2023 年 48 巻 7 号 p. 399-409
2023 年 48 巻 7 号 p. 399-409
Fc-engineering is commonly used to improve the therapeutic potency of antibody (Ab) treatments. Because FcγRIIb is the only inhibitory FcγR that contains an immunoreceptor tyrosine-based inhibition motif (ITIM), Fc-engineered Abs with enhanced binding affinity to FcγRIIb might provide immune suppression in clinical contexts. GYM329 is an anti-latent myostatin Fc-engineered Ab with increased affinity to FcγRIIb which is expected to improve muscle strength in patients with muscular disorders. Cross-linking of FcγRIIb by immune complex (IC) results in phosphorylation of ITIM to inhibit immune activation and apoptosis in B cells. We examined whether the IC of Fc-engineered Abs with enhanced binding affinity to FcγRIIb causes phosphorylation of ITIM or B cell apoptosis using GYM329 and its Fc variant Abs in human and cynomolgus-monkey (cyno) immune cells in vitro. IC of GYM329 with enhanced binding affinity to human FcγRIIb (×5) induced neither ITIM phosphorylation nor B cell apoptosis. As for GYM329, FcγRIIb should work as an endocytic receptor of small IC to sweep latent myostatin, so it is preferable that GYM329 induces neither ITIM phosphorylation nor B cell apoptosis to prevent immune suppression. In contrast, IC of myo-HuCy2b, the Ab with enhanced binding affinity to human FcγRIIb (×4), induced ITIM phosphorylation and B cell apoptosis. The result of the present study demonstrated that Fc-engineered Abs with similar binding affinity to FcγRIIb had different effects. Thus, it is important to also investigate FcγR-mediated immune functions other than binding to fully understand the biological effects of Fc-engineered Abs.
Fc-engineering is commonly used to improve the therapeutic potency of antibody (Ab) treatments (Natsume et al., 2009; Yamin et al., 2021; Kang and Jung, 2019). The Fc domain of an Ab interacts with FcγRs containing immunoreceptor tyrosine-based activation motifs (ITAM), which induces antibody-dependent cell mediated cytotoxicity (ADCC), and antibody-dependent cell-mediated phagocytosis (ADCP) (Takai, 2002). Fc-engineered Abs with enhanced binding affinity to FcγRs showed superior anti-tumor (Kern et al., 2013) and anti-virus efficacy (Yamin et al., 2021). Fc-engineered Abs with increased affinity to neonatal Fc receptor (FcRn) showed prolonged circulation half-life (Mackness et al., 2019; Saxena and Wu, 2016; Monnet et al., 2015). FcγRIIb on liver sinusoidal endothelial cells (LSEC) clears small immune complexes (ICs) (Ganesan et al., 2012). Abs with increased binding affinity to FcγRIIb showed faster clearance of target antigens than natural-type Abs (Igawa et al., 2013). FcγRIIb is the only inhibitory FcγR that contains an immunoreceptor tyrosine-based inhibition motif (ITIM) that controls immune and inflammatory responses (Smith and Clatworthy, 2010).
There are some disadvantages to Fc-engineering. Fc-engineered Abs that enhance the effector function via activating-type FcγRs are associated with a higher risk of severe immune-related adverse events such as cytokine release syndrome (Sylvia Herter, 2018). Thus, we think it is reasonable to use FcRn or FcγRIIb in cases where strong immune responses should be avoided. Because FcγRIIb is an inhibitory FcγR, anti-CD19 Fc-engineered Abs with increased affinity for FcγRIIb had stronger inhibitory potency than anti-CD19 IgG1 against B cells, and have been successfully applied to treatments for autoimmune diseases (Chu et al., 2008; Horton et al., 2011; Chu et al., 2020). On the other hand, Fc-engineered Abs with enhanced binding affinity to FcγRIIb might suppress immunity too much for some clinical indications.
GYM329 is an anti-latent myostatin Fc-engineered IgG1 Ab with increased affinity to FcγRIIb. Myostatin is a negative regulator of muscle growth and strength, and several myostatin inhibitors have been developed as therapeutic agents for muscle disorders such as muscular dystrophy and atrophy (Rybalka et al., 2020; Nielsen et al., 2021; Barrett et al., 2021). GYM329 inhibits the activation of latent myostatin via the Fab domain and sweeps myostatin in the muscle and plasma via the Fc domain (Muramatsu et al., 2021). IC composed of GYM329 and latent myostatin is readily internalized into LSEC and/or immune cells by FcγRIIb, and then latent myostatin is effectively degraded (Muramatsu et al., 2021; Hori et al., 2022). GYM329 is expected to have better efficacy than conventional anti-myostatin agents. After forming IC of GYM329 and latent myostatin, the cross-linking of FcγRIIb by IC might result in the phosphorylation of ITIM, which can inhibit the function of activating receptors in immune cells such as B cells, dendritic cells, macrophages, and activated neutrophils (Takai, 2002; Veri et al., 2010). Cross-linking of FcγRIIb also induced apoptosis in B cells (Pearse et al., 1999). Thus, we decided that GYM329 should be examined for immune suppression before clinical trials.
Previous studies revealed differences between cynomolgus monkey (cyno) and human IgG in FcγR binding and effector functions (Jacobsen et al., 2011; Warncke et al., 2012). It is important to carefully interpret preclinical data obtained from cynos with human Ab therapeutics. GYM329 binds to human FcγRIIb but does not show enhanced binding to cyno FcγRIIb. Therefore, we used myo-HuCy2b, a surrogate Ab with high affinity for cyno FcγRIIb in this study.
The aim of this study is to examine whether IC of Fc-engineered Abs with enhanced binding affinity to FcγRIIb cause phosphorylation of ITIM or B cell apoptosis using GYM329, its Fc variant Abs, and myo-HuCy2b in human and cyno immune cells in vitro.
GYM329 is an anti-latent myostatin Ab with a modified Fc to enhance its binding affinity to human FcγRIIb; myo-HuCy2b is a surrogate Ab against latent myostatin with increased affinity for human/cyno FcγRIIb; myo-Hu2b is an anti-latent myostatin Ab with a modified Fc to enhance its binding affinity to human FcγRIIb; and myo-IgG1 is an anti-latent myostatin with natural human IgG1 constant region. All Fab regions of anti-latent myostatin Abs used in this study were identical. CD19-Hu2b or CD19-Cy2b was used as a positive control in humans or cynos. CD19-Hu2b is an anti-human CD19 Ab with a modified Fc to enhance its binding affinity to human FcγRIIb, and CD19-Cy2b is an anti-cyno CD19 Ab with a modified Fc to enhance its binding affinity to cyno FcγRIIb. A previous study reported that anti-CD19 mAb with strong binding affinity to human FcγRIIb induced ITIM phosphorylation and apoptosis in B cells (Chu et al., 2008; Horton et al., 2011). GYM329 was stably expressed using Chinese hamster ovary cells, and myo-HuCy2b, myo-Hu2b, myo-IgG1, CD19-Hu2b and CD19-Cy2b were transiently expressed from the FreeStyle293-F cell line (Thermo Fisher Scientific, MA, USA). Antibodies were purified from the medium with the protein A affinity chromatography followed by additional purification steps as needed. Recombinant latent myostatin was prepared as described previously (Muramatsu et al., 2021). ICs were prepared from equimolar amounts of Ab and antigen, and ICs were incubated for an hour in RPMI1640 (Nacalai Tesque, Kyoto, Japan) with 10% FBS (Thermo Fisher Scientific) at room temperature. Palivizumab (Synagis) was purchased from AstraZeneca (Cambridge, UK) and was used as a negative control. Palivizumab is a humanized IgG1 targeting the RSV, and no cases of FcγRIIb-mediated clinical immunosuppression have been reported (Chen et al., 2015). Trastuzumab was purchased from Chugai Pharmaceutical (Tokyo, Japan) and was used as an IgG1 control in Biacore analysis.
Biacore analysisThe affinity of each Ab binding to human and cyno latent myostatin at pH 7.4 was determined at 25°C using Biacore T200 (GE Healthcare, IL, USA). Protein L (BioVision, MA, USA) was immobilized onto all flow cells of Biacore sensor chip CM5 (GE Healthcare) using Amine Coupling Kit type2 (GE Healthcare). Each Ab was captured onto the sensor surface by Protein L. Human and cyno latent myostatin was injected at 2 to 32 nM prepared by two-fold serial dilution. The sensor surface was regenerated each cycle by injecting Glycine 1.5 (10 mmol/L glycine–HCl, pH 1.5, GE Healthcare). Kinetic parameters were determined by fitting the data to 1:1 binding model using Biacore T200 Evaluation Software Version 2.0 (GE Healthcare).
Regarding the binding affinity of GYM329, myo-HuCy2b, and myo-IgG1 to human and cyno FcγRIIb at pH 7.4 was determined at 20°C using Biacore T200. Protein L was immobilized onto all flow cells of Biacore sensor chip CM5 using Amine Coupling Kit type2. Each antibody was captured onto the sensor surface by Protein L. Human and cyno FcγRIIb were injected at 1000 nM. The sensor surface was regenerated each cycle by injecting Glycine 1.5. Regarding the binding affinity of myo-Hu2b, CD19-Cy2b, and myo-IgG1 to human and cyno FcγRIIb at pH 7.4 were determined at 25°C using Biacore T200. Protein A/G (PIERCE, Merk, Darmstadt, Germany) was immobilized onto all flow cells of Biacore sensor chip CM5 using Amine Coupling Kit type2. Human and cyno FcγRIIb were injected at 250 nM. The sensor surface was regenerated each cycle by injecting 25 mM NaOH and Glycine 1.5. The amount of human and cyno FcγRIIb binding to Abs (per 1RU) was measured using Biacore T200 Evaluation Software Version 2.0 (GE Healthcare).
Peripheral blood mononuclear cells (PBMCs)Peripheral blood samples were donated by healthy volunteers after informed consent had been obtained, and the samples were anonymized. The use of human-derived test materials was approved by the Research Ethics Committee of Chugai Pharmaceutical Co., Ltd. Fresh cyno blood used in this study was collected from untreated animals. All procedures associated with this study were reviewed and approved by the Institutional Animal Care and Use Committee at Chugai Pharmaceutical Co., Ltd. The test facility has been accredited by AAALAC international. The samples of heparinized blood (heparin 10 U per 1 mL blood) (Heparin sodium, AY Pharmaceuticals, Tokyo, Japan) were kept at room temperature, and PBMCs were isolated from the blood samples by density gradient separation (Ficoll-Paque PLUS, GE Healthcare). Then, PBMCs were washed with D-PBS and the collected PBMCs were counted and used for the assay.
Isolation of human or cyno B cellsHuman B cells were isolated from PBMCs using the EasySep negative B cell enrichment kit (STEMCELL Technologies Inc., Vancouver, Canada) according to the manufacturer's instructions. Cyno B cells were isolated from PBMCs using CD20 Microbeads, non-human primate (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. Then, the collected B cell-enriched cell suspensions were counted and used for the assay.
Phosphorylation of FcγRIIb ITIM in Human or cyno PBMCs (Western Blotting)Human or cyno PBMCs were suspended at 107 cells/mL in RPMI1640 (Nacalai Tesque, Kyoto, Japan) and treated with test articles at 100 μg/mL for 5 min at 37°C. After incubation, cells were washed twice with cold D-PBS, then lysed with Lysis buffer 17 (R&D Systems, MN, USA) containing 1% PIC-2, 3 (ITSI-Biosciences, PA, USA; Sigma-Aldrich, MO, USA) at 2 × 107 cells/mL. Lysates were centrifuged for 5 min at 14000 × g, then supernatants of lysates were collected and stored in a freezer until analysis. For western blotting, approximately 5 μg of proteins were separated by SDS-PAGE (4%–20%) and transferred to a PVDF membrane (Bio-Rad Laboratories, CA, USA). The membrane was blocked by PVDF Blocking Reagent (Toyobo, Osaka, Japan) overnight in a refrigerator and then probed with anti-pCD32B (Abcam, Cambridge, UK) or anti-human/cyno CD32B (Abcam; Sino Biological, Beijing, China) as primary antibodies. Then, after 1 hr incubation at RT, the proteins were probed using HPR-conjugated secondary antibody (Cell Signaling Technology, MA, USA) and visualized with ImageQuant LAS-4000 (FUJI FILM, Tokyo, Japan) and ImageJ (NIH, MD, USA). FcγRIIb was detected at approximately 40 kDa in Western blotting under reducing condition. The phosphorylation levels of ITIM were shown as fold changes from palivizumab. PBMCs from n ≥ 3 donors were used for each treatment. PBMCs from n = 5 cynos were used for each treatment. The distribution of the dataset did not follow normal distribution (Anderson-Darling test, p < 0.05), and the statistical significance was examined with non-parametric Dunn’s test (p < 0.05) to compare several groups with the control group, palivizumab.
B Cell Apoptosis AssayThe B cell apoptosis assay was performed as described by Chu with minor modifications (Chu et al., 2008). Briefly, isolated B cells were re-suspended (0.5–1 × 105 cells/well) in RPMI1640 with 10% FBS and seeded in a 96-well round-bottomed culture plate (100 μL/well) with 1 μg/mL anti-CD79b antibody (clone: 3A2-2E7, BD, NJ, USA) or 10 μg/mL Class B CpG (InvivoGen, CA, USA) for human or cyno B cell activation. To test the effects of Fc-engineered antibodies on B cells via FcγRIIb, serial dilutions of antibodies or ICs were co-incubated with B cell suspensions at 37°C with 5% CO2 overnight. Then, B cells were stained with Annexin V-FITC/7AAD Kit (Beckman Coulter, CA, USA) and with anti-surface marker mAbs (anti-CD45 [BD], anti-CD19 [Beckman Coulter], and anti-CD20 [SONY, Tokyo, Japan]) at 4°C for 15 min. Annexin V+ 7AAD low cells were determined as apoptotic cells, and B cells were defined as CD45+CD19+ and/or CD20+ cells. After washing with binding buffer, B cells were re-suspended in 250 μL of Binding Buffer with 1% paraformaldehyde (FUJIFILM Wako Pure Chemical, Tokyo, Japan) and analyzed with the flow cytometer LE-SP6800Z (SONY). The increase in apoptosis was calculated by subtracting the percentage of apoptosis with no treatment from the percentages with test articles. PBMCs from n ≥ 3 donors were used for each treatment. PBMCs from n = 5 cynos were used for each treatment. Assuming that the distribution of each dataset follows normal distribution (Anderson-Darling test, p > 0.05), significance of the difference in distribution between test articles and palivizumab was examined with parametric Dunnett’s Multiple Comparison Test (p < 0.05).
Binding of GYM329/myo-HuCy2b or their immune complex to human B cellsGYM329, myo-HuCy2b, and human latent myostatin (myo) were labeled with Alexa Fluor (AF) 647 Monoclonal Antibody Labeling Kit (Thermo Fisher Scientific). PBMCs from 3 donors were incubated (1 × 105 cells/well) with test articles (100 μg/mL) in EasySep Buffer (STEMCELL Technologies) for 1 hr at 4°C. Then, surface marker antibodies (anti-CD45, anti-CD19) were added and incubated for 30 min at 4°C. After washing, PBMCs were re-suspended in 1% paraformaldehyde-PBS and analyzed with LE-SP6800Z. B cells were defined as CD45+CD19+ cells. The binding to B cells were shown as geometric means of AF647 in B cells.
Binding properties of each Ab to FcγRIIb are indicated in Table 1. GYM329 showed enhanced binding to human FcγRIIb (×5), but not to cyno FcγRIIb (×1). myo-HuCy2b, a surrogate Ab against latent myostatin with increased affinity for human/cyno FcγRIIb, showed enhanced binding to both human (×4) and cyno (×2) FcγRIIb as expected. GYM329 and myo-HuCy2b were similar in the shape of sensorgrams (Fig. S1). Because the Fc region of myo-Hu2b is identical to that of CD19-Hu2b, the binding affinities of the two Abs to human FcyRIIb are expected to be the same. The binding of myo-IgG1 to human (×0.8) and cyno (×0.8) FcγRIIb are comparable to those of control IgG1, trastuzumab (×1), and palivizumab (×1). All Fab regions of anti-latent myostatin Abs were identical and their binding affinity to human latent myostatin is comparable to that to cyno latent myostatin (Table 2).

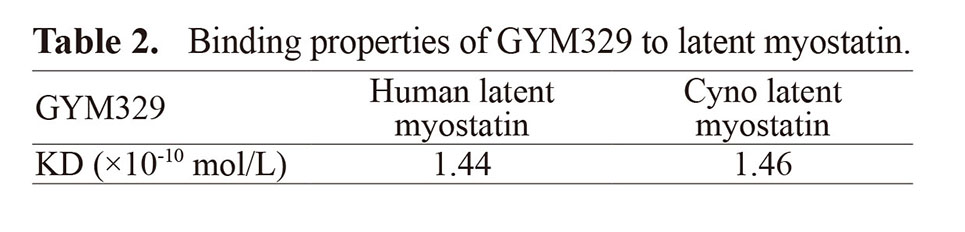 Human FcγRIIb ITIM phosphorylation
Human FcγRIIb ITIM phosphorylation
CD19-Hu2b, the positive control antibody, significantly increased phosphorylation of ITIM (Dunn’s test, p < 0.05), and the phosphorylation levels were 3.3–129.6 times higher than palivizumab, the negative control antibody (Fig. 1A, C). Immune complex formed by latent myostatin and myo-HuCy2b (IC_myo-HuCy2b) or IC_myo-Hu2b significantly increased phosphorylation of ITIM (Dunn’s test, p < 0.05), and the phosphorylation levels were 8.8–19.2 or 16.2–103.7 times higher than palivizumab (Fig. 1A, C). No significant increase in the phosphorylation levels was observed in IC_GYM329 or IC_myo-IgG1 (Fig. 1A, C). On the other hand, no significant increase in the FcγRIIb expression levels was observed in IC_GYM329, IC_myo-IgG1, IC_myo-HuCy2b, IC_myo-Hu2b, or CD19-Hu2b (Fig. 1B, D).
FcγRIIb ITIM phosphorylation and FcγRIIb expression in human PBMCs. PBMCs were incubated with test articles at 100 μg/mL for 5 min at 37°C. Whole cell lysates were analyzed by western blotting using antibodies specific to phospho-ITIM (A) and FcγRIIb (B). The levels of phospho-ITIM (C) and FcγRIIb (D) are shown as fold changes from palivizumab. Data of phospho-ITIM and FcγRIIb are represented as mean + SD (n ≥ 3). Statistical significance of elevation was examined between test articles and palivizumab with Dunn’s test (*: p < 0.05).
Human B cell apoptosis induced by each Ab is indicated in Fig. 2. CD19-Hu2b, the positive control antibody, significantly increased apoptosis in B cells (Dunnett’s test, p < 0.05), and the maximum percentage of apoptosis was higher than palivizumab by 35.3%–54.8%. IC_myo-HuCy2b or IC_myo-Hu2b significantly increased apoptosis (Dunnett’s test, p < 0.05), and the percentages of apoptosis was 4.9–14.0 or 11.5–27.2 times higher than palivizumab. No significant increase was seen in IC_GYM329 or IC_myo-IgG1. IC_myo-HuCy2b, IC_myo-Hu2b, or CD19-Hu2b increased apoptosis at ≥ 100, 1, or 0.01 μg/mL.

Apoptosis in human B cells. B cells were incubated with anti-CD79b and test articles at 37°C overnight. Apoptotic cells were analyzed by flow cytometer. Annexin V+7AAD low cells were defined as apoptotic cells. A, the increase in apoptosis was calculated by subtracting the percentage of apoptosis with no treatment from the percentages of apoptosis with test articles. Maximum increase in apoptosis is represented as mean + SD (n ≥ 3). B, statistical significance of elevation was examined between test articles and palivizumab with Dunnett’s test (*: p < 0.05).
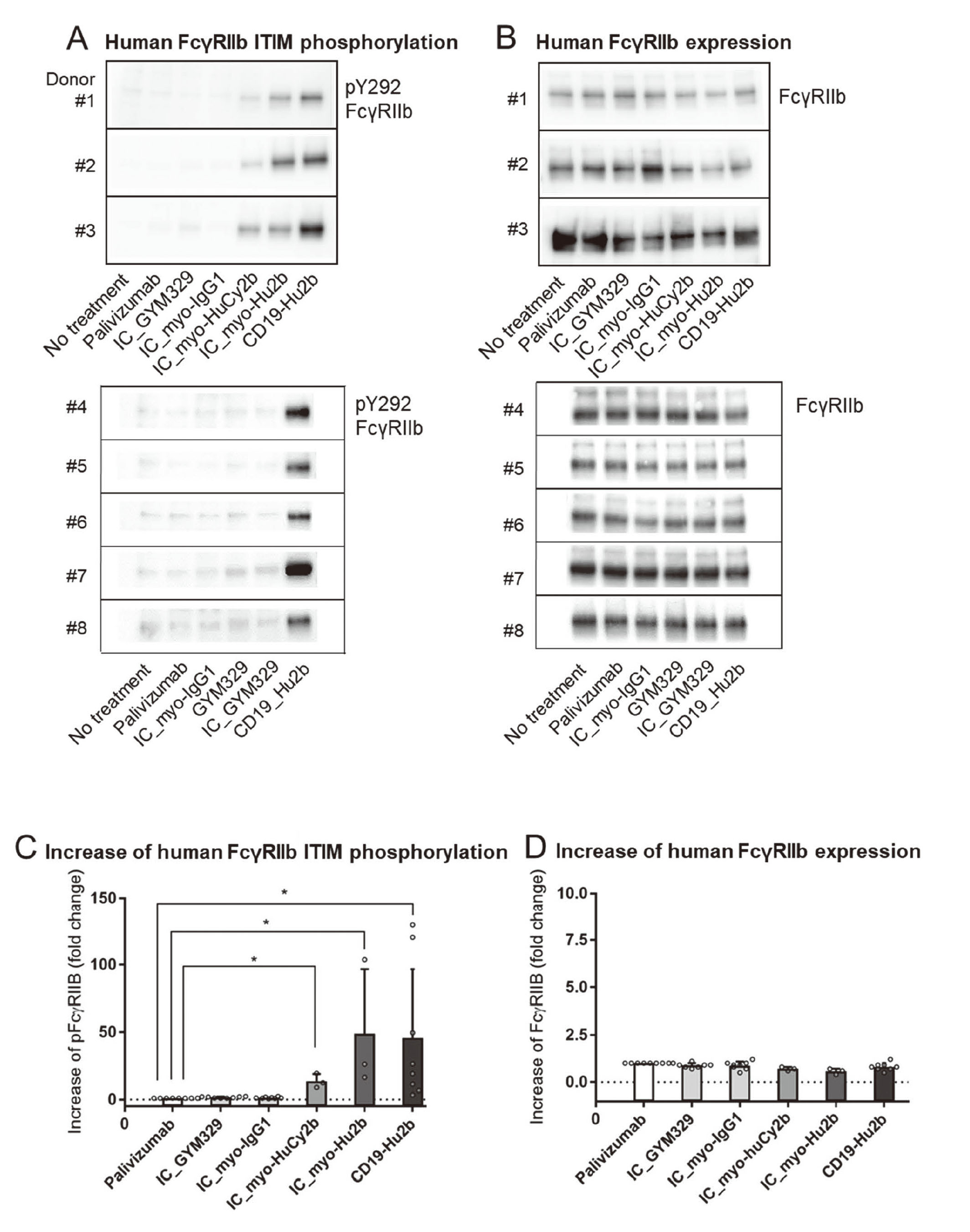
CD19-Cy2b, the positive control antibody, significantly increased phosphorylation of ITIM (Dunn’s test, p < 0.05), and the phosphorylation levels were 4.0–21.2 times higher than palivizumab, the negative control antibody (Fig. 3A, C). IC_myo-HuCy2b significantly increased phosphorylation of ITIM, and the phosphorylation levels were 3.4–5.6 times higher than palivizumab (Fig. 3A, C). No significant increase in the phosphorylation levels was seen in IC_GYM329 or IC_myo-IgG1 (Fig. 3A, C). On the other hand, no significant increase in the FcγRIIb expression levels was seen in any of the treatments (Fig. 3B, D).
FcγRIIb ITIM phosphorylation and FcγRIIb expression in cyno PBMCs. PBMCs were incubated with test articles at 100 μg/mL for 5 min at 37°C. Whole cell lysates were analyzed by western blotting using antibodies specific to phospho-ITIM (A) and FcγRIIb (B). The levels of phospho-ITIM (C) and FcγRIIb (D) are shown as fold changes from palivizumab. Data of phospho-ITIM and FcγRIIb are represented as mean + SD (n = 5). Statistical significance of elevation was examined between test articles and palivizumab with Dunn’s test (*: p < 0.05).
Cyno B cell apoptosis induced by each Ab is indicated in Fig. 4. CD19-Cy2b, the positive control antibody, significantly increased apoptosis in B cells (Dunnett’s test, p < 0.05), and the maximum percentage of apoptosis was higher than palivizumab by 6.4%–11.2%. IC_myo-HuCy2b tended to increase apoptosis in B cells, and the maximum percentage of apoptosis was higher than palivizumab by −0.2%–9.1%. No significant increase was seen in IC_GYM329, IC_myo-IgG1, or IC_myo-HuCy2b. IC_myo-HuCy2b or CD19-Cy2b increased apoptosis at ≥ 100 or 1 μg/mL.
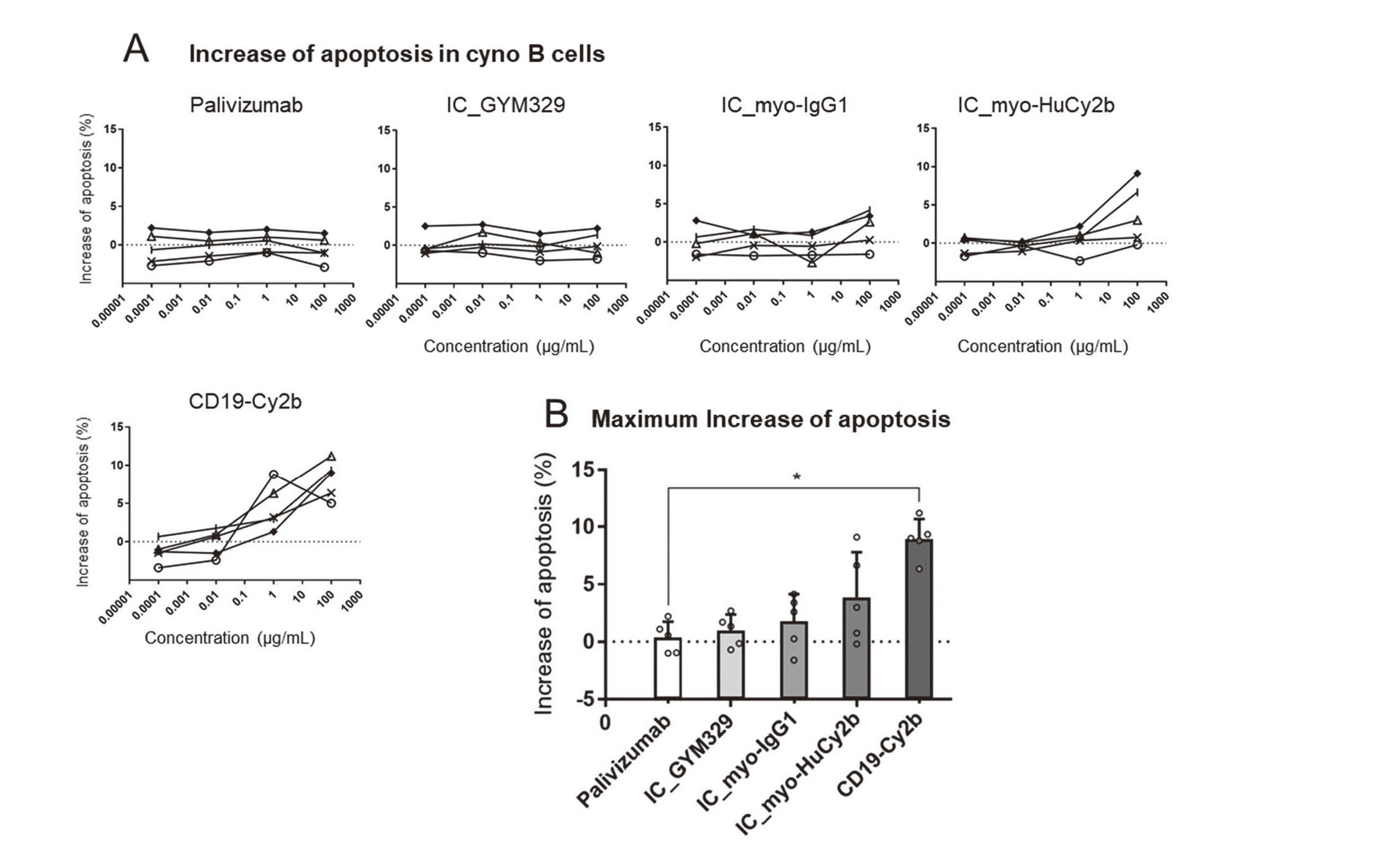
Apoptosis in cyno B cells. B cells were incubated with CpG and test articles at 37°C overnight. Apoptotic cells were analyzed by flow cytometer. Annexin V+7AAD low cells were defined as apoptotic cells. A, the increase in apoptosis was calculated by subtracting the percentage of apoptosis with no treatment from the percentages of apoptosis with test articles. B, maximum increase in apoptosis is represented as mean + SD (n = 5). Statistical significance of elevation was examined between test articles and palivizumab with Dunnett’s test (*: p < 0.05).
Geometric means of B cells treated with AF647-labeled test articles are indicated in Fig. 5. IC_GYM329 and IC_myo-HuCy2b bound to human B cells, and IC_GYM329 showed higher binding than IC_myo-HuCy2b. GYM329, myo-HuCy2b, and myo did not show clear binding to B cells. GYM329 and myo-HuCy2b bound to FcγRIIb with fast dissociation (Fig. S1). Multivalent IgG-antigen ICs enable avidity-based Fc-FcγRIIb interaction (Bournazos et al., 2017). Thus, it is reasonable that the two Abs dissociated from B cells after washing while binding of IC_GYM329 or IC_HuCy2b to B cells was detected with flowcytometry.

Binding of GYM329/myo-HuCy2b or their immune complex to human B cells. B cells were incubated with test articles at 4°C for 1 hr, then incubated with surface markers for 30 min. Then, PBMCs were washed and fixed with 1% paraformaldehyde. Binding od test articles to B cells was analyzed by flow cytometer. CD45+CD19+ cells were defined as B cells. Geometric means of test articles are represented as mean + SD (n = 3).
The result of the present study demonstrated that Fc-engineered Abs with similar binding affinity to FcγRIIb had different effects. IC_GYM329 with enhanced binding affinity to FcγRIIb (×5) induced neither ITIM phosphorylation nor B cell apoptosis in human cells (Fig. 1, 2, Table 1). In contrast, IC_myo-HuCy2b, which has binding affinity to FcγRIIb (×4) similar to that of GYM329, induced ITIM phosphorylation and B cell apoptosis in human cells (Fig. 1, 2, Table 1). Both IC_GYM329 and IC_myo-HuCy2b bound to human B cells (Fig. 5), and GYM329 and myo-HuCy2b showed typical association and fast dissociation kinetics (Fig. S1) (Maenaka et al., 2001). IC of multivalent antigens can crosslink FcγRIIb for receptor triggering (Chen et al., 2022), which results in ITIM phosphorylation to suppress the activating signal cascade (Smith and Clatworthy, 2010), and also apoptosis in B cells (Pearse et al., 1999). Because latent myostatin forms a disulfide-linked C-terminal dimer (Wolfman et al., 2003), GYM329 can form multivalent IC. IC_myo-IgG1 (×0.8) did not induce ITIM phosphorylation and B cell apoptosis whereas IC_myo-Hu2b (×11) and IC_myo-HuCy2b (×4) did (Fig. 1, 2, Table 1). This result is consistent with previous studies in which natural type anti-CD19 IgG1 did not induce ITIM phosphorylation or B cell apoptosis but Abs with increased binding affinity to FcγRIIb did (Horton et al., 2008; Horton et al., 2011). GYM329 was the only Fc-engineered Ab with high binding affinity to FcγRIIb that induced neither ITIM phosphorylation nor B cell apoptosis in this study. Although further investigation is needed to understand why GYM329 did not induce these FcγRIIb-related functions, we suspect it was because the binding of Fc of GYM329 to FcγRIIb could not generate appropriate conformation changes to FcγRIIb to trigger the downstream signaling cascade. This is similar to how some anti-receptor Abs act as antagonists but others as agonists (Graves et al., 2011; Sanders et al., 2010; Taupin et al., 2001). Previous studies indicate that appropriate conformational changes to specific receptor regions are required to trigger downstream signaling by agonistic Abs (Mellado et al., 1997; Müller-Newen et al., 2000). Fc-FcγR complex formation induces structural changes (Sondermann et al., 2000). The Fc region of GYM329 has different amino acid changes compared to myo-HuCy2b (Table S1). GYM329-specific amino acids may impair the appropriate conformational change of Fc-FcγR complex to trigger ITIM phosphorylation and B cell apoptosis.
CD19-Hu2b with enhanced FcγRIIb affinity (×11), the positive control mAb, significantly induced ITIM phosphorylation and B cell apoptosis in humans (Fig. 1, 2, Table 1), which was consistent with the previous studies (Chu et al., 2008; Horton et al., 2011). Palivizumab, the negative control mAb, induced neither ITIM phosphorylation nor B cell apoptosis (Fig. 1, 2). Previous studies indicate that affinity for activating FcγRs is critical to the effector function (Horton et al., 2008; Yamin et al., 2021; Kang and Jung, 2019). However, the present study suggests that the binding affinity of Fc-engineered mAb to FcγRIIb did not necessarily reflect FcγRIIb-induced ITIM phosphorylation and B cell apoptosis when IC consisted of anti-latent myostatin Abs. Verbeek et al. (2019) reported that the endocytic properties of FcγRIIb are independent of the ITIM. As for GYM329, FcγRIIb should work as an endocytic receptor of small IC to sweep latent myostatin, so to prevent immune suppression, it is preferable that GYM329 does not induce ITIM phosphorylation or B cell apoptosis.
The binding affinity of GYM329 to cyno latent myostatin is similar to that in humans (Table 2), but its affinity to cyno FcγRIIb (×1) is much lower than that to human FcγRIIb (×5) (Table 1). It is difficult to extrapolate the effect of GYM329 on FcγRIIb-related functions in cynos to humans because of the species difference in binding affinity to FcγRIIb. Hence, we used myo-HuCy2b with enhanced binding affinity to cyno FcγRIIb (×2); its binding affinity to human FcγRIIb (×4) is similar to that of GYM329 (×5) (Table 1). CD19-Cy2b with enhanced binding affinity to cyno FcγRIIb (×7) (Table 1), the positive control mAb, significantly induced ITIM phosphorylation and B cell apoptosis in cyno cells (Fig. 3, 4). Neither IC_myo-IgG1 nor GYM329 induced ITIM phosphorylation and B cell apoptosis. These results were consistent with the results in human cells (Fig. 1, 2). IC_myo-HuCy2b significantly induced ITIM phosphorylation, and IC_myo-HuCy2b tended to increase B cell apoptosis in cyno cells. IC_myo-HuCy2b significantly induced B cell apoptosis in human cells but not in cyno cells, possibly because the binding affinity to human FcγRIIb (×4) is higher than that to cyno FcγRIIb (×2) (Table 1). myo-HuCy2b showed totally different results in cyno cells from those seen with GYM329 in human cells; myo-HuCy2b (×2) increased ITIM phosphorylation and tended to increase B cell apoptosis in cyno cells while GYM329 (×5) induced neither ITIM phosphorylation nor B cell apoptosis in human cells. We determined that it is difficult to extrapolate the results of cyno toxicology studies using myo-HuCy2b to humans in terms of FcγRIIb-related immune suppression.
The present study indicates that there is at least one case where binding affinity to FcγRIIb does not correlate with FcγRIIb-related functions such as ITIM phosphorylation and B cell apoptosis. Thus, it is important to also investigate FcγR-mediated immune functions other than binding to fully understand the biological effects of Fc-engineered Abs.
The authors would like to acknowledge all our colleagues at Chugai Pharmaceutical Co. Ltd, A. Shioda, H. Nakamura, N. Kobayashi, K. Shimizu, and M. Yano for their technical assistance; A. Ueda and K. Esaki for antibody analysis; T. Kake, H. Muramatsu, H. Ikegami, K. Koyanagi for their support; J. Davis for review of the manuscript; H. Suzuki, T. Kuramochi, and J. Kiyokawa for their invaluable suggestions. We also thank the members at Chugai Research Institute for Medical Science Inc.; S. Hiranabe for antibody production.
Conflict of interestThe authors are employees of Chugai Pharmaceutical Co., Ltd. which is conducting clinical studies on GYM329.