Abstract
Guanfacine hydrochloride extended-release (GXR) is used to treat attention deficit hyperactivity disorder. It is a selective α2A-adrenorecepor agonist that was reported to cause QT prolongation and hypotension in the event of overdosing. We report the case of a 17-year-old man who took 226 tablets of GXR 3 mg for attempted suicide. He was found complaining of dyspnea, and emergency medical services were called. When the patient was transferred to our hospital, his Glasgow coma scale was 12 (E4V3M5). He was agitated and hypoxemic. He was intubated for invasive mechanical ventilation under sedation. His chest X-ray and computed tomography scan showed pulmonary edema. Transthoracic echocardiography showed markedly reduced cardiac function. His serum guanfacine concentration peaked on day 3 after admission. His pulmonary edema improved quickly after a decrease in serum guanfacine concentration, but cardiac decompensation persisted for about 1 month. This case reveals that the decline in cardiac function after guanfacine intoxication is prolonged even after its serum concentration has decreased.
INTRODUCTION
Guanfacine, a selective α2A-adrenorecepor agonist (Uhlén et al., 1995), is a therapeutic agent used to treat attention deficit hyperactivity disorder (ADHD). The major mechanism of action of guanfacine is considered to involve activation of the postjunctional α2A adrenoceptor of glutamatergic pyramidal neurons in the frontal cortex (Fukuyama et al., 2021). The number of children and adolescents being treated with guanfacine is increasing (McGrath and Klein-Schwartz, 2002; Spiller et al., 2013). Symptoms of guanfacine intoxication include bradycardia, hypotension, drowsiness, headache, insomnia, and QT prolongation. Guanfacine is well absorbed orally (Carchman et al., 1987). It is important to be vigilant of guanfacine intoxication because of the early onset of poisoning symptoms, yet there are no published reports in which its serum concentrations were measured in poisoned patients. Here, we report a case of severe guanfacine intoxication that resulted in restlessness, agitation, and pulmonary edema. The serum concentrations of guanfacine were measured throughout the admission period.
Case report
A 17-year-old man with a history of ADHD was transferred to our hospital by ambulance because of dyspnea, 6 hr after taking 226 tablets of GXR (Intunib® 3 mg/tablet) for attempted suicide. On admission, his Glasgow coma scale was 12 (E4V3M5), body temperature was 37.7°C, heart rate (HR) was 68 beats/min, blood pressure was 105/87 mmHg, respiratory rate was 34 breaths/min, and oxygen saturation (SpO2) was 97% during oxygen administration at a rate of 10 L/min via a non-rebreather mask. Excitability and marked hypoxemia were noted. He was intubated for invasive mechanical ventilation with sedation. His chest X-ray after intubation revealed an enlarged cardiac silhouette (cardiothoracic ratio 52.1%) and bilateral diffuse consolidation. A chest computed tomography scan showed bilateral infiltrations, predominantly in the dorsal areas. A transthoracic echocardiogram demonstrated a severely reduced ejection fraction (EF) of 30% and mild left ventricular dilation with diffuse hypokinesis. Right heart catheterization revealed his pulmonary artery wedge pressure was 13 mmHg, pulmonary artery pressure was 32/25 (mean 27) mmHg, right ventricular pressure was 32/12 (mean 19) mmHg, right atrium pressure was 12 mmHg, and cardiac index (CI) was 2.41 L/min/m2. These findings suggested no remarkable increase in the left ventricular filling pressure. The arterial oxygen partial pressure/fractional inspired oxygen (P/F) ratio was below 100, and prone positioning was performed. On day 2 of admission, GXR overdose was revealed by the discovery of empty sheets for a large quantity of pills, and he was therefore administered activated charcoal and laxatives.
The time-courses of the clinical findings and serum guanfacine concentrations are shown in Fig. 1. His pulmonary infiltrations on chest X-ray and oxygenation status improved over time, and he was extubated on day 7 after his persistent agitated state had improved. His HR remained relatively bradycardic since admission, but catecholamines were not required to treat bradycardia or hypotension. QT prolongation worsened over day 6, but once the serum guanfacine concentration had decreased sufficiently, his QT prolongation promptly improved to within the normal range. He was discharged from the intensive care unit once his physical condition had stabilized, and his psychiatric treatments were continued in the psychiatric ward. One month after admission, his cardiac contractility was improving.
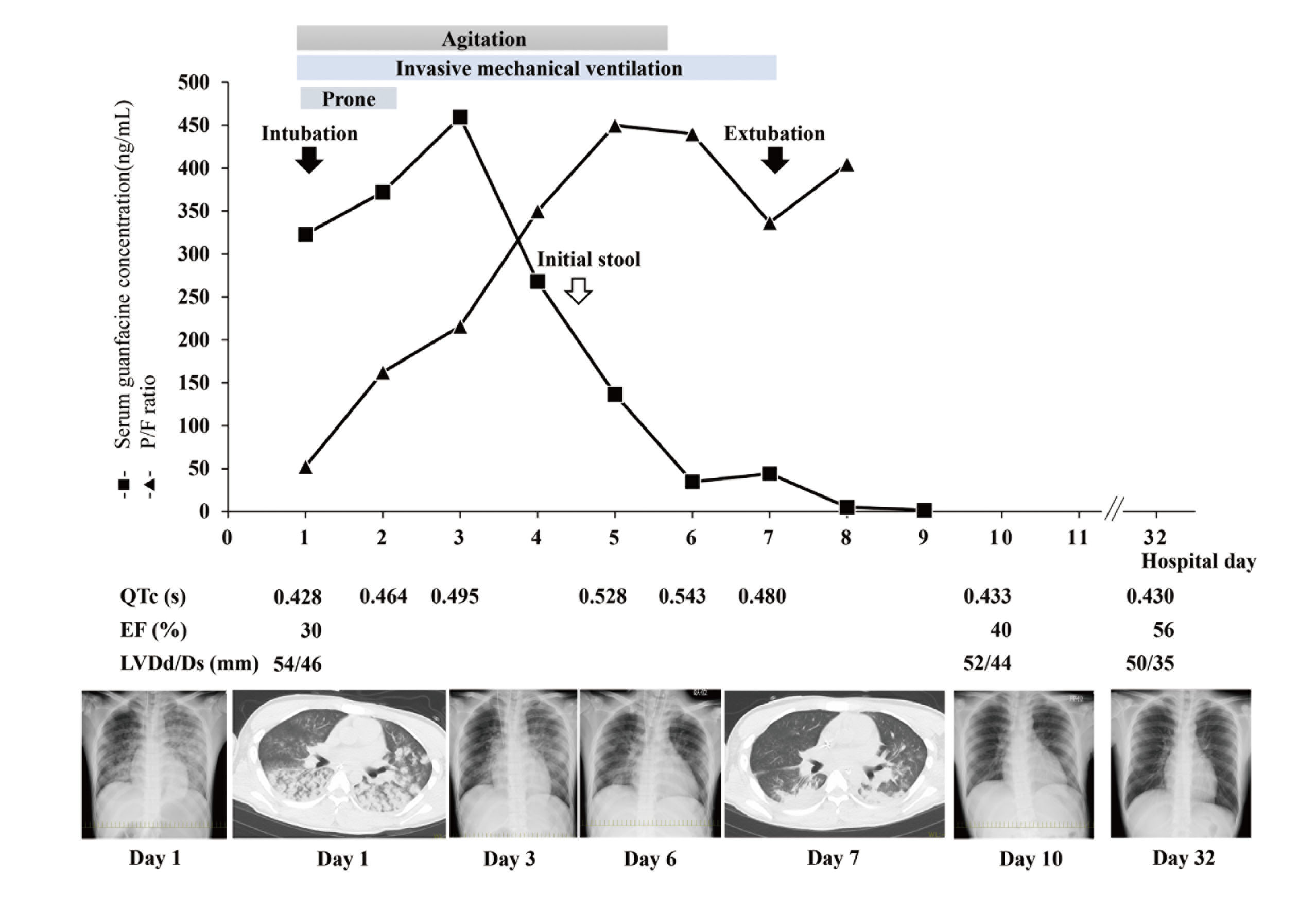
The patient’s serum guanfacine concentration was measured at a later date by liquid chromatograph mass spectrometry (Swearingen et al., 2007). The serum concentration peaked on day 3 after admission (459.5 ng/mL), and then declined. For healthy Japanese adults taking GXR at a daily dose of 6 mg, the estimated maximum serum concentration is 11.7 ± 2.44 ng/mL. In our case, the serum guanfacine concentration decreased below that level on day 8. Invasive mechanical ventilation and prone position therapy helped improve his oxygenation. Subsequently, his clinical course of pulmonary edema seemed to be consistent with the decline in the serum concentration of guanfacine.
DISCUSSION
To our knowledge, this is the first reported case of guanfacine intoxication in which serum guanfacine concentrations were measured. In this case, the trend in serum guanfacine concentrations was consistent with the severity of pulmonary edema in terms of the P/F ratio, chest X-ray, and his agitated state. However, the decrease in EF persisted for about 1 month after the serum concentration had declined.
Prescriptions of GXR, a medication used to treat ADHD, are increasing. The incidence of GXR intoxication is growing, and some case reports have been published (e.g., McGrath and Klein-Schwartz, 2002). Common side effects of GXR include bradycardia, hypotension, drowsiness, headache, insomnia, and QT prolongation, and the most common are bradycardia and hypotension due to sinus bradycardia and AV block (Roach-Fox et al., 2018; Minns et al., 2010; Walton et al., 2014). Although QT prolongation was observed in our case, the patient did not experience other common side effects, but did develop severe pulmonary edema. Bridwell et al. (2021) reported a case of pulmonary edema with decreased cardiac function caused by guanfacine intoxication, similar to our case. Selective α2A-adrenergic receptor agonists have been suggested to have hypertensive effects early in administration through activation of α1 or α2B receptors (Gillis et al., 1985; MacMillan et al., 1996). In vitro, high guanfacine concentrations stimulated the contractility of cardiac papillary muscles (Scholtysik and Brugger 1989). These phenomena might cause pulmonary hypertension and pulmonary edema by increasing afterload and cardiac contractility, as seen in our case and in the case reported by Bridwell et al. (2021). However, in our case, right heart catheterization and echocardiography on admission did not show a marked increase in the left ventricular filling pressure. Therefore, other mechanisms might explain this finding, including the possibility that guanfacine itself may increase pulmonary vascular permeability, given that the patient’s pulmonary edema improved as the serum guanfacine concentration decreased.
The patient’s cardiac function remained impaired even after his serum guanfacine concentration had decreased. The effects of guanfacine on reducing cardiac contraction remain to be elucidated. However, one possible cause of the reduced cardiac function is takotsubo cardiomyopathy; i.e., cardiac function was reduced due to myocardial exhaustion caused by the excessive afterload and enhancement of myocardial contraction. We observed no relationship between serum guanfacine concentration and ECG changes or cardiac contractility in our case. Therefore, we suspect that cardiac dysfunction is not only a direct effect of guanfacine but may also be related to takotsubo cardiomyopathy caused by α1 or α2B receptor stimulation.
The patient was highly agitated while the high serum guanfacine concentrations remained high. Although drowsiness is a known effect of GXR, there are no reports of agitation, and the mechanism involved in GXR overdose-induced agitation is unknown. The patient’s agitation improved from day 6, when his serum guanfacine concentration had dropped. Thus, there may be a relationship between psychiatric symptoms and serum guanfacine concentration. However, there is a lack of data regarding the mechanism underlying these excitatory symptoms.
In conclusion, to our knowledge, this is the first case report in which serum guanfacine concentrations were measured in a patient with guanfacine intoxication. The clinical courses of pulmonary edema and agitation were consistent with the steady reduction in serum guanfacine concentrations. However, continued monitoring of cardiac function is necessary because the decline in cardiac function persists even after the serum guanfacine concentration has declined.
Conflict of interest
The authors declare that there is no conflict of interest.
REFERENCES
- Bridwell, R.E., Larson, N.P., Rosenthal, J.B., Wray, J., Baker, Z., Cibrario, A. and Oliver, J.J. (2021): Guanfacine toxic ingestion with subsequent cardiogenic pulmonary edema. Am. J. Emerg. Med., 39, 256.e5-256.e8.
- Carchman, S.H., Crowe, J.T. Jr. and Wright, G.J. (1987): The bioavailability and pharmacokinetics of guanfacine after oral and intravenous administration to healthy volunteers. J. Clin. Pharmacol., 27, 762-767.
- Fukuyama, K., Nakano, T., Shiroyama, T. and Okada, M. (2021): Chronic administrations of guanfacine on mesocortical catecholaminergic and thalamocortical glutamatergic transmissions. Int. J. Mol. Sci., 22, 4122.
- Gillis, R.A., Gatti, P.J. and Quest, J.A. (1985): Mechanism of the antihypertensive effect of α 2-agonists. J. Cardiovasc. Pharmacol., 7 (Suppl 8), S38-S44.
- MacMillan, L.B., Hein, L., Smith, M.S., Piascik, M.T. and Limbird, L.E. (1996): Central hypotensive effects of the α2a-adrenergic receptor subtype. Science, 273, 801-803.
- McGrath, J.C. and Klein-Schwartz, W. (2002): Epidemiology and toxicity of pediatric guanfacine exposures. Ann. Pharmacother., 36, 1698-1703.
- Minns, A.B., Clark, R.F. and Schneir, A. (2010): Guanfacine overdose resulting in initial hypertension and subsequent delayed, persistent orthostatic hypotension. Clin. Toxicol. (Phila.), 48, 146-148.
- Roach-Fox, E., Welisch, E. and Sarpal, A. (2018): Autonomic instability in a dehydrated child on guanfacine: case report and literature review. Paediatr. Child Health, 23, 89-91.
- Scholtysik, G. and Brugger, P. (1989): In vitro cardiac effects and antiarrhythmic properties of guanfacine. Arzneimittelforschung, 39, 450-454.
- Spiller, H.A., Hays, H.L. and Aleguas, A. Jr. (2013): Overdose of drugs for attention-deficit hyperactivity disorder: clinical presentation, mechanisms of toxicity, and management. CNS Drugs, 27, 531-543.
- Swearingen, D., Pennick, M., Shojaei, A., Lyne, A. and Fiske, K. (2007): A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin. Ther., 29, 617-625.
- Uhlén, S., Muceniece, R., Rangel, N., Tiger, G. and Wikberg, J.E. (1995): Comparison of the binding activities of some drugs on alpha 2A, alpha 2B and alpha 2C-adrenoceptors and non-adrenergic imidazoline sites in the guinea pig. Pharmacol. Toxicol., 76, 353-364.
- Walton, J., Byrum, M., Shumaker, A. and Coury, D.L. (2014): Prolonged bradycardia and hypotension following guanfacine extended release overdose. J. Child Adolesc. Psychopharmacol., 24, 463-465.

