2023 年 48 巻 6 号 p. 311-321
2023 年 48 巻 6 号 p. 311-321
It is well established that platinum-based drugs, including oxaliplatin (L-OHP) and cisplatin (CDDP), as well as microtubule inhibitors paclitaxel (PTX) and vincristine (VCR), are associated with chemotherapy-induced peripheral neuropathy (CIPN). In this study, we examined and compared the characteristics of neuropathies induced by L-OHP, CDDP, PTX, and VCR to evaluate whether Caenorhabditis elegans (C. elegans) could serve as a model organism for human CIPN. Worms were cultured on nematode growth medium plates, and L1 larvae synchronized by gel filtration were employed. We then performed bioassays and examined motility. In the motility test, exposure was performed for 2, 24, and 48 hr, and time-dependent effects were measured for each exposure time and 24 hr after terminating exposure. Herein, we observed that L-OHP and CDDP exerted concentration-dependent effects above a certain concentration, and PTX and VCR exerted concentration-dependent negative effects in the bioassay. Motility recovered in L-OHP-, PTX-, and VCR-treated worms on terminating exposure. However, CDDP exposure tended to reduce motility even 24 hr after terminating exposure. L-OHP exposure could decrease motility 2 hr after exposure, with a trend toward recovery 24 hr after terminating drug exposure. The findings of the present study revealed that C. elegans could exhibit neuropathy characteristics suggested to be similar to those observed in humans, indicating that this organism could be a suitable model to explore human CIPN.
Drug therapy is considered an important component of cancer therapy protocols, along with surgery and radiotherapy. In recent years, anticancer drug therapy has facilitated improved overall survival while maintaining the quality of life (QOL) of patients, which can be attributed to the increased availability of anticancer drug categories, advances in concomitantly employed drugs, and the development of molecular-targeted drugs. However, various clinical side effects have been found to complicate anticancer drug therapy. Chemotherapy-induced peripheral neuropathy (CIPN) has been well associated with anticancer drug use, often causing numbness, pain, and paresthesia in limbs (Loprinzi et al., 2020). Despite the discontinuation of drug therapy, these symptoms may persist, delaying recovery (Yamamoto et al., 2019; Hung et al., 2021). Therefore, CIPN remains a substantial clinical challenge, as it markedly reduces the QOL. Based on a systematic review and meta-analysis of 31 studies (4179 patients) assessing various cancers, the prevalence of CIPN after terminating chemotherapy was as follows: within 1 month, 68.1%; after 3 months, 60%; after ≥ 6 months, 30% (Seretny et al., 2014).
Anticancer agents known to induce CIPN include oxaliplatin (L-OHP) and cisplatin (CDDP), both platinum-based anticancer agents, as well as paclitaxel (PTX) and vincristine (VCR), a taxane and vinca alkaloid, respectively. The incidence of CIPN is known to differ depending on the drug employed, with an incidence of 70–100% associated with platinum-based anticancer drugs, 11–87% with taxanes, and 20% with vinca alkaloids, and further vary depending on the administration regimen (Banach et al., 2016). In addition, the symptoms and mechanisms underlying CIPN differ depending on the anticancer drug. CIPN associated with platinum-based anticancer drugs (L-OHP and CDDP) is classified as neuronopathy and sensory disturbance, with damage to peripheral nerves of the extremities, face, and trunk. CIPN induced by PTX and VCR is classified as axonopathy, causing sensory disturbances and, in some cases, muscle atrophy. Recovery following neuronal cell body injury, associated with CIPN induced by platinum-based anticancer drugs, can be challenging owing to the loss of neuronal cell bodies, with no regeneration of axons and myelination observed (Arakawa et al., 2011). Considering acute CIPN that develops immediately (within a few days) after administration, L-OHP can induce cold exposure (Argyriou et al., 2013). Transient joint and muscle pain, called paclitaxel-associated acute pain syndrome (P-APS), reportedly occurs in ˃ 70% of PTX-treated patients (Reeves et al., 2012). However, the mechanism underlying CIPN-mediated nerve damage remains unknown, and preventive and therapeutic strategies to combat CIPN are yet to be established.
In recent years, alternative methods using cultured cells and the nematode Caenorhabditis elegans (C. elegans), allowing in vivo evaluation, have gained momentum for assessing drug toxicity from the perspective of animal welfare. C. elegans, a multicellular organism with a body length of approximately 1 mm, is transparent and allows easy observation of organ tissues. This cloned organism self-fertilizes and exhibits minimal inter-individual differences. Furthermore, C. elegans can be easily bred in the laboratory using E. coli as a food source (Mitani, 2008). C. elegans can be used to examine behavioral effects, which cannot be explored in cell cultures, and neurotoxicity can be established based on these behavioral effects (Anderson et al., 2004). Moreover, the entire genome sequence of C. elegans has been published and is highly homologous to that of humans, with approximately 30–60% of C. elegans genes having mammalian orthologs or strong homologs (Sonnhammer and Durbin, 1997; Shaye and Greenwald, 2011). Accordingly, C. elegans is considered a suitable model to assess mammalian toxicity (Hunt, 2017; Harlow et al., 2016). Toxicity data from C. elegans could be relevant to human development and diseases. To date, we have examined the effects of various chemical substances using C. elegans (Sakaguchi et al., 2022a, 2022b, 2022c).
Currently, dose reduction or discontinuation of the anticancer drug therapy is considered for mitigating CIPN, and continuation of treatment can be challenging, presenting a notable clinical problem. Herein, we used C. elegans, a model organism, to elucidate the underlying mechanism of nerve damage induced by L-OHP, CDDP, PTX, and VCR, anticancer agents known to induce CIPN. In this study, biological and behavioral assays were performed to evaluate whether C. elegans could serve as a model organism for human CIPN.
In the present study, experiments were performed with wild-type nematode C. elegans (Bristol strain N2), and live E. coli (DH5αFT) was used as the food source.
ReagentsL-OHP (molecular weight [MW]: 397.29, purity: ≥ 98.0%) standards were purchased from Funakoshi Co. Ltd. (Tokyo, Japan). CDDP (MW: 300.05, purity: ≥ 98.0%) and PTX (MW:853.91, purity: ≥ 98.0%) standards were purchased from the FUJIFILM Wako Pure Chemical Corporation (Tokyo, Japan). Standard VCR (MW: 923.04, purity: ≥ 95.0%) was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Each sample was dissolved in dimethyl sulfoxide (DMSO; Nacalai Tesque) and used as a test solution.
NaCl, Na2PO4, agar powder, Tryptone, CaCl2, dry yeast extract, KCl, and NaN3 were purchased from Nacalai Tesque. KH2PO4, KOH, cholesterol, MgSO4, C6H8O7 · H2O, FeSO4 · 7H2O, NaOH, MnCl2 · 4H2O, ZnSO4 · 7H2O, CuSO4 · 5H2O, and Na2EDTA were purchased from Wako Pure Chemical Industries. Sephadex G-25 medium was purchased from GE Healthcare (Tokyo, Japan). All the reagents were of analytical grade.
Breeding of nematode C. elegansMature nematodes were placed on nematode growth medium (NGM) plates and cultured for 3‒4 days in a thermostatic chamber (20°C) to propagate by self-fertilization. To confirm that the next generation of nematodes reached maturity, worms were transferred to new NGM plates and examined under a stereomicroscope.
Preparation and exposure of nematode suspensionsAfter culturing propagated nematodes on NGM plates, L1–L2 worms were isolated by passing them through a column packed with Sephadex G-25. Exposure solutions for each concentration were prepared using liquid medium containing E. coli as the test medium and dispensed into 24-well plates at 0.5 mL/well. L1‒L2 worms were added to wells containing each exposure solution at a rate of 10 worms/well for growth, maturation, and motility tests and one worm/well for the reproductive test. Considering that human nerve cells are exposed to anticancer drugs via the bloodstream, exposure, in the case of C. elegans, was determined on a liquid medium basis.
Lethal toxicity testBriefly, L1‒L2 worms were added to each exposure solution and incubated for approximately 24 hr at 20°C, protected from light. After incubation, both the total number of worms and survivors were counted. To establish whether worms were alive or dead, those that failed to respond to touch with a pick were considered dead.
Growth and maturation assayBriefly, L1‒L2 worms were added to each exposure solution and incubated for approximately 50 hr at 20°C, protected from light. After incubation, sodium azide was added, and the body length of worms and percentage of nematodes with fertilized eggs were measured. Body length was used to evaluate the effect on growth, whereas the percentage of nematodes with fertilized eggs was employed for determining the effect on maturation.
Reproductive assayAfter L1‒L2 worms were added to each exposure solution, incubation was initiated in the dark at 20°C. In each exposure group, parent worms were transferred to a new exposure solution daily after laying the first egg; this process was repeated until day 14. The number of lifetime litters of each worm was measured.
Motility testBriefly, L1‒L2 worms were added to each exposure solution and incubated for approximately 2, 24, and 48 hr at 20°C protected from light. After incubation, the number of body bends per 30 sec and thrashes per 60 sec were determined for each worm. Following incubation in each exposure solution for 2, 24, and 48 hr and incubation in DMSO for an additional 24 hr, the number of body bends per 30 sec and thrashes per 60 sec were measured for each worm. The experimental protocol for the motility test is shown in Fig. 1.

Protocol for assessing the impact of anticancer drug exposure on motility. CDDP, cisplatin; L-OHP, oxaliplatin; PTX, paclitaxel; VCR, vincristine.
Data analysis was performed using Microsoft “Excel Statistics Bell Curve for Excel” (Information Service Co., Ltd., Tokyo, Japan). Dunnett’s test was applied to determine significant differences in growth and maturation, reproductive, and motility tests. Considering the bioassay and behavioral assay, comparisons were performed with the solvent control group. Results regarding growth, maturation, and reproductive impact are presented as mean ± standard deviation. A P-value < 0.05 was deemed statically significant.
We assayed the toxic effects of L-OHP, CDDP, PTX, and VCR in C. elegans. Herein, we observed that the survival rate was markedly reduced following exposure to > 1.8 mM L-OHP, > 4.5 × 10-1 mM CDDP, > 1.8 × 10-1 mM PTX, and > 0.9 mM VCR (Fig. 2). Considering the results of the lethality test, we performed the growth and maturation, reproductive, and motility assays at concentrations that did not decrease the survival rate.

Lethal toxicity test following exposure to anticancer drugs. n = 40. (A) L-OHP: 1.8 × 10-2, 1.8 × 10-1, 1.8, 18 (mM). (B) CDDP: 1.8 × 10-2, 9.0 × 10-2, 4.5 × 10-1, 0.9, 1.35, 1.8 (mM). (C) PTX: 1.8 × 10-2, 1.8 × 10-1, 1.8, 18 (mM). (D) VCR: 1.8 × 10-2, 9.0 × 10-2, 4.5 × 10-1, 0.9, 1.8, 3.6 (mM). CDDP, cisplatin; L-OHP, oxaliplatin; PTX, paclitaxel; VCR, vincristine.
L-OHP significantly inhibited growth at 1.8 × 10-1 mM, with a more remarkable inhibition detected at 1.8 mM. The other examined anticancer drugs remarkably inhibited growth, with CDDP at ≥ 9.0 × 10-3 mM, PTX at ≥ 1.8 × 10-3, and VCR at ≥ 9.0 × 10-3 (Fig. 3). All examined anticancer drugs could shorten the body length of C.elegans.
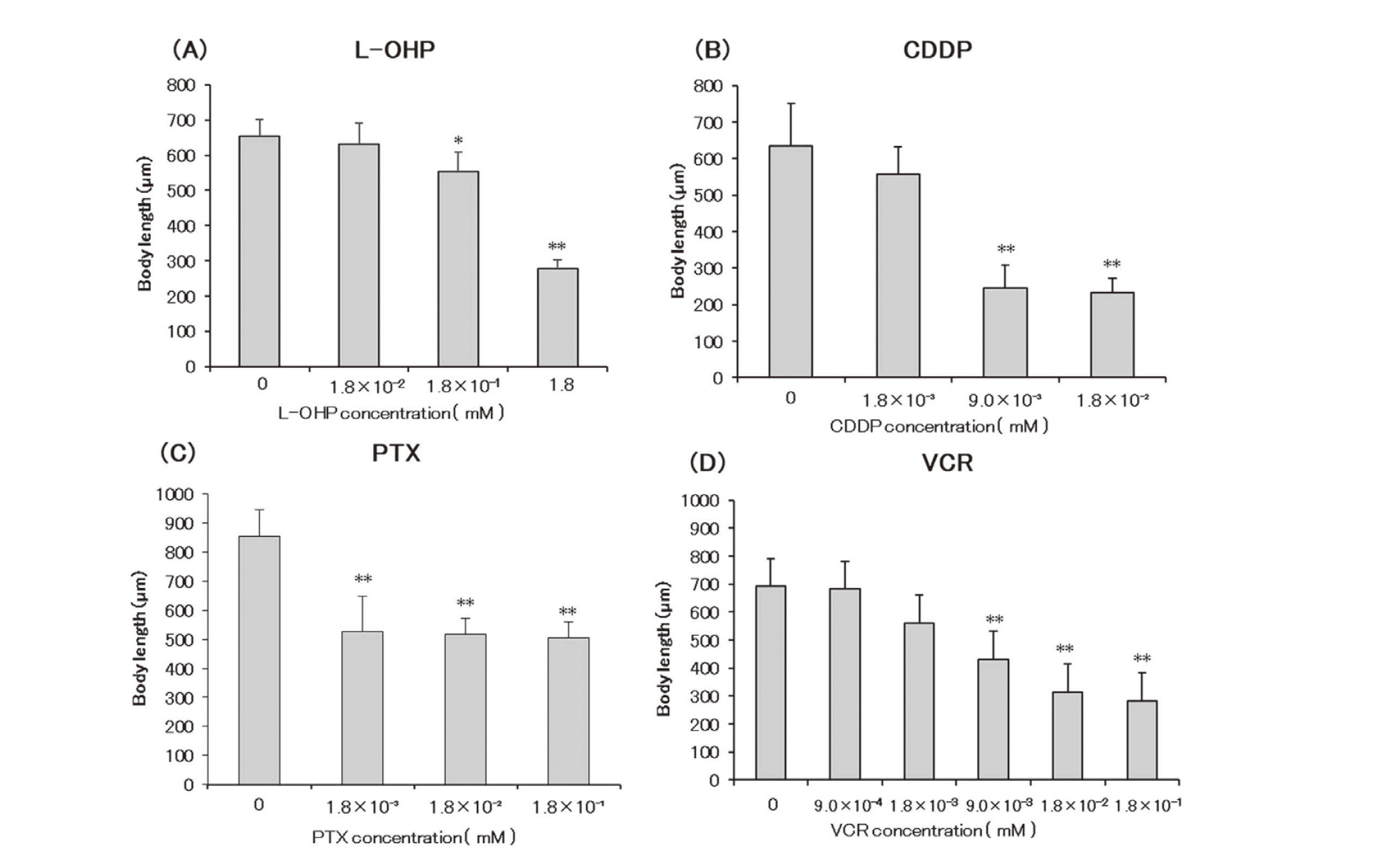
Effects of anticancer drugs on body length (µm) of Caenorhabditis elegans following drug exposure in the growth test. n = 10. Control vs. other concentrations * P < 0.05, ** P < 0.01. CDDP, cisplatin; L-OHP, oxaliplatin; PTX, paclitaxel; VCR, vincristine.
L-OHP and CDDP at concentrations of 1.8 mM and 1.8 × 10-2 mM, respectively, suppressed C.elegans maturation. PTX (at ≥ 1.8 × 10-3 mM) and VCR (at ≥ 9.0 × 10-3) significantly inhibited maturation in a concentration-dependent manner (Fig. 4). Platinum-based drugs inhibited C.elegans maturation above a certain concentration, whereas microtubule inhibitors suppressed C.elegans maturation in a concentration-dependent manner.

Effects of anticancer drugs on the maturation rate (%) of Caenorhabditis elegans following drug exposure in the maturation test. 3 groups (group: n = 10). Control vs. other concentrations * P < 0.05, ** P < 0.01. CDDP, cisplatin; L-OHP, oxaliplatin; PTX, paclitaxel; VCR, vincristine.
L-OHP and CDDP at concentrations of 1.8 mM and 9.0 × 10-3 mM, respectively, could almost completely abolish C.elegans oviposition. PTX at ≥ 1.8 × 10-3 mM significantly inhibited the number of offspring produced over a lifetime. At concentrations of ≥ 9.0 × 10-4 mM, VCR significantly inhibited the number of offspring produced over a lifetime in a concentration-dependent manner (Fig. 5). Similar to the impact on maturation, platinum-based drugs had a significant impact above a certain concentration in the reproduction test. Considering microtubule inhibitors, VCR induced concentration-dependent effects, whereas the PTX showed intermediate effects ranging between platinum-based drugs and VCR.

Effects of anticancer drugs on the total number of Caenorhabditis elegans offspring following drug exposure in the reproduction test. n = 4. Control vs. other concentrations * P < 0.05, ** P < 0.01. CDDP, cisplatin; L-OHP, oxaliplatin; PTX, paclitaxel; VCR, vincristine.
We selected two concentrations to perform the motility test based on concentrations that induced significant effects in the bioassay. Initially, we examined the time-dependent effects of the four anticancer drugs. L-OHP significantly inhibited behavior ≥ 2 hr after exposure when compared with the control, but no time-dependent effects were observed. In contrast, CDDP, PTX, and VCR exhibited a time-dependent decrease in the motility test. Considering all four drugs, we detected a greater reduction in motility at higher concentrations (Fig. 6).

Effects of anticancer drugs on the thrashes and body bends of Caenorhabditis elegans following drug exposure in the motility test. n = 10. DMSO exposure was 100%, and the motor activity at each exposure concentration was calculated. Control vs. other concentrations * P < 0.05, ** P < 0.01. 2-hr exposure vs. 24-hr exposure or 48-hr exposure #: P < 0.05, ##: P < 0.01. CDDP, cisplatin; DMSO, dimethyl sulfoxide; L-OHP, oxaliplatin; PTX, paclitaxel; VCR, vincristine.
Next, we examined the reversibility of neurotoxicity. Motility was significantly recovered 24 hr after a 2-hr exposure to L-OHP (L-OHP 2 hr →24 hr). However, motility failed to recover 24 hr after terminating a 48-hr L-OHP exposure (L-OHP 48 hr →24 hr) (Fig. 7). Twenty hours after terminating exposure, CDDP significantly reduced motility at all exposure times (2, 24, and 48 hr) (Fig. 8). Twenty hours after terminating PTX exposure, motility significantly recovered following exposure for 24 hr (PTX 24 hr →24 hr) and 48 hr (PTX 48 hr →24 hr) (Fig. 9). Considering the VCR-exposed group, motility recovered significantly only 24 hr after terminating a 24-hr VCR exposure (VCR 24 hr →24 hr) (Fig. 10).

Reversibility of Caenorhabditis elegans thrashes and body bends after terminating L-OHP exposure in the motility test. n = 10. Each exposure time vs. 24 hr after the end of exposure * P < 0.05, ** P < 0.01. L-OHP, oxaliplatin.

Reversibility of Caenorhabditis elegans thrashes and body bends after terminating CDDP exposure in the motility test in the motility test. n = 10. Each exposure time vs. 24 hr after the end of exposure * P < 0.05, ** P < 0.01. CDDP, cisplatin.

Reversibility of Caenorhabditis elegans thrashes and body bends after terminating PTX exposure in the motility test. n = 10. Each exposure time vs. 24 hr after the end of exposure * P < 0.05, ** P < 0.01. PTX, paclitaxel.
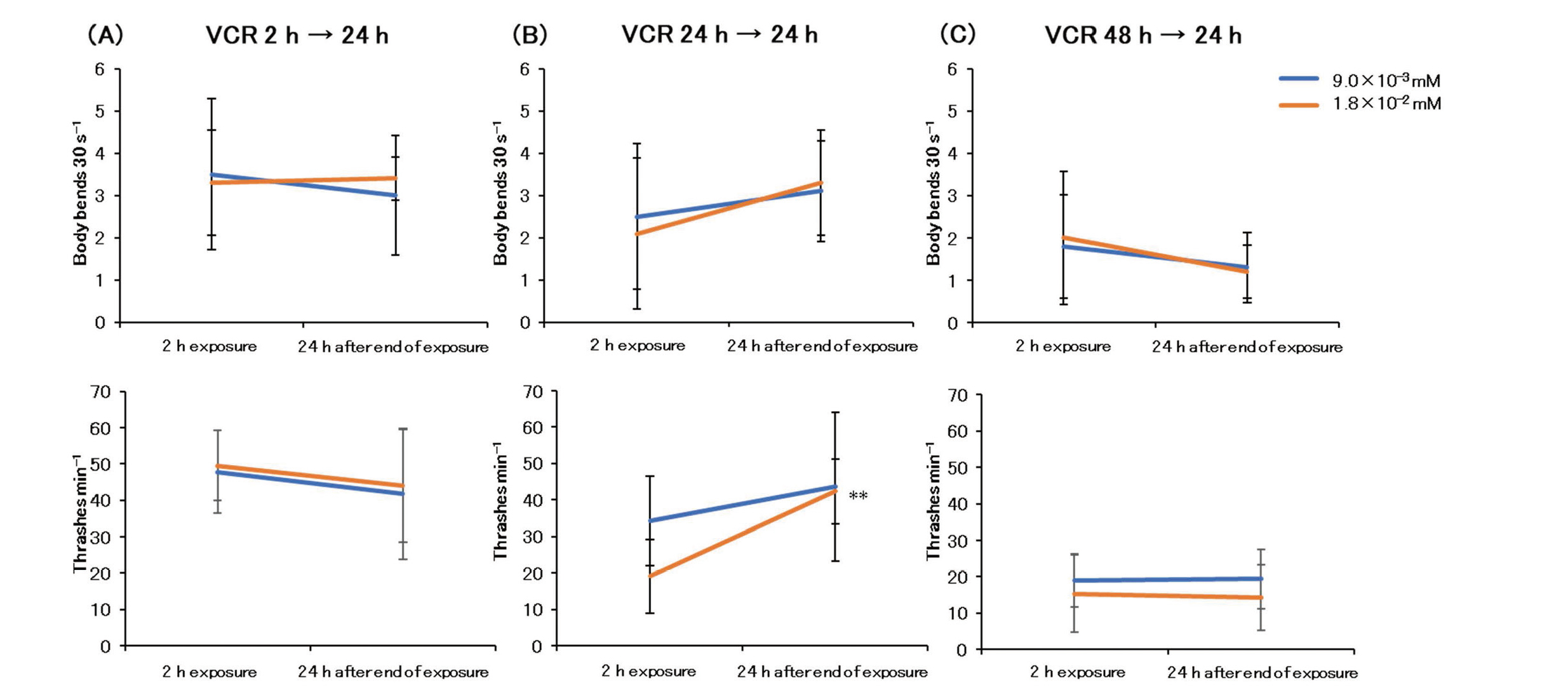
Reversibility of Caenorhabditis elegans thrashes and body bends after terminating VCR exposure in the motility test. n = 10. Each exposure time vs. 24 hr after terminating exposure * P < 0.05, ** P < 0.01. VCR, vincristine.
In the present study, C. elegans was used to assess the biological and behavioral effects mediated by four anticancer drugs (L-OHP, CDDP, PTX, and VCR) well known to induce CIPN. Considering the biological effects, L-OHP and CDDP, both platinum-based drugs, tended to exhibit increased toxicity above a certain concentration, whereas microtubule inhibitors PTX and VCR tended to exert increasing toxicity in a concentration-dependent manner. Only PTX induced increased biotoxicity above a certain concentration in the reproduction test. The motility test, body bend count test, and thrash count test are frequently employed in the field of toxicology to assess neurotoxicity in C. elegans (Tsalik and Hobert, 2003; Buckingham and Sattelle, 2009). Herein, we used C. elegans as a potential model of human CIPN. Using the motility test, we examined C. elegans exposed to drugs for 2 hr to assess acute CIPN and 24 and 48 hr for chronic CIPN. In addition, to evaluate the reversibility of CIPN following drug exposure for 2, 24, and 48 hr, worms were cultured in drug-free medium, and the number of behaviors was measured. We observed that L-OHP could induce concentration-dependent neuropathy, whereas exposure to CDDP, PTX, and VCR resulted in a concentration- and time-dependent neuropathy in C. elegans. Table 1 summarizes the study results.
| Concentration Threshold by Bioassay |
Acute toxicity by motility effects test (recovery) | Chronic toxicity by motility effects test (recovery) | ||
|---|---|---|---|---|
| Platinum-based Drugs | L-OHP | + | + (○) | + (-) |
| CDDP | + | - | + (×) | |
| Microtubule Inhibitors | PTX | ± | ± | + (○) |
| VCR | - | ± | + (○) |
CDDP, cisplatin; L-OHP, oxaliplatin; PTX, paclitaxel; VCR, vincristine.
+: Significant results in some tests.
±: Significant results in limited tests.
-: No significant result
○: recovered
×: got worse
Platinum-based drugs cause somatic neuronal damage. This is a peripheral neuropathy caused by the apoptosis of the dorsal root ganglion (DRG) cells, with secondary damage to the axons and myelin sheaths. DRG cells lack a blood-nerve barrier (BNB), are fragile, and can be easily damaged by drugs. Platinum-based drugs may inhibit DNA synthesis in the DRG neurons and induce neuronal cell apoptosis via the activation of mitogen-activated protein kinase (Li et al., 2021). Consequently, large-diameter myelinated fibers are damaged, thereby resulting in acute axonal degeneration and loss of these nerve fibers. Due to the DRG cells’ death, the nerve cell body itself disappears; therefore, regeneration of the axons and myelin sheaths is absent. Thus, recovery is often not expected even after drug discontinuation (Arakawa et al., 2011), which coincides with the post-treatment exacerbation called coasting reported in humans (Mollman et al., 1988; Grunberg et al., 1989). Although CDDP and L-OHP are both platinum-based drugs, their clinical features of neurotoxicity differ. A characteristic of CDDP is that it accumulates and symptoms often persist long-term, despite discontinuation of administration; moreover, according to reports, coasting is likely to occur (Japanese Association of Supportive Care in Cancer, 2017). In contrast, L-OHP causes acute and chronic symptoms immediately after administration. Acute symptoms are characterized by sensory abnormalities around the extremities and lips, which are exacerbated by cold stimuli; subsequently transient dysphagia and dyspnea may occur. Acute symptoms mostly disappear within a few days, but chronic symptoms may persist for months to years depending on the dosage (Argyriou, et al., 2008). In this study, CDDP significantly reduced worm motility even after exposure ceased. This phenomenon contrasts with the observations of worms exposed to L-OHP, PTX, and VCR, suggesting that CDDP-specific coasting can be examined in C. elegans. Moreover, C. elegans worms exposed to L-OHP for 2 hr showed improvement of behavioral changes after a 24 hr recovery period, and, C. elegans worms treated with L-OHP for 48 hr showed no recovery after 24 hr. These results, could be indicative of reversible acute symptoms and irreversible chronic symptoms characteristic of L-OHP treatment in humans.
Microtubule inhibitors PTX and VCR have been shown to cause axonal damage, and early drug discontinuation could afford recovery. In the present study, the number of behaviors recovered after terminating drug exposure suggests the development of reversible CIPN. PTX can cause transient muscle and joint pain within a few days of human administration, deemed taxane acute pain syndrome (Loprinzi et al., 2011). Recently, this acute symptom has been deemed neuropathic pain (Japanese Association of Supportive Care in Cancer, 2017). Herein, the motility test showed a significant decrease in the number of thrashes 2 hr after PTX exposure, indicating the onset of acute CIPN. Exposure to VCR could induce a decrease in motility similar to that mediated by PTX. However, recovery was not observed in VCR 48 hr →24 hr. In humans, CIPN symptoms often continue after discontinuation of VCR therapy, demonstrating a delay in recovery (Legha, 1986). Herein, we observed a similar phenomenon in the C. elegans model.
In the present study, we evaluated the CIPN induced by four anticancer drugs using C. elegans as a model organism. The results revealed that C. elegans could exhibit neuropathy characteristics possible similar to those observed in humans, indicating that C. elegans is a suitable model of human CIPN. Toxicological evaluation of chemical substances, including pharmaceuticals, requires a comprehensive analysis of the changes in morphology, gene expression, and low-molecular-weight compounds at the metabolic level. In the future, we plan to perform a comprehensive analysis using RNA-Seq and metabolomics on C. elegans, and we aim to elucidate the pathogenesis of CIPN.
Conflict of interestThe authors declare that there is no conflict of interest.