Abstract
Multi-walled carbon nanotubes (MWCNTs), a kind of nanomaterial, are widely used in battery electrodes and composite materials, but the adverse effects associated with their accumulation in the living body have not been sufficiently investigated. MWCNTs are a fibrous material with molecules similar to asbestos fibers, and there are concerns about its effects on the respiratory system. In this study, we conducted a risk assessment by exposing mice using a previously developed nanomaterial inhalation exposure method. We quantified the exposure in the lungs by a lung burden test, evaluated the deterioration due to pneumonia using respiratory syncytial virus (RSV) infection, and measured inflammatory cytokines in bronchoalveolar lavage fluid (BALF). As a result, in the lung burden test, the amount of MWCNT in the lung increased according to the inhalation dose. In the RSV infection experiment, CCL3, CCL5, and TGF-β, which are indicators of inflammation and lung fibrosis, were elevated in the MWCNT-exposed group. Histological examination revealed cells phagocytosing MWCNT fibers. These phagocytic cells were also seen during the recovery period from RSV infection. The present study found that MWCNT remained in the lungs for about a month or more, suggesting that the fibers may continue to exert immunological effects on the respiratory system. Furthermore, the inhalation exposure method enabled the exposure of nanomaterials to the entire lung lobe, allowing a more detailed evaluation of the effects on the respiratory system.
INTRODUCTION
Nanomaterials can be used in various daily necessities due to their fine structures and material properties, but their toxicity remains largely unknown (Najahi-Missaoui et al., 2020). In particular, no studies have been conducted to examine the effects associated with chronic bioaccumulation, which is of greatest concern, and its risk assessment has become a major concern (Xia et al., 2009). Multi-walled carbon nanotubes (MWCNTs), known as one of the most common and widely studied nanomaterials, are used as additives and composite materials for battery electrodes, semiconductor devices, medical materials, automobile/aircraft materials, building materials, etc. (De Volder et al., 2013). MWCNTs are a fibrous substance with a diameter of 10 to 200 nm and a fiber length of 1 to several hundred μm. They contain fibers similar in shape and size to asbestos fibers, which cause pulmonary mesothelioma and chronic inflammation, raising concerns about their impact on the respiratory system (Xia et al., 2009; Uo et al., 2011). In fact, studies on MWCNTs in mice suggest carcinogenicity in vivo (Poland et al., 2008; Takagi et al., 2008). Although the mechanism of carcinogenesis in these mice is unknown, it is feared that the generation of free oxygen radicals and chronic inflammation resulting from biological reactions to MWCNTs may also lead to the initiation or promotion of tumors in humans (Kane and Hurt, 2008).
Respiratory syncytial virus (RSV) causes pneumonia and bronchitis, mainly in infants, children, and elderly people with underlying diseases (Griffiths et al., 2017). In our previous research, we evaluated the effects of various nanomaterials and bacterial particles on the pathology of RSV pneumonia in mice, and investigated the toxicity and effects of these particles on the immune system (Watanabe et al., 2017; Miyauchi et al., 2019). In addition, we confirmed that intranasal administration of MWCNT to RSV-infected mice caused immune system cells in the bronchioles and alveoli to react to the carbon fibers and exacerbated RSV pneumonia (Hashiguchi et al., 2021). At this time, cytokine levels, which are indicators of pneumonia, increased, and these results indicate that MWCNT could be a factor that exacerbates pneumonia. However, conventional intranasal administration of suspensions of MWCNT fibers in the administration solution may cause aggregation, and these large fibers may directly affect the respiratory system. In our daily lives, the most likely opportunity for us to be exposed to MWCNTs is inhaling them as an aerosol. Inhalation of the nebulized substance instead of nasal administration was considered more appropriate for the original toxicity test. Therefore, we confirmed that the lungs of mice were exposed to MWCNT fibers without agglomeration by using a previously developed method that exposed mice to nebulized nanomaterials inhalation (Taquahashi et al., 2013). This time, using the same equipment, we evaluated the effects of MWCNT on living organisms using RSV-infected mice.
MATERIALS AND METHODS
Animals
First, mice were inhaled with MWCNT at the National Institute of Health Sciences (NIHS). RSV infection experiments were performed at Kyushu University of Health and Welfare (KUHW). Animal transportation between facilities was performed by a professional contractor (Japan SLC, Inc., Shizuoka, Japan). Transportation was accomplished within 1 day using animal transport vehicles and airplanes. Since it was a short period of time, no food was given during transportation, and water was allowed to be taken freely from jelly. A polypropylene animal transport container fitted with a sterilizing filter was used, and a Shepherd Shack (Shepherd Specialty Papers, USA) was placed inside to reduce animal stress. Any effects were not identified by post-transport follow-up observations.
Female BALB/cCrSlC mice were purchased from Japan SLC, Inc. After acclimation for one week, 4-week-old mice were subjected to the experimental procedure. The animals were housed in a conventional environmentally controlled animal care facility in NIHS. The room temperature, relative humidity and light cycle were 25 ± 2°C, 50 ± 20%, and 12-hr light/dark, respectively. Mice were kept in individually ventilated cages (Lab Products Inc., Seaford, DE, USA) and allowed free access to water and standard laboratory food (CRF-1, Oriental Yeast Co., Ltd., Tokyo, Japan). At KUHW, breeding was carried out in a facility environment similar to that described above. The mice were allowed free access to the conventional solid diet CRF-1 and water.
The guidelines established by the ethical committee for animal experiments of the NIHS and KUHW were followed for the care and use of animals. All the experimental protocols in this study were reviewed and approved by the Committee for Proper Experimental Animal Use and Welfare, a peer review panel established at the NIHS, with the experimental code #787. At KUHW, the experimental protocol was similarly approved by the Animal Care and Use Committee (approval number 2-1-03).
Cell and virus
The A2 strain of RSV was obtained from American Type Culture Collection (ATCC, Rockville, MD, USA) and grown in HEp-2 cell (human epidermoid carcinoma, ATCC CCL-23) cultures. Viral titers of HEp-2 cells were measured by the plaque method, and expressed as plaque-forming units per milliliter (PFU/mL).
Animal tests
One of the MWCNTs, MWNT-7 (Mitsui Chemicals, Tokyo, Japan), was provided by Dr. A. Hirose, one of the authors, for this study.
MWCNT inhalation of mice followed by RSV infection was performed as described previously (Taquahashi et al., 2013; Watanabe et al., 2008). Briefly, mice were exposed to 53 µm mesh-filtered MWCNT aerosol by the Taquann system (Taquahashi et al., 2013) at the mass concentrations of 2.8 ± 0.1 or 5.2 ± 0.1 mg/m3, 6 hr a day, 3 times every other day. A single inhalation exposure to MWCNT had a low lung burden, and there were also large individual differences in cytokine levels and fiber distribution in the lung tissue, so multiple inhalations were performed. The mass median aerodynamic diameter was approximately 500 nm with a geometric deviation of 4.7-7.0. The control group was supplied only HEPA-filtered clean air with a similar protocol to MWCNT groups.
Three days later, these mice were infected intranasally with 3.0 × 105 PFU of the A2 strain of RSV under anesthesia. In the mock-infected group, mice were administered PBS intranasally. Five or 21 days after infection, each infected group was divided into two and dissected. Bronchoalveolar lavage fluid (BALF) and lungs for histological staining were collected from one infected group, and lungs for lung burden measurements were collected from the other infected group.
To collect mouse lungs and BALF, 5 or 6 mice per group of mock- or RSV-infected mice were sacrificed by overanesthesia 5 or 21 days after infection. BALF was obtained from mice by injecting 0.8 mL of cold PBS into the lungs and aspirating it from the trachea using a tracheal cannula. After BALF acquisition, lungs were excised and fixed in buffered formalin for at least 24 hr for histological examination. Ice-cold BALF was centrifuged at 160 × g at 4°C for 10 min. After centrifugation, the supernatant was stored at -80°C until use. In this study, considering the reduction of the number of experimental animals from the viewpoint of animal welfare, the same individual was used for BALF acquisition and lung tissue observation. This has the advantage of making it easier to determine the correlation between changes in histology and cytokine changes in BALF, but has the disadvantage of removing some inflammatory cells in the alveoli and trachea. However, in addition to comparing all groups under the same conditions, qualitative changes such as thickening of alveolar walls and tissue changes around blood vessels can be detected, so it was judged that there was no problem in this study. For other experiments, 3–7 mice per group were euthanized as above, lungs were isolated, and lung burden measurements were performed. MWCNT extraction from lungs and lung burden measurements were performed as previously reported (Taquahashi et al., 2013; Ohnishi et al., 2016).
ELISA
Levels of CCL3 (MIP-1α) and CCL5 (RANTES) in BALF were measured using specific ELISA kits from R&D Systems (Quantikine, R&D Systems, Inc., Minneapolis, MN, USA) and TGF-β from Invitrogen (Mouse LAP Uncoated ELISA Kit, Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The lower limits of detection of the kits are 1.5 (pg/mL) for CCL3, 2.0 (pg/mL) for CCL5, and 0.2 (ng/mL) for TGF-β. The intra- and interassay coefficients of variation for the ELISA results were less than 10%.
Histological methods and evaluation
For histological examination, mouse lungs were immersed in formalin as described above. The tissue was then embedded in low-melting point paraffin, sectioned at a thickness of 3 μm, and stained with hematoxylin and eosin (HE). Some slides were subjected to Masson's trichrome staining and Periodic Acid Schiff (PAS) staining.
Statistical analysis
Comparisons between the levels of cytokines and chemokines of the control and MWCNT exposed groups were carried out using the Mann-Whitney U-test. A P value of 0.05 or less was considered to be significant.
RESULTS
In this study, to evaluate the effects of MWCNT on the respiratory system using RSV-infected mice, RSV infection was performed after three inhalation exposures to MWCNT. Mice were sacrificed 5 days after RSV infection, and BALF and lungs were collected to confirm the pathology of the acute phase of RSV infection. First, the lung burden was measured using some of the lungs that were collected initially (Fig. 1). In the lung burden measurement, it was found that the amount of residual carbon in the lungs increased in proportion to the amount of MWCNT exposure, and that it accumulated in the lungs. There were no individuals that deviated greatly from the average values, and inhalation exposure was fairly even for all individuals. In addition, the accumulated MWCNT populations decreased over time, indicating that they were gradually excreted, but a large amount of MWCNT still remained in the lungs.

Next, in the collected BALF, we measured CCL3 and CCL5, which are indicators of acute pneumonia, and TGF-β, which is an indicator of airway remodeling and pulmonary fibrosis (Fig. 2). In both groups of RSV-infected mice exposed to MWCNT at 3 mg/kg and 6 mg/kg, the level of CCL3 increased as the exposure concentration increased, indicating a significant difference (Fig. 2a). The group of RSV-infected mice exposed to MWCNT at 3 mg/kg showed a significant increase in the CCL5 levels, while the group exposed to 6 mg/kg showed an increasing trend, but no significant difference was observed (Fig. 2b). In TGF-β, the same tendency as CCL3 was observed, and the level increased as the exposure concentration increased, showing a significant difference in RSV-infected mice (Fig. 2c). In RSV-uninfected mice, levels of CCL3 and TGF-β tended to increase depending on exposure concentration. CCL5 levels were hardly elevated in RSV-uninfected mice.

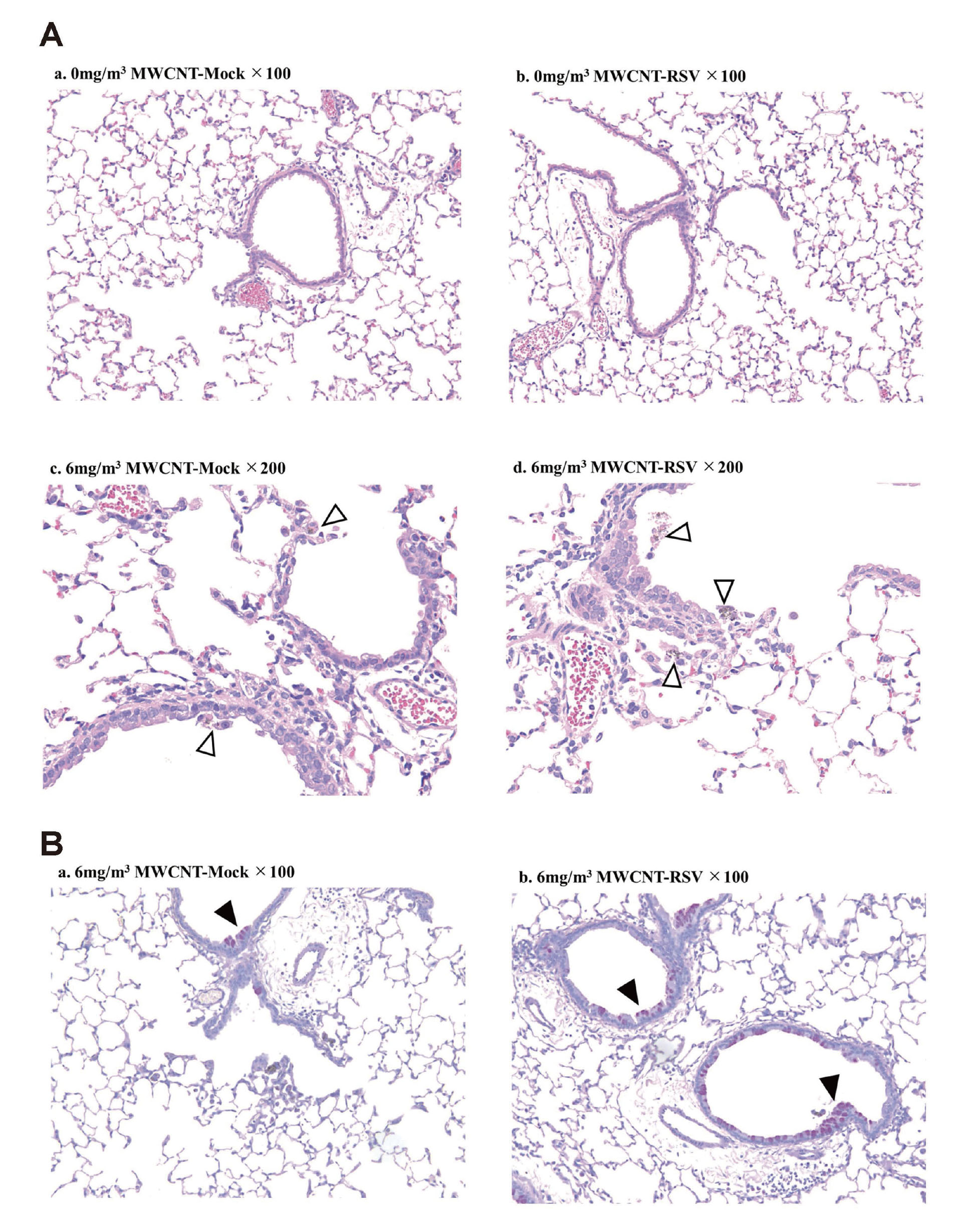
Most of the collected lungs on day 5 after RSV infection were stained with HE, and the histology was observed using a microscope (Fig. 3A). Lymphocytic infiltration and thickening of alveolar walls due to RSV infection were observed in the lungs, but clear exacerbation of pneumonia due to inhalation exposure to MWCNT was not observed (Fig. 3Aa, 3Ab). In the group exposed to MWCNT by inhalation, no clear exacerbation of inflammation was observed, but clusters of carbon-phagocytic macrophages were widely observed (Fig. 3Ac, 3Ad). As the exposure concentration increased, MWCNT fibers were observed more frequently, and small granuloma-like lesions were also observed in many lung lobes (Fig. 3Ae, 3Af). In addition, although carbon was not localized around the pleura, phagocytic macrophages and lymphocytes were confirmed near the pleura (Fig. 3Af). In order to perform a more detailed tissue evaluation, we performed Masson's trichrome staining of the lung tissue, which allows evaluation of lung fibrosis, but collagen fiber proliferation due to MWCNT exposure was not observed (data not shown). Subsequently, PAS staining of lung tissue, which enables evaluation of the proliferation of mucus-secreting cells, was performed (Fig. 3B). With PAS staining, no difference was observed in the lung tissue of the RSV-infected mouse group except for pneumonia symptoms (Fig. 3Ba, 3Bb). However, exposure to MWCNT enhanced airway remodeling-like lesions such as proliferation of goblet cells in the airway epithelium, which was not observed during RSV infection (Fig. 3Bc, 3Bd).

Next, to investigate the long-term effects of MWCNT on the respiratory system, we measured CCL3 and TGF-β in BALF (Fig. 4a, 4b) and evaluated lung tissue lesions (Fig. 5A) 21 days post-infection during the recovery phase of RSV infection. CCL5 was below the detection limit in all specimens and could not be detected (data not shown). In Fig. 4, CCL3 levels were generally low and below the detection limit in the control group, but slightly higher levels were detected in the low-MWCNT-exposed Mock group and increased in the high-concentration exposure group (Fig. 4a). There was no difference in the presence or absence of RSV infection. TGF-β remained at the same level as 5 days after RSV infection (Fig. 4b). The same trend as CCL3 was observed, and the higher the exposure concentration, the higher the value compared to the control group, but there was no difference between RSV-infected and non-infected. In the lung tissue, pneumonia in the RSV-infected control group had almost disappeared, and there was almost no difference from the mock group (Fig. 5Aa, 5Ab). On the other hand, in the group exposed to MWCNT, a concentration of carbon-phagocytic macrophages was observed mainly around the bronchi (Fig. 5Ac). As a difference between the presence or absence of RSV infection, carbon phagocytic macrophages were observed not only in the bronchi but also in some alveoli in the RSV-infected group (Fig. 5Ad). Next, the lung tissue on the 21st day was subjected to PAS staining for more detailed tissue evaluation (Fig. 5B). As a result, in the tracheal epithelium of the MWCNT-exposed group, PAS staining-positive areas such as hypersecretion of viscous polysaccharides by goblet cells, etc., confirmed in the tissue on day 5, were continuously observed (Fig. 5Ba). In addition, these characteristics were observed to be enhanced in RSV infection (Fig. 5Bb).

DISCUSSION
This study revealed that inhalation of MWCNT fibers is highly likely to affect the pathology of RSV infection in mice throughout the lung lobe. Inhalation exposure to MWCNT was less localized than conventional intranasal administration because the carbon fibers could be distributed throughout the lung lobes. Then, when the effects of MWCNT on RSV infection pathology were investigated using the inhalation exposure method, significant increases in CCL3, CCL5 and TGF-β were observed in the acute phase of RSV infection. CCL3 and CCL5 are also called ‘macrophage inflammatory protein alpha (MIP-1α)’ and ‘regulated upon activation normal T cell express Sequence (RANTES)’, respectively, and are known to be markers that indicate pneumonia pathology during the acute phase of RSV infection (Hashiguchi et al., 2015). In addition, TGF-β, a dimeric polypeptide growth factor, is involved in cell proliferation and differentiation, and is said to be an indicator of pulmonary fibrosis (Inui et al., 2021). As other indicators of inflammation, total protein and albumin levels in BALF were measured by FUJI DRI-CHEM 4000v system (FUJIFILM, Tokyo, Japan) and a colorimetric method using BioAssay Systems (Hayward, CA, USA), respectively, but the values were below the detection limit in all samples (data not shown). In this study, lung tissue observation during the acute phase of RSV infection failed to recognize a clear exacerbation of pneumonia, which is thought to be the effect of MWCNT. However, there were differences in inflammatory cytokines, suggesting that they may have affected pneumonia at a more granular level. In particular, MWCNT fibers that remain for a long period of time are likely to leave residual lesions such as pulmonary fibrosis. Inflammatory cells and the cytokines they release are long-lasting and induce lung injury, and viruses and combinations of these factors have been shown to promote lung fibrosis (Huang and Tang, 2021). A study also reported that RSV promotes bleomycin-induced lung fibrosis (Wang et al., 2017). In this study, we thought that it might be possible to observe the histology of accelerated lung fibrosis under the influence of RSV infection by long-term post-exposure observation to MWCNT. As a result, 21 days after RSV infection, it was confirmed that the levels of inflammatory cytokines CCL3 and TGF-β persisted in the MWCNT-exposed group. In particular, the level of TGF-β was confirmed to be about the same as in the acute phase, suggesting the possibility that lung tissue is continuously stimulated by MWCNT. Histological observation with HE staining did not show exacerbation of inflammation by MWCNT. However, in the RSV-infected group, carbon-phagocytic macrophages were scattered not only in the bronchi but also in the alveoli, and many PAS-positive cells remained. We thought that RSV infection might delay MWCNT excretion, and compared the lung burden of MWCNT in RSV-uninfected and RSV-infected mice. Since the data 5 days after RSV infection in Fig. 1 included both data from RSV-infected and non-infected mice, we used that data for analysis. As a result, there was no significant difference between the presence and absence of RSV infection.
Focusing on MWCNT themselves rather than the degree of inflammation confirmed characteristic lesions such as carbon-phagocytic macrophages in lung tissue in the acute phase. These lesions remained even after recovery from RSV infection, suggesting that MWCNT fibers may have long-term effects on respiratory tissues and immune system. The fact that MWCNT fibers remain in the lung tissue has also been demonstrated by lung burden measurement, and the high frequency observation in lung tissue after 21 days suggests that the fibers continue to remain in the lung tissue. It will be interesting to study how these residual fibers affect lung tissue over time. In the study during the recovery period from RSV infection, phagocyte response to MWCNT fibers continued. In addition, PAS-stained histological specimens showed increased airway remodeling-like lesions such as proliferation of goblet cells in the airway epithelium. In humans, RSV-induced lower respiratory tract inflammation is known to cause reactive airway disease (RAD), which has been shown to result in airway remodeling (Sigurs et al., 2005). In this study, long-term stimulation of MWCNT for as long as 21 days after infection may enhance RAD. In the future, we would like to make efforts to elucidate the mechanisms involved in RAD.
This time, acquisition of the new technology of the inhalation exposure method has increased the possibility of obtaining more accurate experimental data in future research investigating the effects of nanomaterials. We previously reported the effects of intranasal administration of MWCNT on the acute phase of RSV infection (Hashiguchi et al., 2021). In that report, similar to this time, increases in CCL3 and CCL5 were observed, and histologically, inflammation around the bronchioles and macrophages phagocytosing MWCNT fibers were confirmed. In this study, as a new toxicological knowledge, inhalation exposure also showed MWCNT infiltration deep into the lung tissue around the pleura, confirming the reaction of macrophages to it (Fig. 3Af). This was thought to be the result of inhalation exposure using a new technique that allows exposure of dispersed MWCNT fibers without agglomeration. One of the problems with the conventional intranasal administration method was that the fiber distribution was highly skewed. In addition, inflammation occurred only in some lung lobes, and there were large individual differences. Using the inhalation exposure method seems to be one solution to such problems. At intranasal administration, MWCNT was administered at 0.025 and 0.25 mg/kg, but at this concentration, the MWCNT fibers were not uniformly dispersed in the administered solution and were observed as clumps in the lung tissue. In this inhalation exposure, an amount of about 5 μg/lung was confirmed as the lung burden, and when converted to the body weight of mice, the same amount of MWCNT in the intranasal administration solution at high concentrations remained in the lungs. In addition, MWCNT was confirmed to be fibrous in histological examination. Based on these facts, it is considered that the conditions of inhalation exposure to aerosols this time were able to establish an experimental system close to the exposure conditions of MWCNT assumed in the actual human body.
In addition, this study found that MWCNT persisted in the lungs for a long period of time, suggesting that the fibers may affect the respiratory immune system. Previous dose-response and time-course studies reported that MWCNT aspiration exposure to mice resulted in dose-dependent and persistent pulmonary inflammation, injury, and granuloma formation (Porter et al., 2010). Intratracheal administration of MWCNT fibers has also been found to result in inflammation, fibrosis, and granuloma formation in mice and rats (Muller et al., 2005; Poulsen et al., 2016). In this study, we could not clearly confirm the strong effect that occurred only with MWCNT exposure, which was confirmed in those reports, but it seems that this was due to the large difference in the amount of exposure. This time, we adopted a research method that evaluated the effects of MWCNT using RSV infection pathology, so the experiment was conducted at a lower exposure level than reported. Studies that have linked MWCNTs to other factors have shown that MWCNTs dose-dependently increased systemic immune responses in a mouse model of asthma (Ronzani et al., 2014). Another report showed that MWCNTs did not cause changes in lung function or structure, but affected the activation of macrophages and dendritic cells in an in vivo mouse model of chronic obstructive lung disease (Beyeler et al., 2020). However, there have been no reports to date linking the RSV infection pathology and MWCNT fibers like this study. We believe that this research is also important in terms of providing a new approach to the effects of nanomaterials on the respiratory system.
ACKNOWLEDGMENTS
The authors are grateful to Yukiko Shimoda, Koichi Morita, Masaki Tsuji, Kousuke Suga, and Asako Aida for their help with the animal experiments. We also thank Ms. Katherine Ono for editing the paper. This study was supported by a Health and Labour Sciences Research Grant (H30-kagaku-shitei-004 and 20KD1004) from the Ministry of Health, Labour and Welfare, Japan.
Conflict of interest
The authors declare that there is no conflict of interest.
REFERENCES
- Beyeler, S., Steiner, S., Wotzkow, C., Tschanz, S.A., Adhanom Sengal, A., Wick, P., Haenni, B., Alves, M.P., von Garnier, C. and Blank, F. (2020): Multi-walled carbon nanotubes activate and shift polarization of pulmonary macrophages and dendritic cells in an in vivo model of chronic obstructive lung disease. Nanotoxicology, 14, 77-96.
- De Volder, M.F., Tawfick, S.H., Baughman, R.H. and Hart, A.J. (2013): Carbon nanotubes: present and future commercial applications. Science, 339, 535-539.
- Griffiths, C., Drews, S.J. and Marchant, D.J. (2017): Respiratory syncytial virus: infection, detection, and new options for prevention and treatment. Clin. Microbiol. Rev., 30, 277-319.
- Hashiguchi, S., Yoshida, H., Akashi, T., Komemoto, K., Ueda, T., Ikarashi, Y., Miyauchi, A., Konno, K., Yamanaka, S., Hirose, A., Kurokawa, M. and Watanabe, W. (2015): Titanium dioxide nanoparticles exacerbate pneumonia in respiratory syncytial virus (RSV)-infected mice. Environ. Toxicol. Pharmacol., 39, 879-886.
- Hashiguchi, S., Miyauchi, A., Komemoto, K., Ueda, T., Tokuda, K., Hirose, A., Yoshida, H., Akashi, T., Kurokawa, M. and Watanabe, W. (2021): Effects of intranasal administration of multi-walled carbon nanotube (MWCNT) suspension on respiratory syncytial virus (RSV) infection in mice. Fundam. Toxicol. Sci., 8, 215-220.
- Huang, W.J. and Tang, X.X. (2021): Virus infection induced pulmonary fibrosis. J. Transl. Med., 19, 496.
- Inui, N., Sakai, S. and Kitagawa, M. (2021): Molecular pathogenesis of pulmonary fibrosis, with focus on pathways related to TGF-beta and the ubiquitin-proteasome pathway. Int. J. Mol. Sci., 22, 6107.
- Kane, A.B. and Hurt, R.H. (2008): Nanotoxicology: the asbestos analogy revisited. Nat. Nanotechnol., 3, 378-379.
- Miyauchi, A., Watanabe, W., Akashi, T., Hashiguchi, S., Yoshida, H., Sugita, C. and Kurokawa, M. (2019): Effect of inactivated Streptococcus pneumoniae as non-pathogenic particles on the severity of pneumonia caused by respiratory syncytial virus infection in mice. Toxicol. Rep., 6, 514-520.
- Muller, J., Huaux, F., Moreau, N., Misson, P., Heilier, J.F., Delos, M., Arras, M., Fonseca, A., Nagy, J.B. and Lison, D. (2005): Respiratory toxicity of multi-wall carbon nanotubes. Toxicol. Appl. Pharmacol., 207, 221-231.
- Najahi-Missaoui, W., Arnold, R.D. and Cummings, B.S. (2020): Safe nanoparticles: are we there yet? Int. J. Mol. Sci., 22, 385.
- Ohnishi, M., Suzuki, M., Yamamoto, M., Kasai, T., Kano, H., Senoh, H., Higashikubo, I., Araki, A. and Fukushima, S. (2016): Improved method for measurement of multi-walled carbon nanotubes in rat lung. J. Occup. Med. Toxicol., 11, 44.
- Poland, C.A., Duffin, R., Kinloch, I., Maynard, A., Wallace, W.A., Seaton, A., Stone, V., Brown, S., Macnee, W. and Donaldson, K. (2008): Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol., 3, 423-428.
- Porter, D.W., Hubbs, A.F., Mercer, R.R., Wu, N., Wolfarth, M.G., Sriram, K., Leonard, S., Battelli, L., Schwegler-Berry, D., Friend, S., Andrew, M., Chen, B.T., Tsuruoka, S., Endo, M. and Castranova, V. (2010): Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology, 269, 136-147.
- Poulsen, S.S., Jackson, P., Kling, K., Knudsen, K.B., Skaug, V., Kyjovska, Z.O., Thomsen, B.L., Clausen, P.A., Atluri, R., Berthing, T., Bengtson, S., Wolff, H., Jensen, K.A., Wallin, H. and Vogel, U. (2016): Multi-walled carbon nanotube physicochemical properties predict pulmonary inflammation and genotoxicity. Nanotoxicology, 10, 1263-1275.
- Ronzani, C., Casset, A. and Pons, F. (2014): Exposure to multi-walled carbon nanotubes results in aggravation of airway inflammation and remodeling and in increased production of epithelium-derived innate cytokines in a mouse model of asthma. Arch. Toxicol., 88, 489-499.
- Sigurs, N., Gustafsson, P.M., Bjarnason, R., Lundberg, F., Schmidt, S., Sigurbergsson, F. and Kjellman, B. (2005): Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am. J. Respir. Crit. Care Med., 171, 137-141.
- Takagi, A., Hirose, A., Nishimura, T., Fukumori, N., Ogata, A., Ohashi, N., Kitajima, S. and Kanno, J. (2008): Induction of mesothelioma in p53+/- mouse by intraperitoneal application of multi-wall carbon nanotube. J. Toxicol. Sci., 33, 105-116.
- Taquahashi, Y., Ogawa, Y., Takagi, A., Tsuji, M., Morita, K. and Kanno, J. (2013): Improved dispersion method of multi-wall carbon nanotube for inhalation toxicity studies of experimental animals. J. Toxicol. Sci., 38, 619-628.
- Uo, M., Akasaka, T., Watari, F., Sato, Y. and Tohji, K. (2011): Toxicity evaluations of various carbon nanomaterials. Dent. Mater. J., 30, 245-263.
- Wang, L., Cheng, W. and Zhang, Z. (2017): Respiratory syncytial virus infection accelerates lung fibrosis through the unfolded protein response in a bleomycin-induced pulmonary fibrosis animal model. Mol. Med. Rep., 16, 310-316.
- Watanabe, W., Shimizu, T., Hino, A. and Kurokawa, M. (2008): A new assay system for evaluation of developmental immunotoxicity of chemical compounds using respiratory syncytial virus infection to offspring mice. Environ. Toxicol. Pharmacol., 25, 69-74.
- Watanabe, W., Hirose, A., Takeshita, T., Hashiguchi, S., Sakata, K., Konno, K., Miyauchi, A., Akashi, T., Yoshida, H., Sugita, C. and Kurokawa, M. (2017): Perinatal exposure to tetrabromobisphenol A (TBBPA), a brominated flame retardant, exacerbated the pneumonia in respiratory syncytial virus (RSV)-infected offspring mice. J. Toxicol. Sci., 42, 789-795.
- Xia, T., Li, N. and Nel, A.E. (2009): Potential health impact of nanoparticles. Annu. Rev. Public Health, 30, 137-150.