2023 年 48 巻 7 号 p. 375-385
2023 年 48 巻 7 号 p. 375-385
Long-term use of proton pump inhibitors (PPIs) is known to clinically induce hypomagnesemia, increasing the risk toward QT-interval prolongation and lethal ventricular arrhythmias, whereas PPIs can directly modulate cardiac ionic currents in the in vitro experiments. In order to fill the gap between those information, we assessed acute cardiohemodynamic and electrophysiological effects of sub- to supra-therapeutic doses (0.05, 0.5 and 5 mg/kg/10 min) of typical PPIs omeprazole, lansoprazole and rabeprazole, using halothane-anesthetized dogs (n = 6 for each drug). The low and middle doses of omeprazole and lansoprazole increased or tended to increase the heart rate, cardiac output and ventricular contraction, whereas the high dose plateaued and decreased them. Meanwhile, the low and middle doses of omeprazole and lansoprazole decreased the total peripheral vascular resistance, whereas the high dose plateaued and increased it. Rabeprazole decreased the mean blood pressure in a dose-related manner; moreover, its high dose decreased the heart rate and tended to reduce the ventricular contractility. On the other hand, omeprazole prolonged the QRS width. Omeprazole and lansoprazole tended to prolong the QT interval and QTcV, and rabeprazole mildly but significantly prolonged them in a dose-related manner. High dose of each PPI prolonged the ventricular effective refractory period. Omeprazole shortened the terminal repolarization period, whereas lansoprazole and rabeprazole hardly altered it. In effects, PPIs can exert multifarious cardiohemodynamic and electrophysiological actions in vivo, including mild QT-interval prolongation; thus, PPIs should be given with caution to patients with reduced ventricular repolarization reserve.
Proton pump inhibitors (PPIs) effectively reduce gastric acid secretion through H+/K+-ATPase blockade, which have been extensively used for acid-related gastrointestinal disease. However, the use of PPIs has been clinically reported to be associated with the increase of mortality and risk for cardiovascular adverse events, including cardiac arrhythmias (Manolis et al., 2020; Xie et al., 2017, 2019; Zhai et al., 2022). Previous clinical studies have shown that long-term use of PPIs may cause hypomagnesemia, since the increase of pH in the intestine can decrease the absorption of magnesium ions through transient receptor potential melastatin 6/7 cation channel (Park et al., 2014; Liao et al., 2019; William and Danziger, 2016). Moreover, hypomagnesemia has been reported to induce QT-interval prolongation and lethal ventricular arrhythmias such as torsade de pointes via the increase of inward L-type calcium channel current (ICaL) and the downregulation of inward-rectifying K+ current (IK1) and transient outward K+ current (Ito) (Lazzerini et al., 2021; Chrysant, 2019; Lazzerini et al., 2018; Shimaoka et al., 2020). Meanwhile, some PPIs, including omeprazole and lansoprazole, have been demonstrated to directly inhibit cardiac ionic currents, including the rapid component of delayed rectifier K+ current (IKr) and ICaL, in vitro (Lazzerini et al., 2021; Nagashima et al., 1999; Naseri and Yenisehirli, 2006), which could modify the ventricular repolarization. Recently, Lazzerini et al. (2021) showed that the mean QTc was more prolonged in a PPI-treated group (446 ms) than in PPI-untreated one (437 ms) in the presence of same degree of hypomagnesemia (< 1.8 mg/dL), and proposed that PPIs can per se prolong QT interval irrespective of hypomagnesemia. However, information is still limited on how much the extent of the direct electrophysiological effects of PPIs contributes to the onset of QT-interval prolongation and ventricular arrhythmias in vivo.
To fill the gap of information in translating the in vitro basic findings to clinical observation, in this study we tried to assess the electropharmacological effects of three representative PPIs, omeprazole, lansoprazole and rabeprazole, using halothane-anesthetized dogs. The canine model has been known to be useful for estimating the drug-induced electropharmacological responses in healthy human subjects (Sugiyama, 2008), and classified into the follow-up study in E14/S7B Q&A (Strauss et al., 2021). We administered those PPIs with a single dosing to the animals to separate the acute cardiovascular effects of PPIs from PPIs-associated various chronic actions, including electrolyte abnormalities. We simultaneously analyzed multiple electropharmacological variables in vivo to better estimate the underlying potential mechanisms of QT-interval prolongation by PPIs. For example, we recorded His bundle electrograms and monophasic action potentials (MAP) besides the surface lead II electrogram to exactly analyze electrophysiological effects of PPIs. Moreover, we measured the MAP duration and effective refractory period at the same site of the ventricle, and directly compared the drug effects on the repolarization and refractoriness to quantify the electrical vulnerability of the ventricle (Sugiyama, 2008; Sugiyama and Hashimoto, 1998).
Experiments were performed using male and female beagle dogs weighing 9-14 kg (n = 18). The dogs were obtained from Chugai Research Institute for Medical Science, Inc. (Shizuoka, Japan). The dogs were individually housed in stainless steel dog cages on a 12 hr light (6:00-18:00)-dark (18:00-6:00) cycle, and were given 200 g/day of standard pellet diet (CD-5M, CLEA Japan, Inc., Tokyo, Japan) and free access to tap water. The animal rooms were maintained at a temperature of 23 ± 2°C and a relative humidity of 50 ± 30%. All experiments were approved by the Animal Care and User Committee of Yamanashi Research Center of Clinical Pharmacology (Yamanashi, Japan) (No. 2003-02), and performed at the University of Yamanashi according to the Guideline for the Care and Use of Laboratory Animals of University of Yamanashi.
Surgical preparationBeagle dogs (n = 18) were initially anesthetized with thiopental sodium (30 mg/kg, i.v.). After intubation with a cuffed endotracheal tube, anesthesia was maintained by inhalation of halothane (1% v/v) vaporized in oxygen with a volume-limited ventilator (SN-480-3, Shinano Manufacturing Co., Ltd., Tokyo, Japan). Tidal volume and respiratory rate were set at 20 mL/kg and 15 breaths/min, respectively. Four clinically available catheter sheath introducer sets (FAST-CATHTM, St. Jude Medical, Daig Division, Inc., Minnetonka, MN, USA) were used for introducing the catheters as described below. Two 6-F ones were inserted in the right and left femoral arteries toward aorta, whereas 6-F and 7.5-F ones were done in the right and left femoral veins toward inferior vena cava, respectively. Heparin calcium (100 IU/kg) was administered to prevent the blood clotting through a flush line of the catheter sheath placed at the right femoral vein.
Measurement of cardiohemodynamic variablesA 5-F pig-tail catheter was placed at the left ventricle through the right femoral artery to measure the left ventricular pressure, whereas the aortic pressure was measured at a space between the inside of the 6-F catheter sheath and outside of the pig-tail catheter through a flush line. We have confirmed that the insertion of 5-F pig-tail catheter did not affect the aortic pressure waveform obtained through 6-F catheter sheath (Supplementary Fig. S1). The maximum upstroke velocities of the left ventricular pressure (LVdP/dtmax) and the left ventricular end-diastolic pressure were obtained during sinus rhythm to estimate the isovolumic systolic function and the preload to the left ventricle, respectively. A thermodilution catheter (132F5, Edwards Lifesciences, Irvine, CA, USA) was positioned at the right side of the heart through the right femoral vein. The cardiac output was measured by using a standard thermodilution method with a cardiac output computer (MFC-1100, Nihon Kohden Corporation, Tokyo, Japan). The total peripheral vascular resistance was calculated with the following basic equation: total peripheral vascular resistance=mean blood pressure/cardiac output.
Measurement of electrophysiological variablesThe lead II electrocardiogram was obtained from the limb electrodes. The PR interval, QRS width and QT interval were measured. The QT interval was corrected with Van de Water’s formula: QTcV = QT−0.087 × (RR−1,000) with RR given in ms (Van de Water et al., 1989). A standard 6-F quad-polar electrodes catheter (Cordis-Webster Inc., Baldwin Park, CA, USA) was positioned at the non-coronary cusp of the aortic valve through the left femoral artery to obtain the His bundle electrogram. A bidirectional steerable MAP recording/pacing combination catheter (1675P, EP Technologies, Inc., Sunnyvale, CA, USA) was positioned at the endocardium of the interventricular septum in the right ventricle through the left femoral vein to obtain MAP signals and to electrically drive the right ventricle. The MAP signals were amplified with a DC preamplifier (model 300, EP Technologies, Inc.). The duration of the MAP signals was measured as an interval, along a horizontal line corresponding to the diastolic baseline, from the MAP upstroke to the desired repolarization level. The interval (ms) at 90% repolarization was defined as MAP90. The heart was electrically driven with a cardiac stimulator (SEC-3102, Nihon Kohden Corporation) via pacing electrodes of the combination catheter placed in the right ventricle. The stimulation pulses were rectangular in shape, 2-2.5 V (about twice the threshold voltage) and of 1-ms duration. The MAP90 of the ventricle was measured during sinus rhythm (MAP90(sinus)) and ventricular pacing at pacing cycle lengths of 400 ms (MAP90(CL400)) and 300 ms (MAP90(CL300)). The ventricular effective refractory period (VERP) was assessed with the following programmed electrical stimulation. The pacing protocol consisted of 5 beats of basal stimuli in a cycle length of 400 ms followed by an extra stimulus of various coupling intervals. Starting in the late diastole, the coupling interval was shortened in 5-ms decrements until the additional stimulus could no longer elicit a response. The VERP was defined as the shortest coupling interval that could produce a response. The duration of terminal repolarization period of the ventricle, reflecting phase-3 repolarization of the action potential, was calculated as the difference between the MAP90(CL400) and VERP at the same site to estimate the extent of local electrical vulnerability of the ventricular muscle (terminal repolarization period=MAP90(CL400)–VERP).
Experimental protocolThe aortic pressure, left ventricular pressure, electrocardiogram, His bundle electrogram and MAP signals were monitored with a polygraph system (RM-6000, Nihon Kohden Corporation) and analyzed by using a real-time, fully automatic data analysis system (Win VAS 3 for Windows ver. 1.1R24v; Physio-Tech Co., Ltd., Tokyo, Japan). Three recordings of consecutive complexes were used to calculate mean for the electrocardiographic indices, MAP duration as well as atrio-His (AH) and His-ventricular (HV) intervals. The cardiovascular variables were assessed in the following order. Firstly, the electrocardiogram, His bundle electrogram, aortic pressure, left ventricular pressure and MAP signals were recorded under sinus rhythm. Secondly, the cardiac output was measured 3 times. Thirdly, the MAP signals were recorded during the ventricular pacing at cycle lengths of 400 and 300 ms. Finally, the VERP was measured. All parameters described above were usually obtained within 2 min at each time point. After the basal assessment, omeprazole (n = 6), lansoprazole (n = 6) or rabeprazole (n = 6) in a low dose of 0.05 mg/kg was intravenously infused over 10 min, and each variable was assessed at 5, 10, 15, 20 and 30 min after the start of administration. Then, each drug in a middle dose of 0.5 mg/kg was intravenously infused over 10 min, and each variable was assessed in the same manner. Finally, each drug in a high dose of 5 mg/kg was infused over 10 min, and each variable was assessed at 5, 10, 15, 20, 30, 45 and 60 min after the start of administration.
DrugsClinically recommended intravenous doses of omeprazole and lansoprazole are 20 mg/body and 30 mg/body, of which Cmax were described as 1,260 ng/mL (3.65 µM) and 2,262 ng/mL (6.12 µM), respectively, according to the information from their manufacturers (AstraZeneca K.K., Osaka, Japan and Teva Takeda Yakuhin Ltd., Nagoya, Japan, respectively). In addition, since the protein binding ratio of omeprazole and lansoprazole was described as 96% and 98% in those manufactures’ information, their free Cmax values could be calculated as 50.4 ng/mL (0.15 µM) and 45.2 ng/mL (0.12 µM), respectively. Given that the body weight of a patient is 50 kg, their therapeutic doses can be calculated as 0.4 mg/kg and 0.6 mg/kg, respectively. Meanwhile, since a clinically recommended oral dose of rabeprazole is 10-20 mg/body and its bioavailability was reported to be 51.8% in the information from the manufacture (Eisai Co., Ltd., Tokyo, Japan), the intravenous dose could be roughly estimated as 0.1-0.2 mg/kg. Also, Cmax of rabeprazole following a single intravenous administration of 20 mg/body to healthy subjects was reported to be 1,309 ng/mL (3.64 µM) (Wang et al., 2011). Accordingly, we set the doses of 0.05, 0.5 and 5 mg/kg as subtherapeutic, clinically-relevant and supratherapeutic doses, respectively, for assessing those PPIs in this study. Omeprazole sodium hydrate (Omepral® Injection20, AstraZeneca K.K.) was purchased and diluted with saline to 5 mg/mL as omeprazole. Lansoprazole and rabeprazole sodium were provided by Astellas Pharma Inc. (Tokyo, Japan). Lansoprazole was dissolved in 0.1 M of NaOH solution, which was neutralized with 0.1 M of HCl solution and diluted with saline to 5 mg/mL. Rabeprazole sodium was dissolved with saline to 5 mg/mL as rabeprazole. These solutions were diluted with saline to 0.05 and 0.5 mg/mL for low and middle doses, respectively. Each drug solution was intravenously infused at a rate of 1 mL/kg/min over 10 min.
Statistical analysisData are presented as mean ± S.E. Differences within a parameter were evaluated with one-way, repeated-measures analysis of variance (ANOVA) followed by Contrast as a post hoc-test for mean values comparison. Meanwhile, differences in each parameter at pre-treatment basal control among groups were assessed with one-way, ordinary ANOVA followed by Tukey’s test as a post hoc-test for multiple comparison. These statistical analyses were performed by using GraphPad prism 8 (ver. 8.43, GraphPad Software, LLC, La Jolla, CA, USA). A p value < 0.05 was considered to be significant.
No animals exerted any lethal ventricular arrhythmia or hemodynamic collapse leading to their death during the experimental period. No significant difference was observed among groups in any of the pre-treatment basal control values.
Effects on cardiohemodynamic variablesThe time courses of changes in the heart rate, mean blood pressure, cardiac output, total peripheral vascular resistance, LVdP/dtmax and left ventricular end-diastolic pressure are shown in Fig. 1. Their pre-treatment control values (C) in each treatment group are summarized in Table 1.

Time courses of changes in the heart rate (HR), mean blood pressure (MBP), cardiac output (CO), total peripheral vascular resistance (TPR), maximum upstroke velocity of the left ventricular pressure (LVdP/dtmax) and left ventricular end-diastolic pressure (LVEDP) after the start of administration of 0.05, 0.5 and 5 mg/kg of omeprazole (left), lansoprazole (center) and rabeprazole (right). Data are presented as mean ± S.E. (n = 6 for each drug). Closed symbols represent statistically significant differences from each pre-treatment basal control value (C) by p < 0.05.
| Omeprazole | Lansoprazole | Rabeprazole | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Heart rate (bpm) | 117 | ± | 6 | 121 | ± | 7 | 117 | ± | 3 |
| Mean blood pressure (mmHg) | 119 | ± | 9 | 122 | ± | 4 | 111 | ± | 5 |
| Cardiac output (L/min) | 1.50 | ± | 0.25 | 1.66 | ± | 0.23 | 1.76 | ± | 0.10 |
| Total peripheral vascular resistance (mmHg·min/L) | 86 | ± | 9 | 78 | ± | 8 | 64 | ± | 4 |
| LVdP/dtmax (mmHg/s) | 2,311 | ± | 282 | 2,202 | ± | 227 | 2,180 | ± | 120 |
| Left ventricular end-diastolic pressure (mmHg) | 11 | ± | 1 | 9 | ± | 2 | 12 | ± | 2 |
| PR interval (ms) | 100 | ± | 3 | 109 | ± | 8 | 101 | ± | 6 |
| QRS width (ms) | 63 | ± | 2 | 63 | ± | 3 | 62 | ± | 3 |
| QT interval (ms) | 242 | ± | 3 | 257 | ± | 13 | 242 | ± | 12 |
| QTcV | 284 | ± | 4 | 300 | ± | 12 | 284 | ± | 12 |
| AH interval (ms) | 76 | ± | 3 | 76 | ± | 6 | 74 | ± | 5 |
| HV interval (ms) | 28 | ± | 3 | 31 | ± | 1 | 30 | ± | 1 |
| MAP90(sinus) (ms) | 214 | ± | 5 | 239 | ± | 15 | 229 | ± | 10 |
| MAP90(CL400) (ms) | 218 | ± | 5 | 244 | ± | 14 | 224 | ± | 7 |
| MAP90(CL300) (ms) | 203 | ± | 4 | 222 | ± | 10 | 206 | ± | 6 |
| VERP (ms) | 183 | ± | 7 | 198 | ± | 9 | 191 | ± | 9 |
| Terminal repolarization period (ms) | 35 | ± | 4 | 47 | ± | 6 | 33 | ± | 4 |
Data are presented as mean ± S.E. (n = 6 for each group). LVdP/dtmax: maximum upstroke velocity of the left ventricular pressure; QTcV: QT interval corrected with Van de Water's formula; MAP90: monophasic action potential duration at 90% repolarization level; and VERP: ventricular effective refractory period
The low dose of omeprazole decreased the total peripheral vascular resistance for 10-20 min after the start of administration, whereas no significant change was detected in the other variables. Its middle dose increased the heart rate for 10-30 min and cardiac output for 5-30 min, but further decreased the total peripheral vascular resistance for 5-30 min, whereas no significant change was observed in the other variables. Its high dose kept the heart rate and cardiac output increased for 5-20 min, but made the total peripheral vascular resistance decreased for 5-10 min followed by increase at 60 min, whereas no significant change was found in the other variables. Thus, the low and middle doses of omeprazole altered the heart rate, cardiac output and total peripheral vascular resistance in a dose-related manner, whereas those effects were plateaued after the high dose administration and returned to their basal levels.
The low dose of lansoprazole decreased the total peripheral vascular resistance at 20 min, but increased the LVdP/dtmax for 20-30 min after the start of administration, whereas no significant change was detected in the other variables. Its middle dose increased the heart rate for 15-30 min, cardiac output and LVdP/dtmax for 5-30 min, but decreased the total peripheral vascular resistance for 5-30 min and left ventricular end-diastolic pressure for 20-30 min, whereas no significant change was observed in the mean blood pressure. Its high dose decreased the heart rate for 45-60 min, and kept the total peripheral vascular resistance decreased and the cardiac output increased at 10 and 20 min. It tended to decrease the LVdP/dtmax when compared with that at 30 min after the middle dose, although significant increase from basal control level was detected for 5-15 min. It hardly altered the mean blood pressure or left ventricular end-diastolic pressure. Thus, the low and middle doses of lansoprazole altered the heart rate, cardiac output, total peripheral vascular resistance, LVdP/dtmax and left ventricular end-diastolic pressure in a dose-related manner, whereas those effects were returned to their basal levels after the high dose administration except that the heart rate decreased significantly below the basal level.
The low dose of rabeprazole decreased the mean blood pressure for 15-30 min, whereas no significant change was detected in the other variables. Its middle dose also reduced the mean blood pressure at 15 and 30 min, whereas no significant change was observed in the other variables. Its high dose decreased the heart rate at 45 min and the mean blood pressure for 5-30 min, and tended to decrease the LVdP/dtmax for 5-20 min, whereas no significant change was found in the other variables.
Effects on electrophysiological variablesThe time courses of changes in the PR interval, QRS width, QT interval and QTcV; and the AH interval, HV interval, MAP90(sinus), MAP90(CL400), MAP90(CL300), VERP and terminal repolarization period are shown in Figs. 2 and 3, respectively. Their pre-treatment control values (C) in each treatment group are summarized in Table 1. Typical tracings of the His bundle electrogram, electrocardiogram, aortic pressure, left ventricular pressure and MAP signals during the programmed electrical stimulation on the right ventricle for the assessment of VERP in the omeprazole-treated animal are depicted in Fig. 4.

Time courses of changes in the PR interval (PR), QRS width (QRS), QT interval (QT) and QTcV after the start of administration of 0.05, 0.5 and 5 mg/kg of omeprazole (left), lansoprazole (center) and rabeprazole (right). QTcV = QT−0.087 × (RR−1,000) with RR given in ms (Van de Water et al., 1989). Data are presented as mean ± S.E. (n = 6 for each drug). Closed symbols represent statistically significant differences from each pre-treatment basal control value (C) by p < 0.05.

Time courses of changes in the AH interval (AH), HV interval (HV), MAP90 during sinus rhythm (MAP90(sinus)), MAP90 during ventricular pacing at cycle lengths of 400 ms (MAP90(CL400)) and 300 ms (MAP90(CL300)), ventricular effective refractory period (VERP) and terminal repolarization period (TRP) after the start of administration of 0.05, 0.5 and 5 mg/kg of omeprazole (left), lansoprazole (center) and rabeprazole (right). MAP90: monophasic action potential duration at 90% repolarization level. TRP = MAP90(CL400)–VERP. Data are presented as mean ± S.E. (n = 6 for each drug). Closed symbols represent statistically significant differences from each pre-treatment basal control value (C) by p < 0.05.
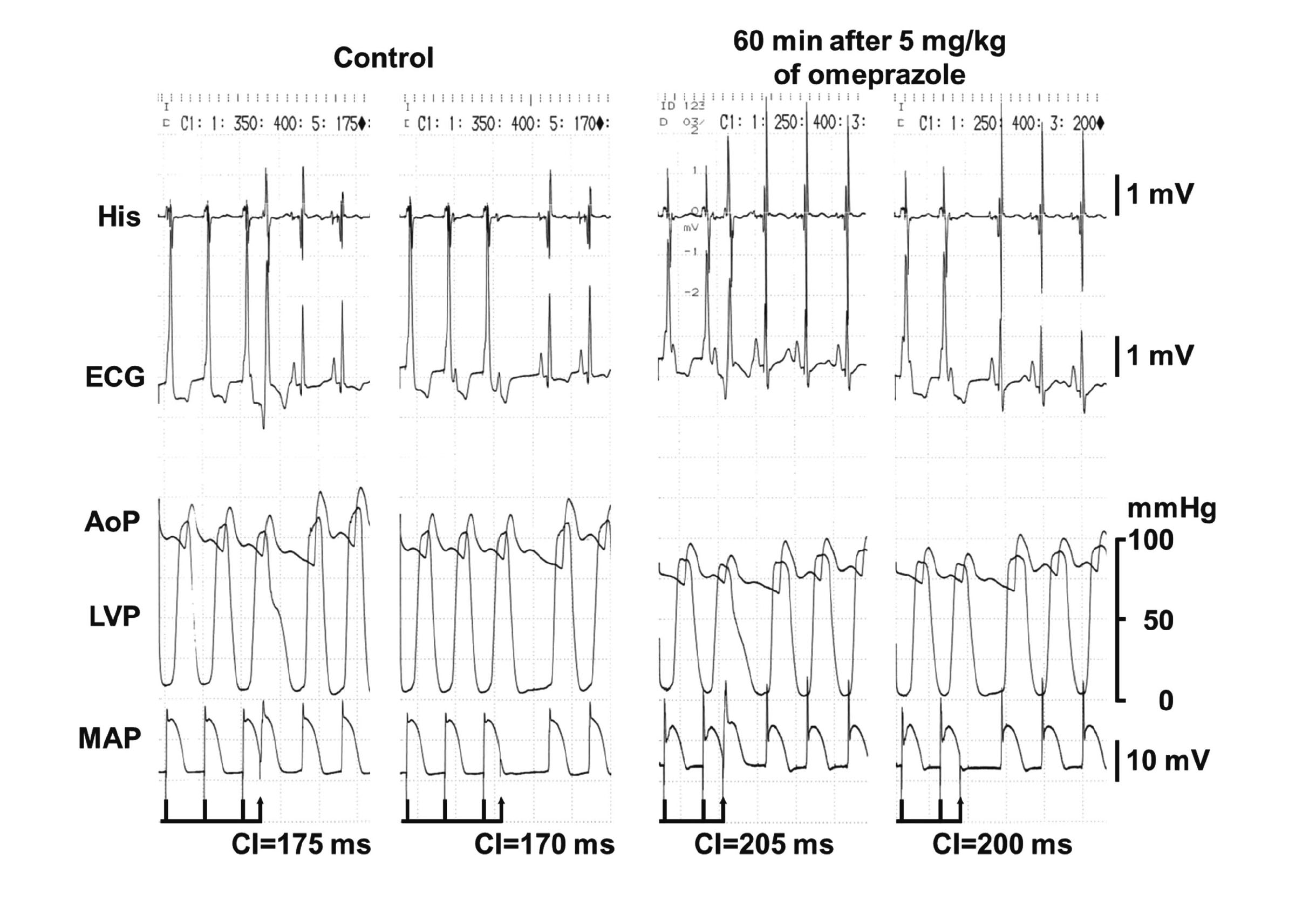
Typical tracings of the His bundle electrogram (His), electrocardiogram (ECG), aortic pressure (AoP), left ventricular pressure (LVP) and monophasic action potential (MAP) during programed electrical stimulation for the assessment of ventricular effective refractory period (VERP) at pre-drug control (Control) and 60 min after the start of intravenous administration of 5 mg/kg of omeprazole (60 min after 5 mg/kg of omeprazole). At Control, a premature right ventricular stimulation with a coupling interval (CI) of 175 ms following basal stimuli induced electrical activities in ECG and MAP (left panel), whereas that of 170 ms failed to develop either of them (right panel), indicating that the VERP was 175 ms. Sixty min after 5 mg/kg of omeprazole, the right ventricular stimulation with a CI of 205 ms induced the electrical activities in ECG and MAP (left panel), whereas that of 200 ms did not develop either of them (right panel), indicating that the VERP was 205 ms.
The low dose of omeprazole hardly altered any of variables. Its middle dose prolonged the QRS width for 10-30 min, whereas no significant change was detected in the other variables. Its high dose prolonged the QRS width and VERP for 5-60 min, but shortened the terminal repolarization period for 15-60 min, whereas no significant change was observed in the other variables.
The low or middle dose of lansoprazole hardly altered any of the variables. Its high dose prolonged the MAP90(sinus) for 30-60 min and VERP at 5 min and for 20-60 min, whereas no significant change was detected in the other variables.
The low dose of rabeprazole hardly altered any of the variables. Its middle dose prolonged the QT interval at 30 min and QTcV at 5 min and for 20-30 min, whereas no significant change was found in the other variables. Its high dose prolonged the QT interval and QTcV for 5-60 min and VERP at 5 min and for 15-60 min, whereas no significant change was detected in the other variables.
In order to bridge the information gap between previous in vitro studies and clinical investigation, we examined in vivo acute cardiovascular effects of omeprazole, lansoprazole and rabeprazole using halothane-anesthetized dogs for estimating their direct and reflex-mediated indirect mechanisms of actions. Those PPIs in subtherapeutic to supratherapeutic doses exerted multifarious cardiohemodynamic and electrophysiological effects in vivo, which were discussed as below.
Cardiohemodynamic effectsThe low and middle doses of omeprazole and lansoprazole decreased the total peripheral vascular resistance, indicating their vasodilator effects in vivo, which could be partly explained by a previous in vitro study that omeprazole and lansoprazole relaxed the human internal mammary artery precontracted with phenylephrine in a concentration-related manner with IC50 values of 142 and 186 µM, respectively (Naseri and Yenisehirli, 2006). The vasodilator action of omeprazole on the rat aorta was attenuated by pretreatment of L-NAME, nitric oxide (NO) synthase inhibitor (Kelicen et al., 2002), whereas lansoprazole-induced relaxation on the isolated human internal mammary artery was hardly affected by L-NAME (Naseri and Yenisehirli, 2006). Moreover, in isolated cardiac myocytes of guinea pigs, 300 µM of omeprazole inhibited ICaL by 39% (Nagashima et al., 1999). Also, 300 µM of lansoprazole completely inhibited Ca2+-induced contraction of the isolated human internal mammary arteries (Naseri and Yenisehirli, 2006), also indicating its ICaL inhibitory action. Thus, our findings may reflect that the subtherapeutic and clinically-relevant doses of omeprazole can dilate the vessels possibly through both NO generation and ICaL inhibition, whereas those of lansoprazole may reduce the vascular resistance mainly through the latter. However, estimated free plasma concentrations after the administration of omeprazole and lansoprazole in this study would be much smaller than those reported for inhibiting ICaL in vitro, suggesting that the other mechanisms might be also associated with their vasodilator effects. Meanwhile, the total peripheral vascular resistance began to increase after the high dose of omeprazole and lansoprazole administration, suggesting that other vasoconstrictor mechanisms might have counteracted those direct actions, which needs to be elucidated.
The middle to high doses of omeprazole significantly increased the heart rate along with moderate elevation of the left ventricular contractility, whereas the middle dose of lansoprazole significantly increased the heart rate and left ventricular contractility along with the mitigation of left ventricular preload. The vasodilator action of omeprazole and lansoprazole discussed above is considered to trigger the reflex-mediated increase of sympathetic tone, which would overcome omeprazole and lansoprazole-induced direct cardiac suppression. Thus, the increase of the heart rate and left ventricular contraction may increase the cardiac output, maintaining the blood pressure around the basal control level. The high dose of omeprazole and lansoprazole returned the heart rate, left ventricular contractility and cardiac output to their basal control level, partly suggesting their cardiac ICaL inhibitory action. Since the negative chronotropic action was more potent for lansoprazole than for omeprazole, the magnitude of inhibitory action on the sinus automaticity might be greater for lansoprazole.
Rabeprazole tended to reduce the total peripheral vascular resistance and significantly decreased the mean blood pressure in a dose-related manner, possibly inducing the reflex-mediated increase of sympathetic tone. However, the low and middle doses of rabeprazole did not increase the heart rate or left ventricular contractility, indicating that positive chronotropic and inotropic action via the reflex-mediated increase of sympathetic tone may be counteracted by its direct suppressive action on the heart. Moreover, the high dose of rabeprazole decreased the heart rate and tended to decrease the left ventricular contraction, suggesting that its direct suppressive action on the heart, which would be possibly induced through ICaL inhibition, may have overcome the indirect stimulatory action by the sympathetic reflex.
These results suggest that PPIs may have common cardiovascular profile that includes the vasodilatory effect and negative chronotropic as well as inotropic action. It should be noted that their magnitude of vasodilatory effect and cardio-suppressive action might be different in vivo one another; namely, the former may be in the order of omeprazole ≈lansoprazole > rabeprazole, and the latter will be rabeprazole > lansoprazole ≥ omeprazole.
Electrophysiological effectsThe single administration of omeprazole and lansoprazole tended to prolong the QT interval and QTcV, and that of rabeprazole significantly but mildly prolonged them in a dose-related manner, indicating that they can directly modulate the cardiac ionic currents in vivo. The extents of prolongation of MAP90(sinus) (at a rate of 104-135 bpm), MAP90(CL400) (150 bpm) and MAP90(CL300) (200 bpm) were +19 ms, +7 ms and +4 ms for omeprazole; +31 ms, +7 ms and +1 ms for lansoprazole; and +15 ms, +13 ms and +13 ms for rabeprazole at 60 min after the administration of their high dose, respectively. Since IKr inhibitory action is known to be greater at slower beating rate; namely, reverse frequency-dependent blockade (Jurkiewicz and Sanguinetti, 1993), those findings suggest that the IKr inhibition may play a major role for both the omeprazole and lansoprazole-induced prolongation of the ventricular repolarization period in vivo. In a previous patch-clamp assay, omeprazole in concentrations of 10 µM and 100 µM inhibited IKr expressed in HEK293 cells by 11% and 32%, whereas those of lansoprazole did it by 23% and 51%, respectively (Lazzerini et al., 2021). Their supra-therapeutic doses in this study would provide free plasma concentrations of approximately 1.5 µM and 1.2 µM, respectively, which were still several times smaller than those for significantly inhibiting IKr in vitro. Accordingly, in the case of single administration, IKr inhibition by omeprazole or lansoprazole alone may not fully explain the QT-interval and QTc prolongations. Thus, it would be possible that the other mechanisms might be involved in those prolongations, including hERG-trafficking inhibition (Yokoyama et al., 2009) and IK1 suppression (Takanari et al., 2013). Meanwhile, rabeprazole prolonged the ventricular repolarization period in a frequency-independent manner, suggesting that rabeprazole might have inhibited not only IKr but also other outward current, including the slow component of delayed rectifier K+ current (IKs), in vivo (Bosch et al., 1998). The high dose of omeprazole, lansoprazole and rabeprazole might have potential to modestly block inward cardiac ICaL in vivo as discussed above, partly counteracting the prolonging effects of those drugs on the ventricular repolarization period (Shiina et al., 2000; Liu et al., 2012), which may limit excessive QT-interval prolongation. In addition, omeprazole delayed the intra-ventricular conduction, indicating INa inhibitory action in vivo. Lack of significant prolongation of the PR interval or AH interval suggests that PPIs-induced direct inhibitory action on the atrioventricular node might be well-balanced by the reflex-mediated increase of sympathetic tone in vivo.
In our previous studies using the same protocol, amiodarone, dronedarone, donepezil and oseltamivir prolonged the QTcV by 19, 41, 42 and 20 (Matsukura et al., 2017; Motokawa et al., 2018; Hagiwara-Nagasawa et al., 2021; Kitahara et al., 2013). Meanwhile, these drugs did not induce TdP in the chronic atrioventricular block dogs, one of the well-established proarrhythmia models (Yoshida et al., 2002; Kambayashi et al., 2021; Hagiwara-Nagasawa et al., 2021; Nakamura et al., 2016). Since in this study, the extents of the maximum prolongation of QTcV induced by the high dose of omeprazole (+16), lansoprazole (+9) and rabeprazole (+15) were less great than those by amiodarone, dronedarone, donepezil and oseltamivir, potential torsadogenic risk of those PPIs may be low.
The high dose of omeprazole, lansoprazole and rabeprazole significantly prolonged the VERP along with the slight prolongation of MAP90(CL400), which shortened or hardly altered the terminal repolarization period. The terminal repolarization period is known to reflect phase-3 repolarization, prolongation of which will increase the chance of conduction delay at less complete repolarization level when the triggered activity occurs, facilitating the onset of re-entrant ventricular arrhythmias (Sugiyama, 2008; Sugiyama and Hashimoto, 2002). The maximum changes in the terminal repolarization period were −18 ms, −16 ms and +8 ms after the administration of omeprazole, lansoprazole and rabeprazole, respectively, suggesting that omeprazole and lansoprazole may have a potential to prevent the onset of re-entrant ventricular arrhythmias. The shortening of terminal repolarization period by omeprazole largely depended on the prolongation of VERP, for which its INa inhibitory action as described above would play an important role.
Clinical implication and study limitationThe single administration of omeprazole, lansoprazole and rabeprazole mildly prolong the QT interval in halothane-anesthetized dogs, suggesting that these PPIs by themselves may have a potential to similarly exert QT-interval prolongation in healthy human subjects. Thus, PPIs should be administered with caution in patients having a decreased repolarization reserve due to congenital long QT syndrome, structural cardiovascular disease, concomitant use of drugs that may enhance the QT-interval prolongation and/or electrolyte disturbance, including hypomagnesemia (Varró and Baczkó, 2011; Roden, 2004; Nachimuthu et al., 2012). On the other hand, there are some limitations in this study. First, we did not directly assess the molecular mechanisms of cardiovascular effects of PPIs using the in vitro approach. Such information will directly explain the PPIs-induced cardiovascular adverse effects. Second, we did not compare the acute effects of PPIs with their chronic ones using the same animal model. Such information would help better understand which PPIs' effects play a more important role in the onset of QT-interval prolongation and ventricular arrhythmias.
ConclusionThe current translational study of cardiovascular adverse events indicates that omeprazole, lansoprazole and rabeprazole can exert multifarious cardiohemodynamic and electrophysiological actions on halothane-anesthetized dogs, including mild QT-interval prolongation. Thus, caution has to be paid regarding patients who have decreased repolarization reserve, when PPIs are prescribed with single as well as repeated dosing.
The authors thank Mrs. Yuri Ichikawa for her technical assistances during preparation of the manuscript. This study was supported in part by research grants from Astellas Pharma Inc., and from Japan Society for the Promotion of Science (JSPS KAKENHI) grant number 20K16136 (to R.K.).
Conflict of interestThe authors declare that there is no conflict of interest.