT-treated group with 2-point blood sampling (Groups 5 and 6). Each group consisted of 3 males and 3 females. The animals received a single oral administration of the vehicle or 75 mg/kg of ANIT that was expected to cause hepatotoxicity to mice (Tang et al., 2016). Dosing volume was set at 10 mL/kg. At the commencement of this investigation, the experimental protocol was reviewed and approved by Institutional Animal Care and Use Committee according to the following guideline of the test facility, LSIM Safety Institute Corporation: Guidelines for Animal Studies.
 Blood sampling for assumed TK sampling
Blood sampling for assumed TK sampling
Blood samples for assumed TK sampling were collected from the tail vein using a disposable winged injection needle (25G, CLEA Japan, Inc., Tokyo, Japan) and hematocrit tube (Paul Marienfeld GmbH & Co. KG, Lauda-Koenigshofen, Germany). Blood sampling was conducted at 0.5, 1, 2, 4, 8, and/or 24 hr (as shown in Table 1) after treatment without anesthesia. The sampling volume was 50 µL/time point from the tail vein. The concentration of ANIT in these samples was not determined.
Clinical observation and body weight and food consumption measurementsAnimals were observed for clinical signs once daily. Body weight and food consumption were measured on the day of treatment and the day after treatment. Since 3 animals were kept in 1 cage, the amount of food consumption by each animal was divided by 3.
Hematology and blood chemistryAnimals were anesthetized with isoflurane (ISOFLURANE Inhalation Solution [Pfizer], Mylan N.V.), and blood samples of approximately 0.8 mL for hematology and blood chemistry examination were collected after overnight fasting from the posterior vena cava at terminal necropsy on 2 days after administration. Approximately 0.3 mL of blood was dispensed into blood sampling tubes containing EDTA-2K and used for the hematological test. These blood samples were analyzed using a hematology analyzer (XT-2000iV, Sysmex Corporation, Hyogo, Japan) for the following parameters: red blood cells (RBC), hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet, reticulocyte ratio, white blood cell count, lymphocyte count, neutrophil count, eosinophil count, basophil count, and monocyte count. Approximately 0.5 mL each of blood samples was dispensed into blood sampling tubes containing heparin lithium and then centrifuged to obtain plasma samples. The plasma samples were analyzed using an automated clinical chemistry analyzer (TBA-2000FR, Canon Medical Systems Corporation., Tochigi, Japan) for aspartate aminotransferase (ASAT), alanine transaminase (ALAT), glutamate dehydrogenase (GLDH), γ-glutamyl transpeptidase (γGT), alkaline phosphatase (ALP), total bilirubin, total bile acid, urea nitrogen, creatinine, glucose, total cholesterol, triglyceride, total protein, Ca, albumin, inorganic phosphorus, Na, K, and Cl using an automated electrophoresis analyzer (Epalyzer 2, Helena Laboratories, Saitama, Japan) for A/G ratio.
Organ weight measurement, necropsy, and histopathologyAs shown in the previous section, animals were anesthetized, euthanized by exsanguination, and necropsied. Then, the thymus, liver, and spleen were excised, weighed, and the relative organ weights were calculated based on the body weight at necropsy. The thymus, liver, gallbladder, and spleen were examined microscopically.
Statistical analysisThe data of body weight, organ weight (absolute and relative weight), hematology, and blood chemistry were subjected to statistical analysis. Food consumption was not analyzed statistically.
Comparison between Group 1 and Group 2 and between Group 1 and Group 3: The data were tested by the F test (significance level: 5%) for homogeneity of variance. When the variances were homogeneous, the Student t-test (significance level: 1% and 5%, two-tailed test) was used, and when the variances were heterogeneous, the Aspin-Welch t-test (significance level: 1% and 5%, two-tailed test) was performed.
Comparison between Group 2 and Groups 4 to 6 and between Group 3 and Groups 4 to 6: The data were first analyzed by Bartlett’s test (significance level: 5%). When the variances were homogeneous, Dunnett’s test (significance level: 1% and 5%, two-tailed test) was performed. When the variances were heterogeneous, Steel test (significance level: 1% and 5%, two-tailed test) was performed.
No differences were observed between all groups in body weights. The ANIT-treated groups (Groups 3 to 6) showed a trend toward lower food intake from the day of treatment to the day after treatment compared to the vehicle control groups (Groups 1 and 2); however, there was no difference among Groups 3 to 6. No significant changes were observed in the general condition in any group (data not shown).
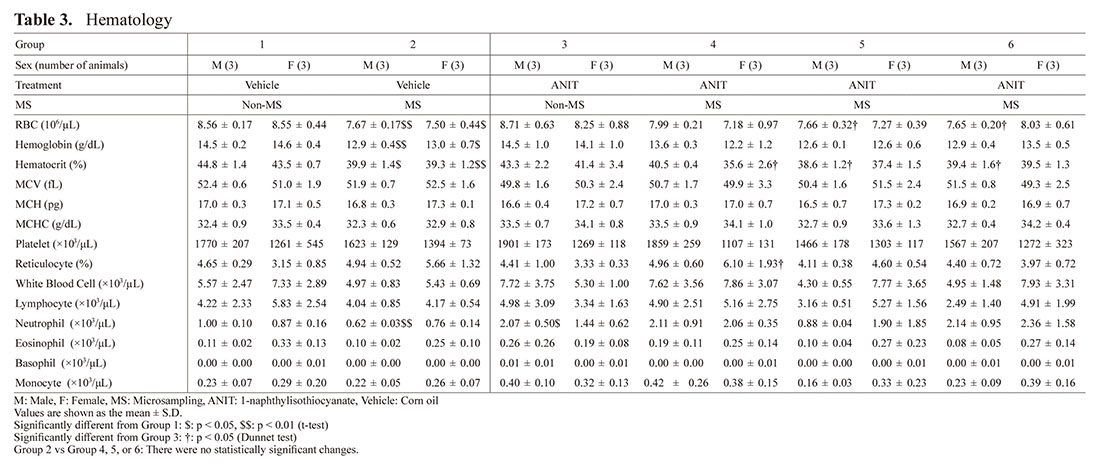
 Hematology (Table 3)
Hematology (Table 3)
Increases in neutrophil count and monocyte count were noted in the ANIT-treated groups (Group 3, 4, 5, or 6) compared to the vehicle control groups, which were considered to be related to liver and gallbladder injury by ANIT.
In detail, a statistically significant higher neutrophil count was noted in Group 3 males compared to Group 1 males. Similarly, a higher tendency for neutrophil count was found in Groups 3 and 5 females and Groups 4 and 6 males and females compared to the vehicle control groups. A trend towards increased monocyte count was noted in Groups 3 and 4 in males and Groups 4 to 6 in females compared to the vehicle control groups.
Regarding influences of MS, decreases in erythrocytic parameters and an increase in reticulocyte ratio were noted in groups with MS compared to the groups without MS. Precisely, RBC, hematocrit, and hemoglobin values were lower with statistical significance in Group 2 with MS compared to Group 1 without MS. Statistically significant lower RBC values were noted in Groups 5 and 6 males than those in Group 3. Statistically significant lower hematocrit values were noted in Groups 5 and 6 males and Group 4 females compared to Group 3. A statistically significant higher reticulocyte ratio was noted in Group 4 females compared to Group 3 females as a reactivity change for lower erythroid parameters.
 Blood Chemistry (Table 4)
Blood Chemistry (Table 4)
Changes in ASAT, ALAT, GLDH, γGT, ALP, total bilirubin, total bile acid, glucose, total cholesterol, triglyceride, albumin, and A/G ratio were noted in the ANIT-treated groups (Group 3, 4, 5, or 6) compared to the vehicle control groups, possibly due to hepatobiliary disorders caused by ANIT.
In detail, higher tendencies compared to the vehicle control groups were noted as follows: ASAT in Groups 3 to 6 males and females; ALAT, GLDH, and total bile acid in Group 3 females and Groups 4 to 6 males and females; ALP and total bilirubin in Groups 3 to 6 females; γGT in Group 6 males and Groups 4 to 6 female; total cholesterol in Group 6 males and Groups 3 to 6 females. ASAT, ALAT, GLDH, ALP, total bilirubin, and total bile acid in Groups 3 to 6 females were higher than in each male group and γGT in Groups 4 to 6 females were higher than in each male group. A statistically significant lower value of glucose was noted in Group 3 males and females and Group 6 males compared to the vehicle control groups. A statistically significant lower value in albumin was noted in Group 3 males and females and lower tendency was also noted in Groups 4 to 6 males and females compared to the vehicle control groups. A statistically significant lower value in A/G ratio was noted in Groups 3 to 6 males and females compared to the vehicle control groups. Statistically significant lower values of triglyceride were noted in Groups 4 and 6 females compared to Group 2 females. In addition, Groups 3 and 5 females and Groups 3 to 6 males showed a trend toward lower triglyceride levels compared to Group 1 or 2. Statistically significant higher values of Na were noted in Group 3 males and Group 2 females compared to Group 1.
Regarding influences of MS, there were statistically significant lower values of inorganic phosphorus in Group 2 males compared to Group 1 males, and statistically significant lower values of K in Group 2 males compared to Group 1 males; however, these were slight changes.
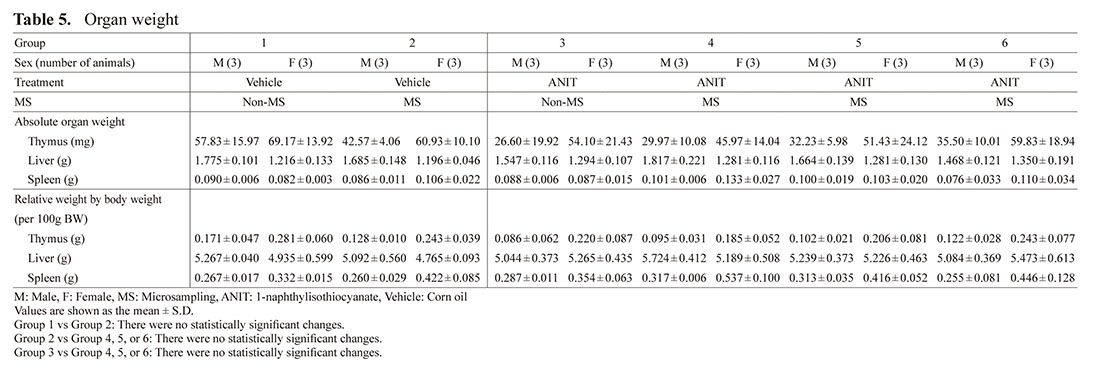
 Organ Weight (Table 5)
Organ Weight (Table 5)
There were no statistically significant differences in Group 2 compared to Group 1 or in Groups 4, 5, and 6 compared to Groups 2 and 3.
 Pathology (Table 6)
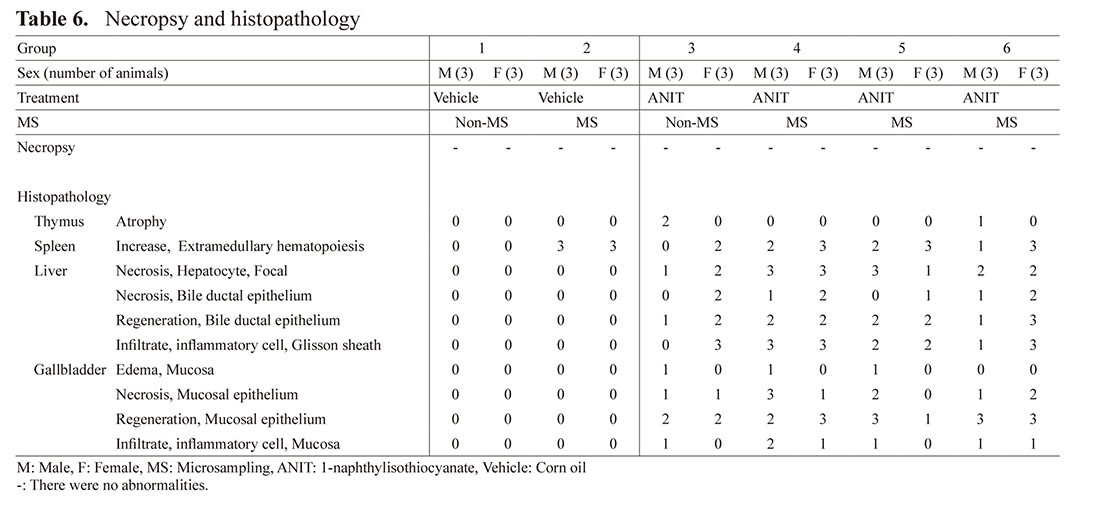
Pathology (Table 6)
No abnormalities were noted at necropsy.
Histopathological examination revealed injuries in the liver and gallbladder, which were considered to be due to ANIT. In detail, focal necrosis of hepatocytes, necrosis of bile ductal epithelium, regeneration of bile ductal epithelium, and inflammatory cell infiltration in the Glisson sheath were observed in the liver in Groups 3 to 6. Mucosal edema, necrosis of mucosal epithelium, regeneration of mucosal epithelium, and inflammatory cell infiltration in the mucosa were observed in the gallbladder in Groups 3 to 6. Other than the above, there was atrophy of the thymus in Groups 3 and 6.
Regarding MS effects, an increased number of mice affected with extramedullary hematopoiesis in the spleen was apparently observed in Group 2 (compared with Group 1) and Groups 4 to 6 (compared with Group 3). In addition, an increased number of male mice affected with focal hepatocyte necrosis and inflammatory cell infiltration in the liver as well as mucosal epithelium necrosis in the gallbladder was observed in Groups 4 and 5 males compared with Group 3 males.
We investigated whether the toxicity of ANIT could be properly evaluated when TK blood samples were collected by MS. The results are discussed on the following 3 points.
First, we discuss whether the toxicity by ANIT, a hepatotoxic substance, was consistent with a previous report (Tang et al., 2016). In the report, C57BL/6J mice were treated with ANIT once at 75 mg/kg. As a result, there were increases in ALAT, ALP, total bile acid, and total bilirubin, suggesting a severe liver injury with cholestasis 2 days after treatment. Periportal hemorrhage, diffuse vacuolization, inflammatory infiltration, and parenchymal necrosis were observed in ANIT-treated livers in the histopathology. In our current study, changes in ASAT, ALAT, GLDH, γGT, ALP, total bilirubin, total bile acid, glucose, total cholesterol, triglyceride, albumin, and A/G ratio were noted 2 days after treatment in blood chemistry, which were considered to be due to various hepatobiliary disorders caused by ANIT. The higher values of neutrophil and monocyte counts observed in ANIT-treated groups were considered to be related to liver and gallbladder injuries. Histopathological examination revealed injuries in the liver and gallbladder. These changes were generally consistent with the previous report. Therefore, ANIT toxicity itself could be detected in this report. In blood chemistry for items related to the liver, ASAT, ALAT, GLDH, ALP, total bilirubin, and total bile acid in Groups 3 to 6 females were higher than in each male group and γGT in Groups 4 to 6 females was higher than in each male group. Expression and its induction of cytochrome P450 isoforms in mice is known to be sex-dependent for some compounds (Sekimoto et al., 2011). The isoforms of cytochrome P450 involved in the ANIT are unknown, but it was considered that sex differences in the metabolism/induction of drug-metabolizing enzymes may have led to sex differences in toxicity expression.
Second, we discuss about the influence of MS. Hepatobiliary disorders seemed to be increased in the affected male mice in Groups 4 and 5 compared with those in Group 3. Regarding influences of MS on anemia, the tendency was noted in mice following 2- or 3-point blood sampling. In fact, hematological tests showed that erythrocytic parameters were lower in Group 2 with MS compared to Group 1 without MS, and in Groups 4, 5, and 6 with MS compared to Group 3 without MS. The increased extramedullary hematopoiesis in the spleen on histopathological examination was considered to be due to MS (and also to ANIT) treatment, probably as secondary change reacted to the anemia. If the changes were due to ANIT treatment, they were probably related to liver or gallbladder injury caused by ANIT treatment. Although there were exceptions such as some hepatobiliary disorders in males, the hepatotoxicity caused by ANIT could be almost evaluable even when blood was collected by MS.
Lastly, we discuss whether MS can be applied to mouse toxicity studies. In Groups 2 and 4, in which blood was collected by 3-point blood sampling, the percentage of blood collected was estimated to be 6.1% to 6.4% and 8.0% to 8.1% of the circulating blood volume in males and females, respectively, when the average blood volume of mice is regarded as 72 mL/kg (Diehl et al., 2001). Moreover, in Groups 5 and 6, in which blood was collected by 2-point blood sampling, the percentage of blood collected was 4.0% and 5.4% of the circulating blood volume in males and females, respectively. According to a previous report (Hattori et al., 2020), when hematological tests were performed in rats with a 1-day interval after completion of serial blood sampling, the maximum unaffected blood volume was 3% of the circulating blood volume. In this study, blood sampling time points were 2 or 3 per mouse, so the collected blood volume exceeded 3% of the circulating blood volume of the mice, which is thought to have affected the hematology results. Using a single blood sampling time point per mouse can reduce the collected blood volume to less than 3% of the circulating blood volume, and thus the effect on hematology results is expected to be minor. In other words, when applying MS to mouse toxicity studies, sparse sampling with a single blood sampling time point per mouse is recommended as previously described (Bonnie Wang et al., 2020). For example, sparse sampling with a single blood time point resulted in using a group of 12 animals, in which blood is collected from animals No. 1-3 at 1 hr after administration, from animals No. 4-6 at 3 hr after administration, from animals No. 7-9 at 6 hr after administration and from animals No. 10-12 at 24 hr after administration. This makes it possible to compare TK results with the results of various examinations such as hematology and blood chemistry in the same individual and is expected to contribute to the 3Rs with a minimum amount of blood collection and also with reduction of satellite animals.
One limitation of the present investigation was that only a high dose level of ANIT was tested. The possibility cannot be denied that MS may affect to a different degree in cases with doses close to the no observable adverse effect level (NOAEL).
The current results indicate that ANIT hepatotoxicity could be evaluated in mice in which blood samples were collected by MS for most parameters; however, parameters in anemia and pathology in the liver and gallbladder were influenced in the groups with MS. Therefore, MS application in mice should be carefully considered. It was suggested that properly planned sparse sampling may be a feasible way to apply MS to toxicity studies in mice.
We wish to thank Eiji Murata and Yui Akagawa for their work in these experiments.
Conflict of interestAuthors except for Yoshiro Saito are employees of LSIM Safety Institute Corporation. The authors declare that there is no conflict of interest on this study.