2023 年 48 巻 4 号 p. 179-189
2023 年 48 巻 4 号 p. 179-189
In this study, the toxicity effects on circulatory system and respiratory system, and the acute toxicity test of recombinant neorudin (EPR-hirudin, EH) in cynomolgus monkeys were evaluated to provide reference information for clinical studies. Eighteen cynomolgus monkeys were randomly divided into three groups for single intravenous administration of 3, 30 mg/kg EH and normal saline, respectively. The changes of respiratory frequency, respiratory intensity, blood pressure and electrocardiogram before and after administration were recorded. In acute toxicity test, six cynomolgus monkeys were intravenously received EH at a single dose of 171, 257, 385, 578, 867 and 1300 mg/kg respectively. The vital signs, hematology, serum biochemistry, coagulation indexes and electrocardiogram indexes of the animals were determined before administration and on the 7th and 14th day after administration. As the results showed that there were no significant abnormal changes in respiratory frequency, respiratory intensity, blood pressure or electrocardiogram in cynomolgus monkeys after receiving EH at 3 mg/kg and 30 mg/kg, and there was no statistical difference between the treated groups and normal saline group. In the acute toxicity test, no significant abnormalities were observed in vital signs, hematology, serum biochemistry, coagulation indexes and electrocardiogram indexes of six cynomolgus monkeys at day 7 and 14 after EH administration. Furthermore, autopsies of all cynomolgus monkeys showed no abnormalities. The results of toxicokinetics showed that AUClast of the drug increased in proportion to the EH dose in the range of 171–578 mg/kg, and increased in over proportion to the EH dose in the range of 578–1300 mg/kg. The variation of Cmax was basically consistent with AUClast. In a sum, A single intravenous injection of 3 and 30 mg/kg of EH did not affect the circulatory system and respiratory system in cynomolgus monkeys and the maximum tolerated dose of EH in cynomolgus monkey is over 1300 mg/kg (equivalent to 619–1300 times of the proposed clinical equivalent dose).
Hirudin is the strongest natural specific inhibitor of thrombin discovered so far (Dong et al., 2020; Junren et al., 2021), but its clinical application is greatly limited because of its association with severe bleeding (Antman, 1996; Greinacher and Warkentin, 2008; Lubenow and Greinacher, 2002). By comparison, recombinant neorudin (EPR-hirudin, EH) is developed as a prodrug of hirudin by introducing an EPR short peptide (Glu-Pro-Arg), which completely inhibits the antithrombin activity of hirudin, so EH is normally inactive (Zhang et al., 2008). However, the EPR short peptide can be recognized and cleaved by FXIa and/or FXa in the local thrombus, releasing free hirudin, thus playing a targeted antithrombotic role. Therefore, the bleeding associated with EH application evidently decreased, in that EH harbors the unique structure and action mechanism (Dong et al., 2020).
Previous studies confirmed that in animal pharmacodynamic experimental models, EH had the characteristics of low bleeding while playing an anticoagulant role, such as deep vein thrombosis in rats, arteriovenous bypass thrombosis in rabbits (Liu et al., 2022a), coronary artery thrombosis in dogs (Liu et al., 2022b) and other thrombosis models. Nevertheless, it is acceptable to discover the potential toxicity and the maximum tolerated dose of EH in order to provide references for clinical studies. Thus, in this current study, the EH potential toxicity on the circulatory system and respiratory system of cynomolgus monkeys was investigated, and the acute toxic reaction and the maximum tolerated dose of EH in cynomolgus monkeys was explored as well.
EH (20 mg/bottle) was obtained from Beijing Institute of Radiation Medicine. 0.9% normal saline was purchased from Siyao Co., Ltd. (Shijiazhuang, China).
EMKA non-invasive telemetry system for animal electrocardiograph (ECG) and blood pressure was purchased from EMKA Technologies Inc. (Paris, France); TCS-01-150 electronic platform scale was purchased from Shanghai Yiyu Electronic Technology Co., Ltd. (Shanghai, China); Omron electronic thermometer was purchased from Omron Co. Ltd. (Dalian, China); Digital single channel automatic ECG machine (ECG-6951E) was purchased from Nihon Kohden Co. Ltd. (Tokyo, Japan); MEK-7222K automatic five-category blood analyzer was purchased from Nihon Kohden Co. Ltd. (Tokyo, Japan); STAGO STA-4 semi-automatic four-channel hemagglutination machine was purchased from STAGO Co. Ltd. (Paris, France).
Twenty-four cynomolgus monkeys (body weight of 3.4 ± 0.4 kg, age of 3–5 years and half male and half female) were supplied by the Center of Experimental Animal of Beijing Institute of Radiation Medicine. All experimental procedures were approved by Management and Use Committee of Experimental Animals (IACUC) of Beijing Union Jianhao Medical Technology Development Co., Ltd. (Beijing, China) (No. IACUC-2018.01.15). Animal experiments were conducted according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
The toxicity effects on circulatory system and respiratory system Dose and groupingEighteen cynomolgus monkeys were randomly divided into three groups with three male and three female monkeys in each, and were single intravenously administrated EH at 3, 30 mg/kg body weight or equal volume of normal saline, respectively. EH was dissolved with normal saline to a desired concentration and each monkey was given EH in a volume of 10 mL/kg body weight.
Experiment preparationThe ECG monitor was first connected and the data acquisition and analysis software of IOX2 was then opened. Thereafter, the Transmitters were calibrated using emKA ECG and blood pressure corrector.
On the day of the detection, the fur was shaved on both sides of the chest of the animal and the shaved area was subject to the suitable ECG electrode (about 5 cm × 5 cm). After the skin was prepared, electrodes were stuck on the shaved site, and then ECG electrode wires were connected in sequence. Thereafter, an elastic vest was first put on the animal, the chest breathing belt was connected, and then an external vest was put on the animal. The signal transmitters (Transmitters) were finally connected and fixed on the external vest. After the animals and instruments were ready, the connection was confirmed by the monitoring results for the formal experiment.
ECG and respiration measurementThe changes in respiratory rate, respiratory intensity, blood pressure (SBP, DBP, MBP), heart rate and electrocardiogram (RR interval, PR interval, QRS duration, QT interval, QTcF) were recorded before and after drug administration. Monkeys were given drugs after above monitor data were stable for 30 min. The time point of drug administration was set as 0 min, and above monitor data were measured at the points of −15 min, 0 min, 10 min, 30 min, 60 min and 120 min. If significant changes occurred, the monitoring time could be extended or the monitoring time point could be increased as required. The statistical differences of data recorded after drug administration were analyzed compared with those of before administration. The QT interval (QTcF) was corrected by the Fredericia method.
QTcF = QT/RR1/3
Acute toxicity test Dose and groupingThe approximate lethal dose method was used in this acute toxicity study of EH in order to fully expose the toxicity of the drug. Total six cynomolgus monkeys with half male and female were injected intravenously EH once for each animal from the beginning dose of 171 mg/kg to 1300 mg/kg according to 50% increment method. EH was dissolved and was given animals as above described. The observation was continued for 14 days after administration. If possible, the lowest lethal dose and the highest non-lethal dose was determined, and a middle dose between them was selected to give an animal. Then the range between the dose and the lowest lethal dose was the approximate lethal dose range if the animal died at the dose; the range between the dose and the maximum non-lethal dose was the approximate lethal dose range if the animal died at that dose. If no animal died when the dose reached 1300 mg/kg, the dose was not increased further. Experimental groups are shown in Table 1.
 Detection of various indicators
Detection of various indicators
Animal observation
The appearance, activity and fecal traits of all animals were observed once a day.
Body weight and temperature measurement
Determination was performed twice before drug administration and once on day 7 and 14 after drug administration.
Determination of ECG
ECG determination was performed before and on day 14 after administration. ECG II was recorded and the following indexes were calculated: heart rate, PR interval, QT interval and QRS interval.
Hematological and blood biochemical examination
Blood samples were collected from the subcutaneous vein to perform hematological assay and sera were separated for blood biochemical detection before and on day 14 after drug administration.
Coagulation parameter determination
Blood was collected and plasma was separated by centrifugation for coagulation index detection before and at 1 hr after drug administration and on the 14th day after drug administration.
Toxicokinetic analysis
Intravenous plasma was collected before drug administration, and at 5 min, 20 min, 1 hr, 3 hr, 8 hr and 24 hr after drug administration, respectively, and drug concentration in the plasma was analyzed by the ELASA method.
Histopathological examination
All living animals were euthanized on the 14th day of the experiment by intravenous injection of 2% pentobarbital sodium (40 mg/kg). After inspection, the abnormal tissues were fixed with 10% neutral formaldehyde for histopathological examination.
Statistical analysisAll data were expressed as mean ± standard deviation 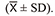 SPSS software was used for ANOVA variance analysis of the obtained data, with P < 0.05 as the significant difference.
SPSS software was used for ANOVA variance analysis of the obtained data, with P < 0.05 as the significant difference.
There were no significant abnormal changes in respiratory frequency and respiratory intensity of the monkeys at 10 min, 30 min, 60 min and 120 min after administration of EH or normal saline compared with those before administration, and no significant differences were detected among the groups (Fig. 1).

Effects on respiratory frequency and respiratory intensity in cynomolgus monkeys after administration (n = 6). The range of animal respiratory frequency was about from 30–60 times per minute, and the average was about 40 times/minute. The range of animal respiratory intensity was about from 2–15%, and the average was about 8%. After administration of neorudin at 3 mg/kg and 30 mg/kg, the respiratory frequency and respiratory intensity of the monkeys did not alter significantly, demonstrating that intravenous administration of neorudin at 30 mg/kg did not influence evidently the monkeys’ respiratory system.
As shown in Fig. 2, the systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial blood pressure (MBP) of the cynomolgus monkeys showed no significant abnormal changes at 10 min, 30 min, 60 min and 120 min after drug administration compared with those before administration, and there were no statistical differences among groups.
Effects on blood pressure in cynomolgus monkeys after administration (n = 6). The systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial blood pressure (MBP) of the cynomolgus monkeys showed no significant abnormal changes after administration of neorudin at 3 mg/kg and 30 mg/kg, proving that intravenous administration of neorudin at 30 mg/kg was safe for monkeys’ blood pressure.
There were no significant changes in RR interval (ms), heart rate (times/min), PR interval (ms), P wave duration (ms), QRS duration (ms), QT interval (ms) and QTcF at 10 min, 30 min, 60 min and 120 min after drug administration compared with before administration. There were no obvious differences in above data between the cynomolgus monkeys administrated with EH and normal saline (Fig. 3).

Effects on ECG function in cynomolgus monkeys after administration (n = 6). There were no significant changes in ECG, including RR interval (ms), heart rate (times/min), PR interval (ms), P wave duration (ms), QRS duration (ms), QT interval (ms) and QTcF, after neorudin administration at 3 mg/kg and 30 mg/kg. The results demonstrated that it was tolerable of intravenous administration of neorudin at 30 mg/kg for the heart function of cynomolgus monkeys.
Animal observation
The hemostasis time at the site of blood collection was significantly prolonged in all animals after EH administration, but was recovered at 1 hr after drug administration. There were no other abnormalities were observed except that there was slight redness and swelling in injection local of the monkeys on the first day after EH administration at 385, 867 and 1300 mg/kg body weight. The detailed results are shown in Table 2.

Body weight and temperature
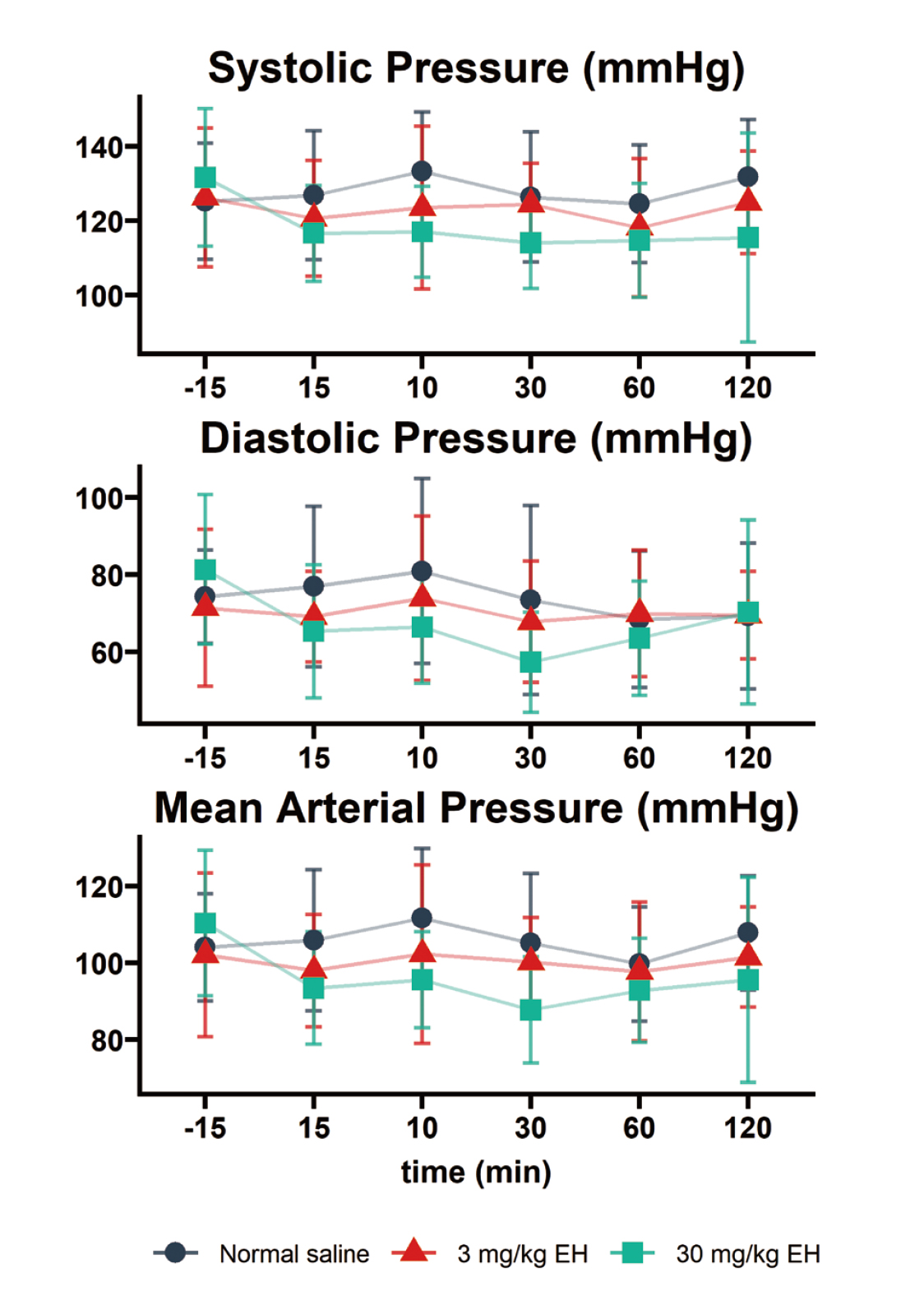
As shown in Fig. 4, there were no obvious abnormalities in body weight and temperature in all animals on day 7 and 14 after drug administration.
The acute toxic effects of neorudin on the body weight and temperature of cynomolgus monkeys. There were no statistic difference of the body weight and temperature in all animals on day 7 and 14 after intravenous administration of neorudin, compared with before drug administration.
ECG
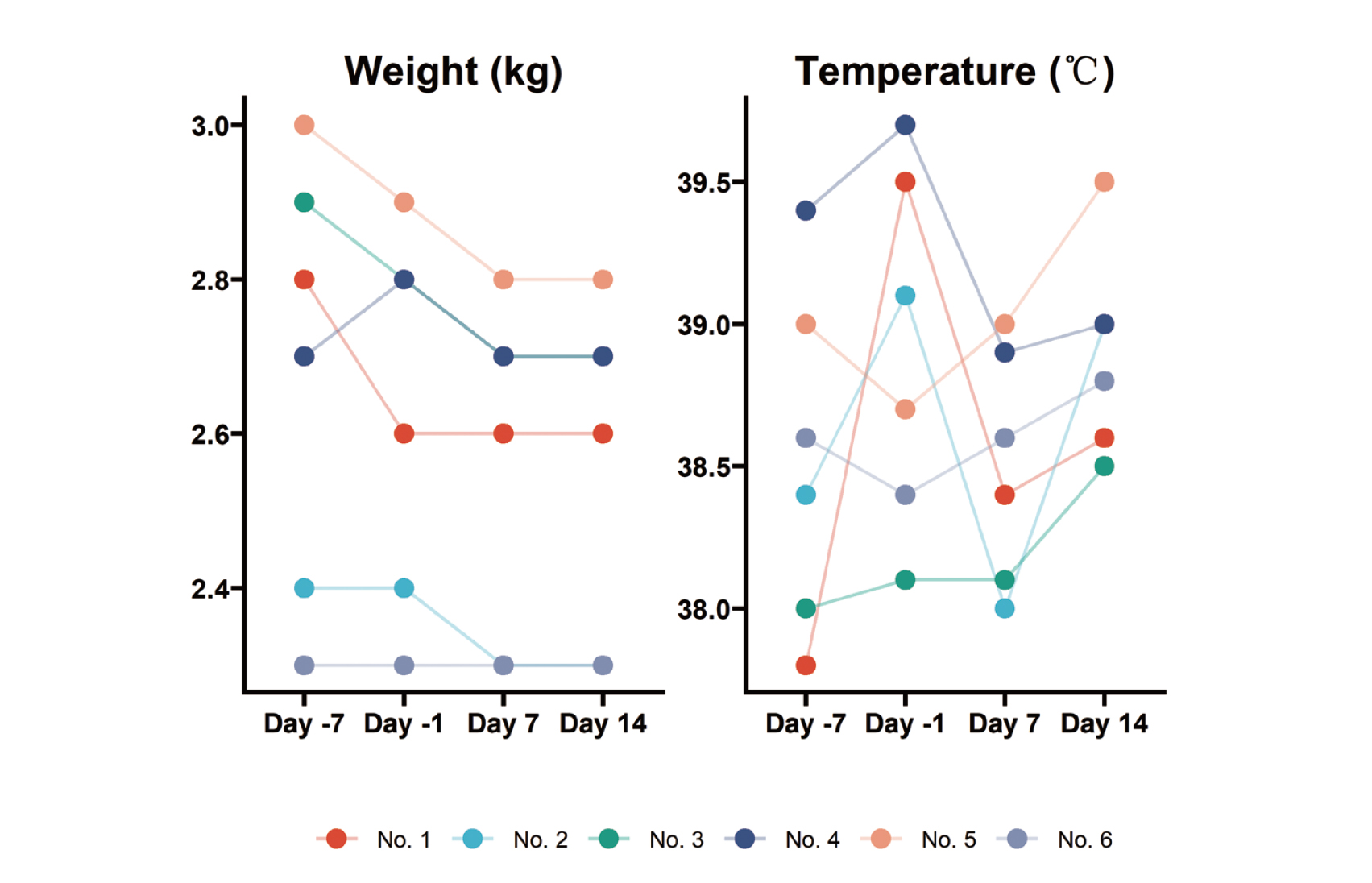
The results in Fig. 5 show no obvious abnormalities in heart rate, PR interval, QT interval and QRS duration in all animals on day 14 after EH injection compared with before administration. The typical ECG waveform is shown in the Fig. 6.
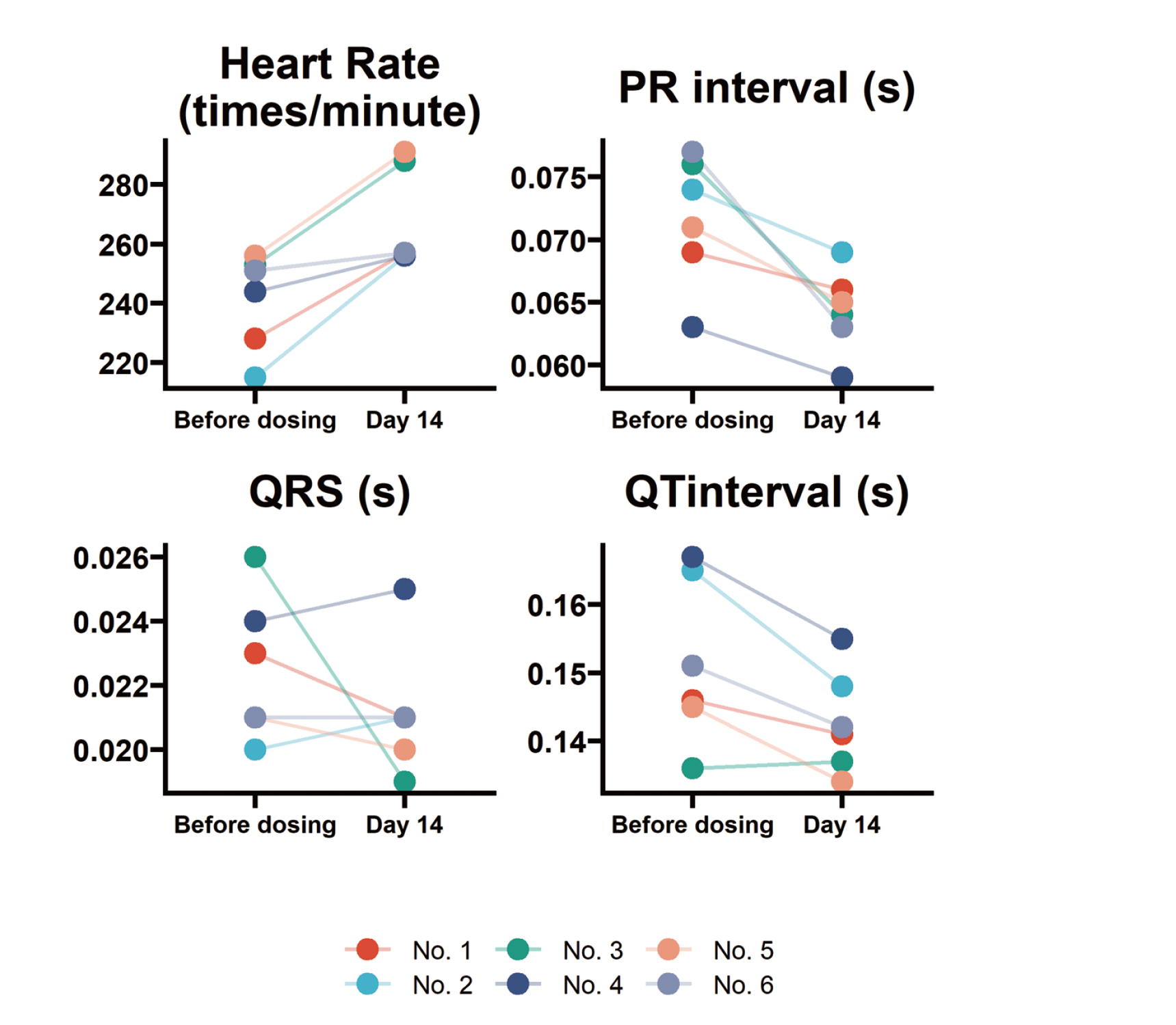
The acute toxic effects of neorudin on ECG function of cynomolgus monkeys. Compared with before administration, there were no obvious abnormalities in heart rate, PR interval, QT interval and QRS duration in all animals determined on day 14 after neorudin injection.
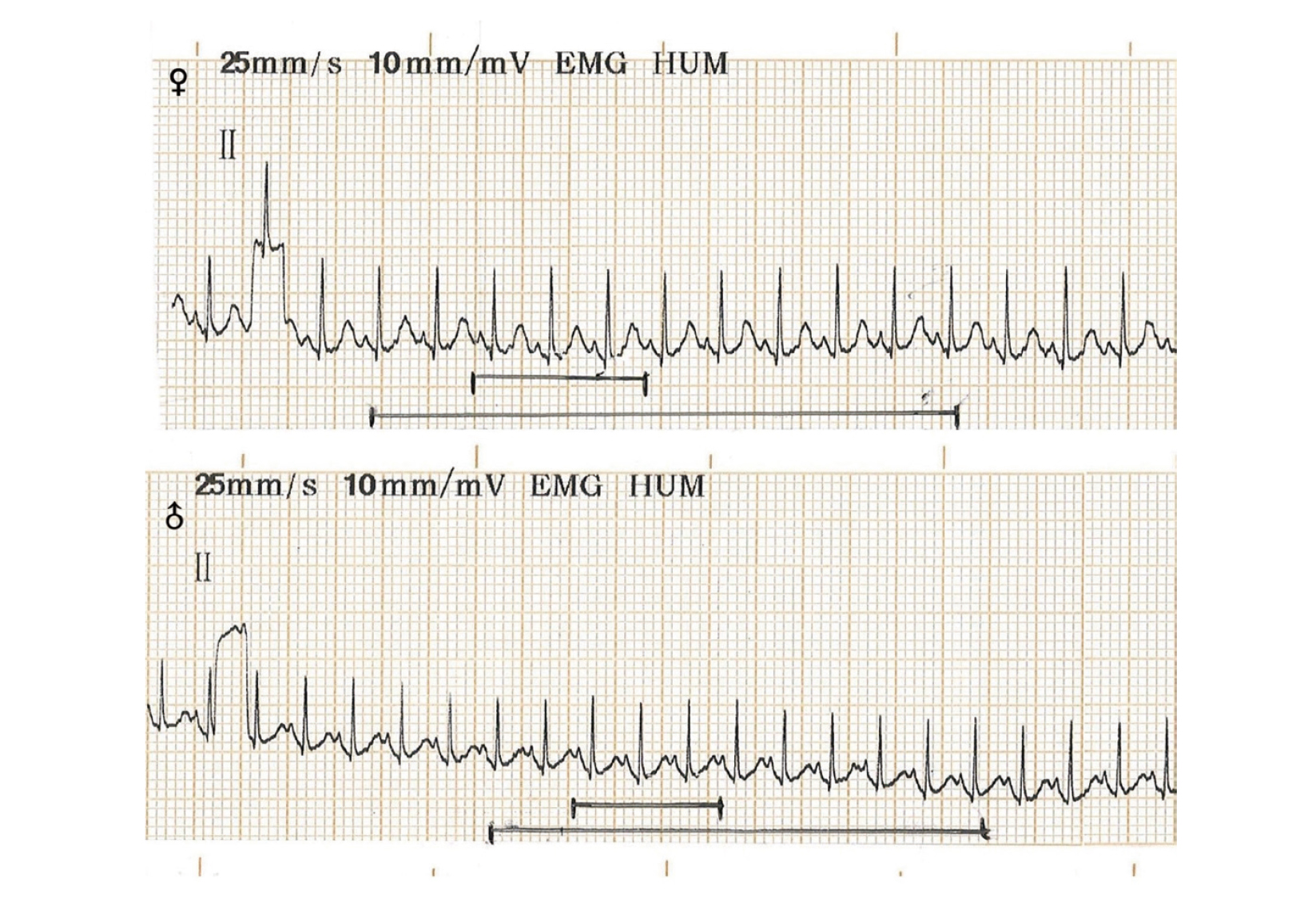
The typical ECG waveform of cynomolgus monkeys. The upper part is the typical ECG of male cynomolgus monkeys, and the lower part is female ECG.
Haematological index
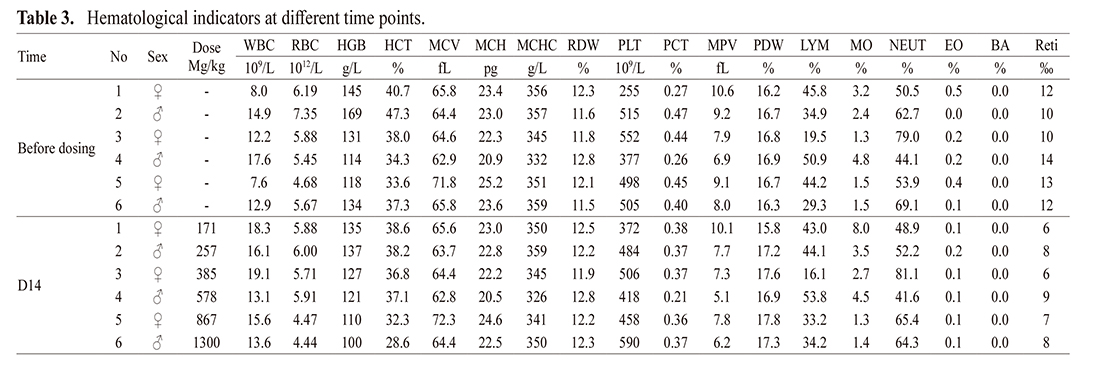
Hematologic indexes, including WBC, RBC, hemoglobin, RBC specific volume and mean RBC volume, showed no obvious abnormalities in all animals on day 14 after EH injection compared with before administration (Table 3).
Serum biochemical indexes
As shown in Table 4, serum biochemical indexes including alanine aminotransferase, albumin, alkaline phosphatase and aspartate aminotransferase showed no obvious abnormalities in all animals on day 14 after EH injection compared with before administration.

Coagulation indicator
TT of all animals at 1 hr after drug administration was > 80 s (superior limit of the detection kit); PT and APTT were significantly prolonged and Fib was significantly decreased in all monkeys at 1 hr after drug administration compared with before dosing. On the 14th day after drug administration, Both TT and APTT recovered, Fib recovered partially, but PT was lower than before drug administration (Fig. 7).

The acute toxic effects of neorudin on coagulation indicator in cynomolgus monkeys. TT of all animals at 1 hr after drug administration was > 80 s (superior limit of the detection kit); PT and APTT were significantly prolonged and Fib was significantly decreased in all monkeys at 1 hr after drug administration compared with before dosing. On the 14th day after drug administration, TT, PT and APTT were all recovered, but Fib recovered partially. Furthermore, the increase of PT and APTT at 1 hr after drug administration was dose-dependent.
As shown in Table 5, the body exposure was measured by AUClast, and the exposure dose increased in proportion to the EH dose in the range of 171–578 mg/kg, while increased in excess of the EH dose in the range of 578–1300 mg/kg. The variation of the Cmax value is basically consistent with AUClast. The changes of MRTlast and T1/2 in the whole dose range were not obvious.
 Histopathological examination
Histopathological examination
No abnormalities were found in the sections of the main organs, including the heart, liver, spleen, lung, kidney and brain, in the autopsies of all the animals.
Evaluation of the toxicity profile of a promising therapeutic agent is unarguably at the forefront of any drug discovery process. Acute toxicity is generally the first type of toxicity study in vivo during preclinical drug developments (Abubakar et al., 2022). It is aimed at providing a guide for the safe dose range of a new therapeutic agent and providing some preliminary information on the mode of toxic action of the agent in question (Jin et al., 2021; Mohs and Greig, 2017; Takeshita et al., 2014). This study described was aimed at assessing the safety and acute toxicity in cynomolgus monkeys at dosages up to 1300 mg/kg in order to support a clinical trial of EH for the treatment of thrombotic disease.
In general, EH was found to be safe and well tolerated. No meaningful toxic changes in body weight, temperature, blood pressure, respiratory function, ECG and haematological index were observed in the treated groups and as well as the normal saline group. Furthermore, in this acute toxicity study, no other anticoagulant-related toxic changes in hematology and clinical chemistry were detected apart from the slight local swelling, the increase of TT, PT and APTT and the decrease of Fib after EH administration whose alterations ascribed to the pharmacodynamics of EH and were described in our preclinical animal thrombosis models (Liu et al., 2022a, 2022b). APTT, TT and PT are routinely measured to assess blood coagulation in toxicity studies for anticoagulants (Kim et al., 2007; Kurata and Horii, 2004). Slight local redness and swelling at the injection site after administration suggested that there was minor bleeding at the injection site and it should be paid close attention in future clinical practices.
When the blood contacts the surface of the foreign body and activates the FXII, the endogenous coagulation system is activated on the one hand and the fibrinolytic system is also activated through the activation of the FXIIa on the other hand (Brien, 2019; Declerck, 2011; Gando et al., 2010; Reiner and Bell, 1984), so that the coagulation and fibrinolytic system can coordinate and maintain a balance. In this acute toxic study, when animals were intravenously injected with a high dose of EH, the coagulation system might be activated and the activity of the fibrinolytic system was enhanced, which might cause Fib decomposition. However, the underlying definite mechanism of the Fib decrease resulting from EH administration needs to be further unveiled.
Owing to evident toxic reactions such as bleeding, anticoagulants are generally used at a low dose in acute toxicological tests in animals. For example, as a classical anticoagulant and the most frequently used anticoagulant to treat various blood clot diseases (Camporese and Bernardi, 2009; Hull and Pineo, 2000), Low molecular weight heparin (LMWH) produced obvious adverse effects at the dose of 20 mg/kg in rats (Borelli and Bertoli, 1986). The acute toxic experiment of warfarin reported that its lethal dose (LD50) was 500 mg/kg for mice and 420 mg/kg body mass for rats (Manolov et al., 1993; Ohishi et al., 2010). However, there were no meaningful drug-related toxic reactions in monkeys with the EH dosages up to 1300 mg/kg. Thus, the results in this study indicated that EH has a sufficient margin of safety and a larger security widow for anticoagulant therapy.
Additionally, we performed the statistical analysis of sex differences of EH administration, and there was no significant sex difference in the toxic influence of EH on monkeys the same as other anticoagulants (Agewall, 2017; Carnicelli et al., 2022; Seki et al., 2008). In contrast with humans (Sedlak et al., 2012; Vink et al., 2018), there are no significant differences of QT interval between male and female monkeys (Seki et al., 2008). However, this might result from individual difference in such few samples that the male QT seemed to be longer than the female QT in this acute toxicity study.
In conclusion, a single intravenous injection of 3 and 30 mg/kg of EH did not affect the circulatory system and respiratory system in cynomolgus monkeys and the maximum tolerated dose of EH in cynomolgus monkey is over 1300 mg/kg.
This study was partially supported by the grant from Chinese National Major Projects for New Drug Discovery, Grant/Award Number: 2018ZX09301010-001.
Conflict of interestThe authors declare that there is no conflict of interest.