2023 年 48 巻 8 号 p. 457-467
2023 年 48 巻 8 号 p. 457-467
Cadmium is an environmental pollutant and a risk factor for atherosclerosis. In the atherosclerotic intima, dermatan sulfate chains accelerate accumulation and oxidation of LDL cholesterol. The major type of dermatan sulfate proteoglycan that is synthesized by vascular endothelial cells is biglycan. In the present study, we analyzed the effect of cadmium on the biglycan synthesis using cultured bovine aortic endothelial cells. Cadmium did not induce biglycan mRNA and core protein expression; however, it elongated the chondroitin/dermatan sulfate chains of biglycan. Among elongation enzymes of the chondroitin/dermatan sulfate chain, chondroitin sulfate synthase 1 (CHSY1) mRNA and protein expression were dose- and time-dependently upregulated by cadmium depending on protein kinase Cα. This finding suggests that CHSY1-dependent elongation of chondroitin/dermatan sulfate chains of biglycan may exacerbate cadmium-induced atherosclerosis.
Proteoglycans (PGs) are composed of a core protein and glycosaminoglycan side chains consisting of amino sugars and uronic acids repeated on a tetrasaccharide linker (-xylose-galactose-galactose-glucuronic acid) attached to specific serine residues on the core protein. PGs are broadly classified based on the type of constituent glycosaminoglycan chains (Ruoslahti, 1988). Vascular endothelial cells synthesize heparan sulfate PGs perlecan, syndecan-1, and syndecan-4 (Saku and Furthmayr, 1989; Kojima et al., 1992) and chondroitin/dermatan sulfate (CS/DS) PG biglycan (Järveläinen et al., 1991). PGs bind growth factors/cytokines, receptors, and lipids via core proteins or glycosaminoglycans; regulate their activities; and are involved in cell growth, regulation of vascular permeability, lipid metabolism, and the blood coagulation-fibrinolytic system in vascular endothelial cells (Berenson et al., 1984; Camejo, 1982). Especially, CS/DS chains that are synthesized by vascular endothelial and smooth muscle cells and accumulate in plaque in the subendothelial layer of vascular endothelial cells show a high affinity for low-density lipoprotein (LDL) cholesterol and form an insoluble complex (Hurt-Camejo et al., 1992; Salisbury et al., 1985). Since this complex promotes oxidation of LDL cholesterol, CS/DS chains promote atherosclerosis with lipid plaque formation and accelerate endothelial cell injury.
The DS chain is constructed by elongation of the CS chain which consists of N-acetylgalactosamine and glucuronic acid alternately ligated to the linker tetrasaccharide, epimerization of the glucuronic acid in CS to iduronic acid (Maccarana et al., 2006), and sulfation of the C-4 position 4 of N-acetylgalactosamine or C-2 position of iduronic acid (Kobayashi et al., 1999; Mikami et al., 2003; Evers et al., 2001). Since epimerization is a mosaic reaction, the finally synthesized glycan chain is a hybrid of CS and DS (CS/DS). In the elongation process of this sugar chain, CS N-acetylgalactosaminyltransferase (CSGalNAcT) 1 and 2 are involved in the transfer of N-acetylgalactosamine (fifth sugar) to glucuronic acid at the linker, and hetero complexes of any of chondroitin synthase (CHSY) 1, CHSY3, chondroitin polymerizing factor (CHPF), or CHPF2 are involved in the subsequent synthesis of the glucuronic acid and N-acetylgalactosamine repeating structure (Kitagawa et al., 2001, 2003; Izumikawa et al., 2007, 2008; Uyama et al., 2002, 2003). Regarding the elongation of CS/DS chains in vascular cells, we have reported that transforming growth factor (TGF)-β1 increases biglycan core protein with elongated CS/DS chains in vascular endothelial cells only at high density in culture (Kaji et al., 2000). In vascular smooth muscle cells, platelet-derived growth factor elongates CS/DS chains without inducing biglycan mRNA, while TGF-β1 has been reported to elongate CS/DS chains with an increase in biglycan mRNA in vascular smooth muscle cells (Schönherr et al., 1993). Because both growth factors are strongly associated with atherosclerosis, CS/DS chain elongation is considered to be important in the development of early lesions.
Cadmium is a heavy metal to which humans are unintentionally exposed through rice and cigarettes, and animal studies have shown that it causes atherosclerotic lesions with lipid plaque formation (Oliveira et al., 2019). In addition, epidemiological studies have reported that cadmium is a risk factor for atherosclerosis (Tellez-Plaza et al., 2012). Lipid plaques induce various cardiovascular diseases such as myocardial infarction and stroke, and their formation is initiated by the accumulation and oxidation of LDL cholesterol in the vessel wall. The arterial vessels are segmented (starting from the inside) as intima, media, and adventitia, which are mainly composed of vascular endothelial cells, vascular smooth muscle cells, and fibroblasts, respectively. Injury to vascular endothelial cells is a trigger for atherosclerosis (Ross et al., 1977). We have shown that cadmium exhibits strong cytotoxicity to vascular endothelial cells (Kaji et al., 1992).
Endothelial perlecan levels are increased by cadmium (Ohkawara et al., 1997). On the other hand, previous studies on CS/DSPGs have been limited to the increase of CS/DS chains by cadmium (Kaji et al., 1994). The present study aimed to characterize the increase in the CS/DS chain in cadmium-exposed vascular endothelial cells.
Bovine aortic endothelial cells were purchased from Cell Applications (San Diego, CA, USA). Tissue culture dishes and plates were obtained from AGC Techno Glass (Shizuoka, Japan). Dulbecco’s modified Eagle’s medium (DMEM) and Ca2+- and Mg2+-free phosphate-buffered saline (PBS) were obtained from Nissui Pharmaceutical (Tokyo, Japan). Fetal bovine serum (FBS), the BCA protein assay and high-capacity cDNA reverse transcription kit, and CHSY1 antibody (PA5-48601) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Cadmium chloride, 4α-phorbol 12,13-didecanoate (4α-PDD), and mouse monoclonal antibody against β-actin were obtained from Fujifilm Wako Pure Chemical Industries (Osaka, Japan). Rabbit monoclonal antibody against biglycan (ab94460), phorbol 12-myristate 13-acetate (PMA), and the classical protein kinase C (PKC) inhibitor GF109203X were obtained from Abcam (Bristol, UK). Mouse monoclonal antibody against PKC (sc-80) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Horseradish peroxidase (HRP)-conjugated anti-rabbit (#7074) and HRP-conjugated anti-mouse (#7076) IgG antibodies were procured from Cell Signaling Technology (Beverly, MA, USA). Chondroitinase ABC, papain, and diethylaminoethyl (DEAE)-Sephacel were obtained from Sigma-Aldrich (St Louis, MO, USA). Heparinase II (derived from Flavobacterium heparinum) and heparinase III (EC 4.2.2.8, derived from F. heparinum) were obtained from IBEX Technologies (Montreal, Quebec, Canada). [35S]Na2SO4 ([35S]sulfate) and D-[6-3H(N)]glucosamine hydrochloride ([3H]glucosamine) were obtained from PerkinElmer (Waltham, MA, USA). Sepharose CL-4B, Sepharose CL-6B, PD-10 columns, and Amersham Hybond P PVDF 0.2 were purchased from Cytiva (Marlborough, MA, USA). Negative control siRNA, ISOGEN II, and GeneAce SYBR qPCR mix α were obtained from Nippon Gene (Tokyo, Japan). Other reagents, of the highest grade available, were purchased from Nacalai Tesque (Kyoto, Japan).
Cell culture and treatmentsVascular endothelial cells were cultured in a DMEM containing 10% FBS and maintained in a humidified atmosphere with 5% CO2 at 37°C until confluent. The cells were then transferred into 6-well plates or 100-mm dishes and cultured until confluent. After the medium was discarded, the cells were washed twice with serum-free DMEM and treated with cadmium (0.1, 0.2, 0.5, 1, or 2 μM) for 4, 6, 8, 12, or 24 hr in the presence or absence of GF109203X (an inhibitor for PKCα/β/γ; 5 or 10 μM). The detailed conditions are described in corresponding figure legends. The experiments performed after the treatment are detailed in the following sections.
siRNA transfectionThe transfection of siRNAs was performed using Lipofectamine RNAiMAX as described previously (Hara et al., 2021a), with slight modifications. Briefly, annealed siRNA duplexes and Lipofectamine RNAiMAX were dissolved in Opti-MEM in separate tubes and incubated for 5 min, followed by mixing and incubation for 20 min. Vascular endothelial cells were grown to near subconfluence in DMEM supplemented with 10% FBS and then incubated at 37°C in a fresh DMEM supplemented with 10% FBS in the presence of the siRNA/Lipofectamine RNAiMAX mixture. The final concentrations of siRNA and Lipofectamine RNAiMAX were 18 nM and 0.09%, respectively. After 24 hr, the medium was changed to DMEM, and the cells were treated with cadmium for 24 hr. The siRNA sequences were as follows: bovine PKCα siRNA (siPKCα), 5′-AGCAGUGCGUGAUCAACGUdTd-3′ (sense), and 5′-ACGUUGAUCACGCACUGCUdTdT-3′ (antisense) bovine PKCβ siRNA (siPKCβ), 5′-GCAUGCAUUUUUCCGGUAUdTdT-3′ (sense), and 5′-AUACCGGAAAAAUGCAUGCdTdT-3′ (antisense), and bovine PKCγ siRNA (siPKCγ), 5′-CCCCAACGGUCUCUCCGAUdTdT-3′ (sense), and 5′-AUCGGAGAGACCGUUGGGGdTdT-3′ (antisense).
Characterization of PGsThe [35S]sulfate and [3H]glucosamine-labeled PGs were extracted from the cell layer. The conditioned medium of dense vascular endothelial cells was exposed to cadmium (2 µM) for 24 hr in a serum-free DMEM in the presence of [35S]sulfate (2 MBq/mL) and [3H]glucosamine (2 MBq/mL) and characterized using DEAE-Sephacel after desalting as we previously reported (Ohkawara et al., 1997). The fractions obtained from DEAE-Sephacel were collected and further separated using Sepharose CL-4B or Sepharose CL-6B after digestion by chondroitinase ABC, heparinase II/III, or papain as described previously (Hara et al., 2023).
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)Extraction of the total RNA from vascular endothelial cells was performed using ISOGEN II as previously described (Hara et al., 2021b). Complementary DNA was synthesized with a high-capacity cDNA reverse transcription kit on extracted total RNA. qRT-PCR was performed using GeneAce SYBR qPCR mix α with 1 ng/µL cDNA and 0.1 µM primers (Table 1) in a CFX connect real-time PCR system (BioRad, Hercules, CA, USA). Biglycan, CHSY1, CHSY3, CHPF, CHPF2, CSGalNAcT1, CSGalNAcT2, exostosin-1 (EXT1), EXT2, EXT-like-1 (EXTL1), EXTL2, EXTL3, PKCα, and beta-2 microglobulin (B2M) transcript levels were analyzed quantitatively using the relative standard curve method. The fold change in intensity of the target gene was normalized to that of B2M. qRT-PCR was performed in three technical replicates and reproducibility was confirmed using samples obtained from independent experiments, at least twice.
| Gene | Forward primer (5’ – 3’) | Reverse primer (5’ – 3’) |
|---|---|---|
| Biglycan | GCTGCCACTGCCATCTGAG | CGAGGACCAAGGCGTAG |
| CHSY1 | ATCAATGCCAACGCCAAGA | CAGAAGCAGCAAGTCCAGAATG |
| CHSY3 | GGTTCTCAGGAGGATGGTGCC | ATGGATTTTGCTGTTGTGAAGGTC |
| CHPF | AAGTTGCTCGTGGCGGTG | CTCAGTGTAGGTGGCATCGGTA |
| CHPF2 | CGACCAGCACTGCCCCTC | TTTCCCCACAGCCTCTACGC |
| CSGalNAcT1 | GGTCTTGCTCGTGCTTCTCTG | CGGTGACGCTCCTCCCA |
| CSGalNAcT2 | GAGAAAGAATGTAGGGGCTAATGG | TCAGGATGGCGAGTGAGACC |
| EXT1 | GAAAAACGGCTTCAAAGTCTACG | CTCAGGACAAAGAGGCACGC |
| EXT2 | TGCCTGTTCGTCCCGTCCA | CGTAGCCTTGCCGATAAGTCCAC |
| EXTL1 | GGCTTGCCTCCTCATCCTCGTC | GCCACCATCGCCTGTCCCA |
| EXTL2 | AGATGCTCACTTTGCGTAGGGA | ATTCTGTTTGTGGTCTGTGCTTTG |
| EXTL3 | TTTGAGGAGGAGATGGAGGGG | CGGCGTGATGATGAGGGC |
| PKCα | AGCCGCAAGCAGTGTTCTATG | TGTGTCCTTCCGAGTCCAG |
| B2M | CCATCCAGCGTCCTCCAAAGA | TTCAATCTGGGGTGGATGGAA |
PGs accumulated in the cell layer were extracted from the whole cell lysate, and the conditioned medium of vascular endothelial cells were concentrated as previously reported (Hara et al., 2020). Concentrated PGs were digested with 1.67 U/mL chondroitinase ABC in 50 mM Tris–HCl buffer (pH 8.0) containing 0.1 mg/mL bovine serum albumin and 3 mM sodium acetate for 3 hr at 37°C. The biglycan core protein was detected by SDS-PAGE and Western blot analysis as well as other proteins after CS/DS chain digestion (Hara et al., 2021a). Western blot analysis was performed using samples obtained from multiple independent.
Statistical analysisData were analyzed for statistical significance using a Student’s t-test or Tukey’s method after ANOVA analysis. Results with p < 0.05 were considered statistically significant.
First, we investigated the effect of cadmium on PG synthesis by vascular endothelial cells using DEAE-Sephacel anion exchange chromatography to analyze PG characteristics based on differences in charge density. The PGs synthesized in the cell layer and conditioned medium were separated into three peaks. Although [35S]sulfate incorporation was not significantly altered, [3H]glucosamine incorporation was increased by cadmium exposure in all peaks in both the cell layer and conditioned medium (Fig. 1). Since we have reported that the CS/DSPGs elute with approximately 0.55 M NaCl in conditioned medium (Kaji et al., 2004), the fractions were collected and analyzed in detail for the components. The collected fractions were concentrated and digested with chondroitinase ABC, heparinase II/III, or papain, and separated using Sepharose CL-4B size exclusion chromatography. The PG subclass eluted at Kav = 0.40 in this subclass was sensitive to digestion with both chondroitinase ABC and papain but resistant to digestion with heparinase II/III (Fig. 2), suggesting that this fraction contains CS/DSPGs, as we have previously reported (Kaji et al., 2000).
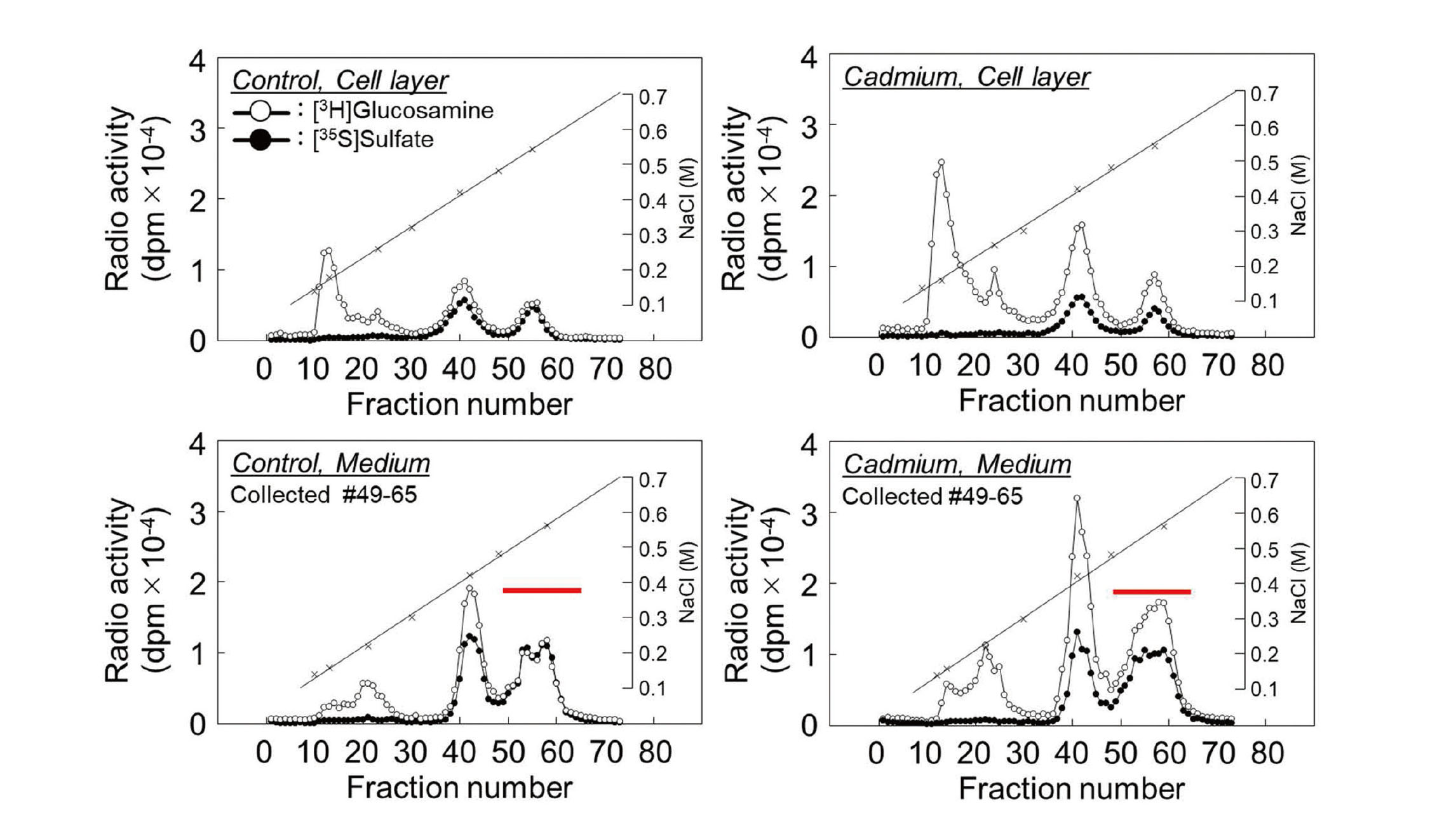
Results of DEAE-Sephacel ion exchange chromatography of [3H]glucosamine- and [35S]sulfate-labeled molecules extracted from the cell layer and the conditioned medium of dense vascular endothelial cells after exposure to cadmium. Confluent cultures of bovine aortic endothelial cells were incubated with (right panels) or without (left panels) cadmium at 2 µM for 24 hr in the presence of [3H] glucosamine and [35S] sulfate. Red horizontal bars indicate the fractions which were pooled and chromatographed on a Sepharose CL-4B and CL-6B column (shown in Figs. 2 and 3, respectively).
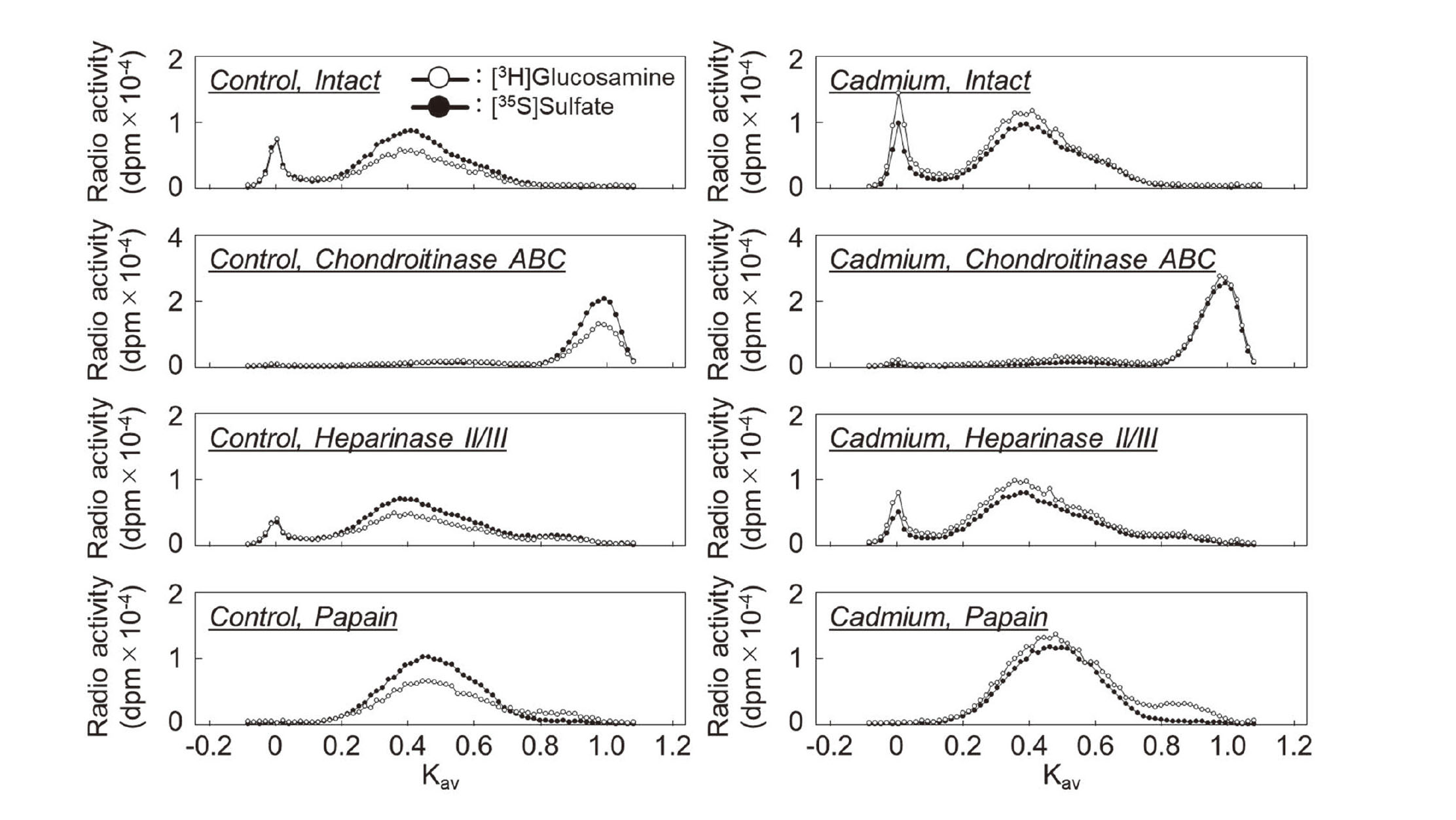
Results of Sepharose CL-4B gel filtration chromatography of chondroitin/dermatan sulfate proteoglycans. The fractions of conditioned medium derived from DEAE-Sephacel chromatography (shown in Fig. 1) were concentrated and separated using Sepharose CL-4B column after digestion with chondroitinase ABC, heparinase II/III, or papain.
To examine the effect of cadmium on the length of CS/DS chains secreted into conditioned medium, a part of the DEAE-Sephacel fraction was separated using Sepharose CL-6B size exclusion chromatography, after digestion with papain. The both peaks of [3H]glucosamine and [35S]sulfate eluted at Kav = 0.40 in the control group shifted to Kav = 0.35 in the cadmium-treated group (Fig. 3). This indicates that cadmium elongates CS/DS chains by about 20 sugars, increasing the relative molecular mass from 35100 to 40000 (Wasteson, 1971).
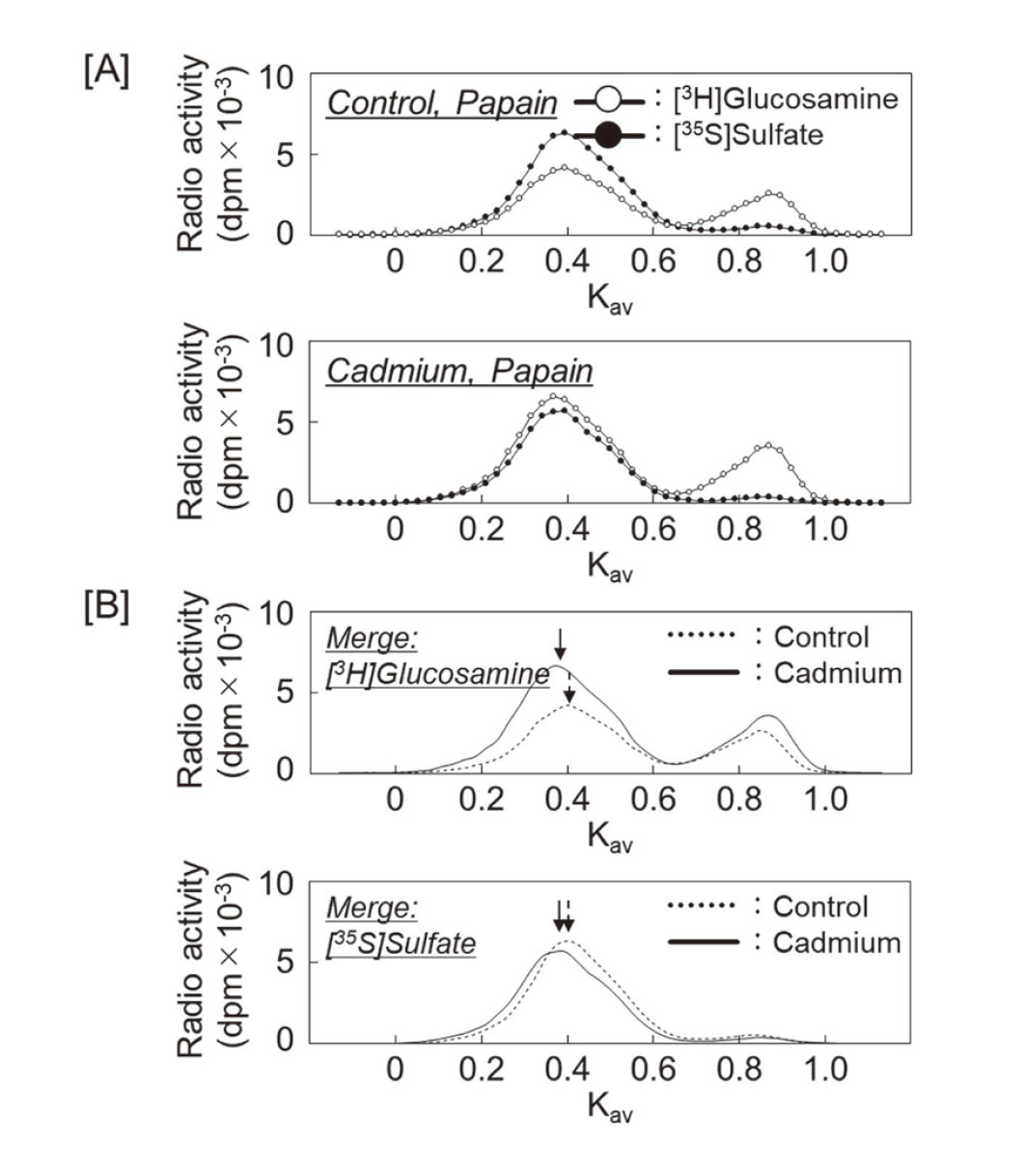
Results of Sepharose CL-6B gel filtration chromatography of chondroitin/dermatan sulfate chains. [A] The fractions of conditioned medium derived from DEAE-Sephacel chromatography (shown in Fig. 1) were concentrated and separated using Sepharose CL-6B column after digestion with papain. [B] The values obtained from each nuclide were overlaid. Arrows indicate the peak of elution.
We analyzed biglycan expression in cadmium-treated vascular endothelial cells but found no significant changes in its mRNA or core protein expression (Figs. 4A and 4B). Subsequently, we investigated the effect of cadmium on the expression of glycosyltransferases to clarify the cause of cadmium-induced elongation of CS/DS chains. The screening for expression of elongation enzymes for CS/DS chains, including CHSY1, CHSY3, CHPF, CHPF2, CSGalNAcT1, and CSGalNAcT2, showed a concentration-dependent induction of CHSY1 mRNA expression in the treatment with cadmium (Fig. 5A). Although the expression of mRNAs coding for enzymes involved in heparan sulfate chain elongation, including EXT1, EXT2, EXTL1, EXTL2, and EXTL3, was also investigated, no change was observed before and after cadmium exposure (Fig. 5B). Especially, CHSY1 mRNA expression was induced by cadmium in a time-dependent manner, and CHSY1 protein expression also increased (Figs. 6A and 6B).
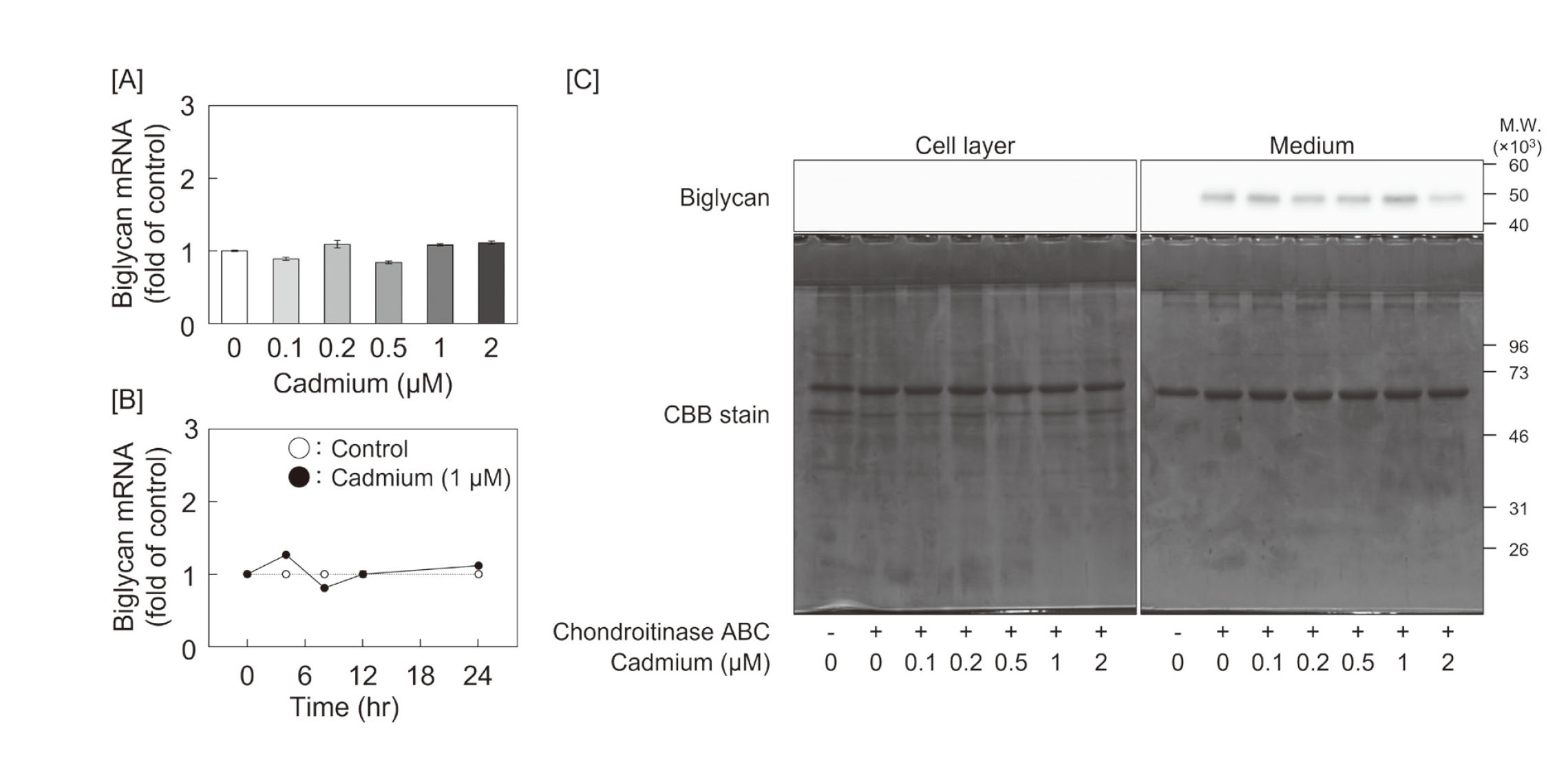
Expression of biglycan mRNA and core protein in vascular endothelial cells exposed to cadmium. Vascular endothelial cells were exposed to cadmium [A and C] at 0.1, 0.2, 0.5, 1 and 2 µM for 24 hr or [B] at 1 µM for 4, 8, 12, and 24 hr. Values are means ± standard error (S.E.) of three samples.

Effect of cadmium on [A] chondroitin/dermatan sulfate and [B] heparan sulfate elongation enzymes mRNA expression in vascular endothelial cells. Vascular endothelial cells were treated with cadmium at 2 µM for 24 hr. Values are means ± S.E. of three technical replicates. *p < 0.05; **p < 0.01 vs. the corresponding control.
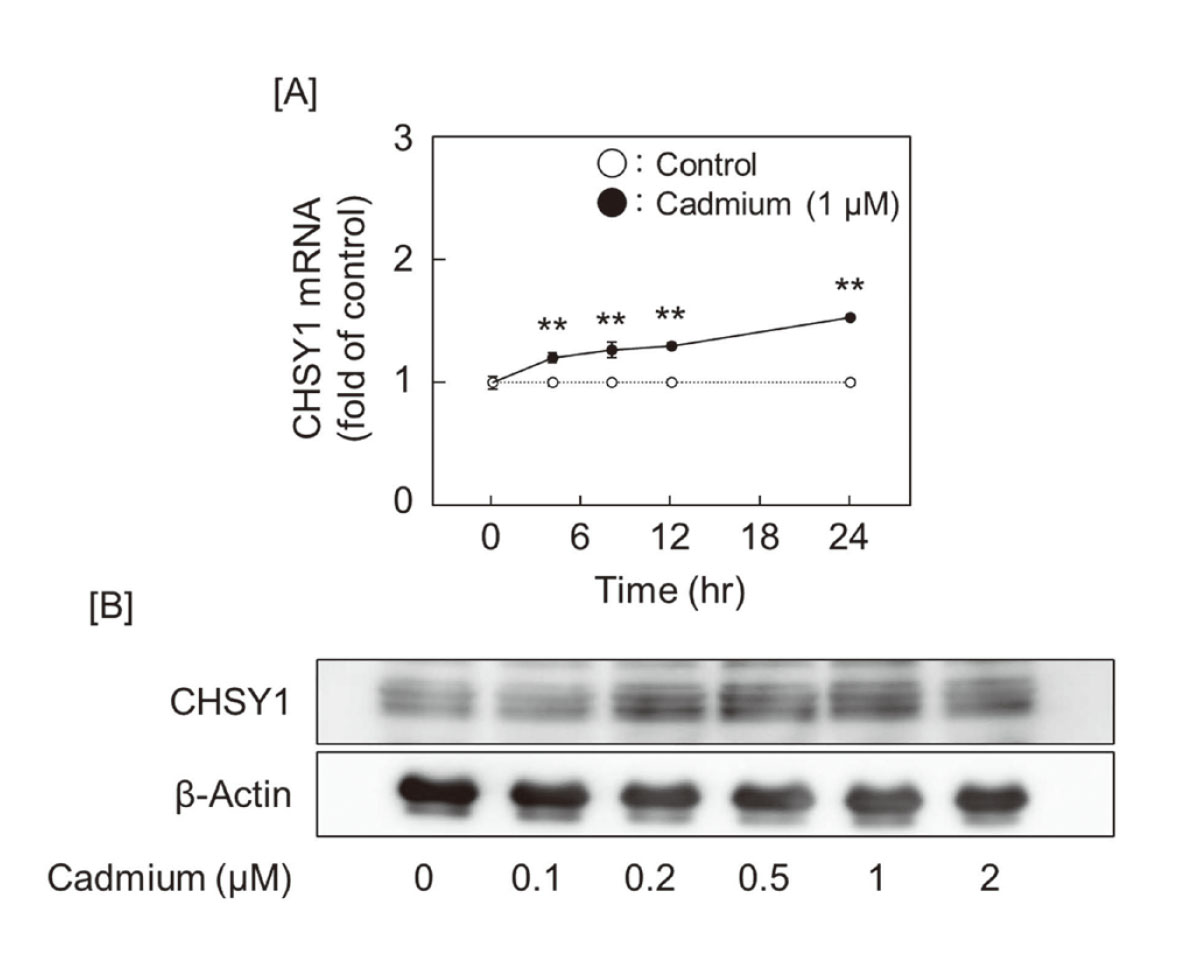
Cadmium induces CHSY1 mRNA and protein expression in vascular endothelial cells. Vascular endothelial cells were treated with cadmium [A] at 1 µM for 4, 8, 12, or 24 hr, and [B] at 0.1, 0.2, 0.5, 1, or 2 µM for 24 hr. Values are means ± S.E. of three technical replicates. **p < 0.01 vs. the corresponding control.
Since cadmium activates PKC (Romare and Lundholm, 1999), we investigated the involvement of PKC in the expression of CHSY1. CHSY1 mRNA and protein were induced by treatment with PMA, a broad-spectrum PKC activator, in a concentration-dependent manner in vascular endothelial cells (Figs. 7A and 7B). However, CHSY1 mRNA was not induced by treatment with 4α-PDD, a phorbol ester lacking PKC activating ability (Fig. 7C). These results indicate that PKC contributes to CHSY1 expression. The induction of CHSY1 by cadmium abolished pretreatment with GF109203X, a specific inhibitor of PKCα/β/γ (Figs. 7D and 7E).
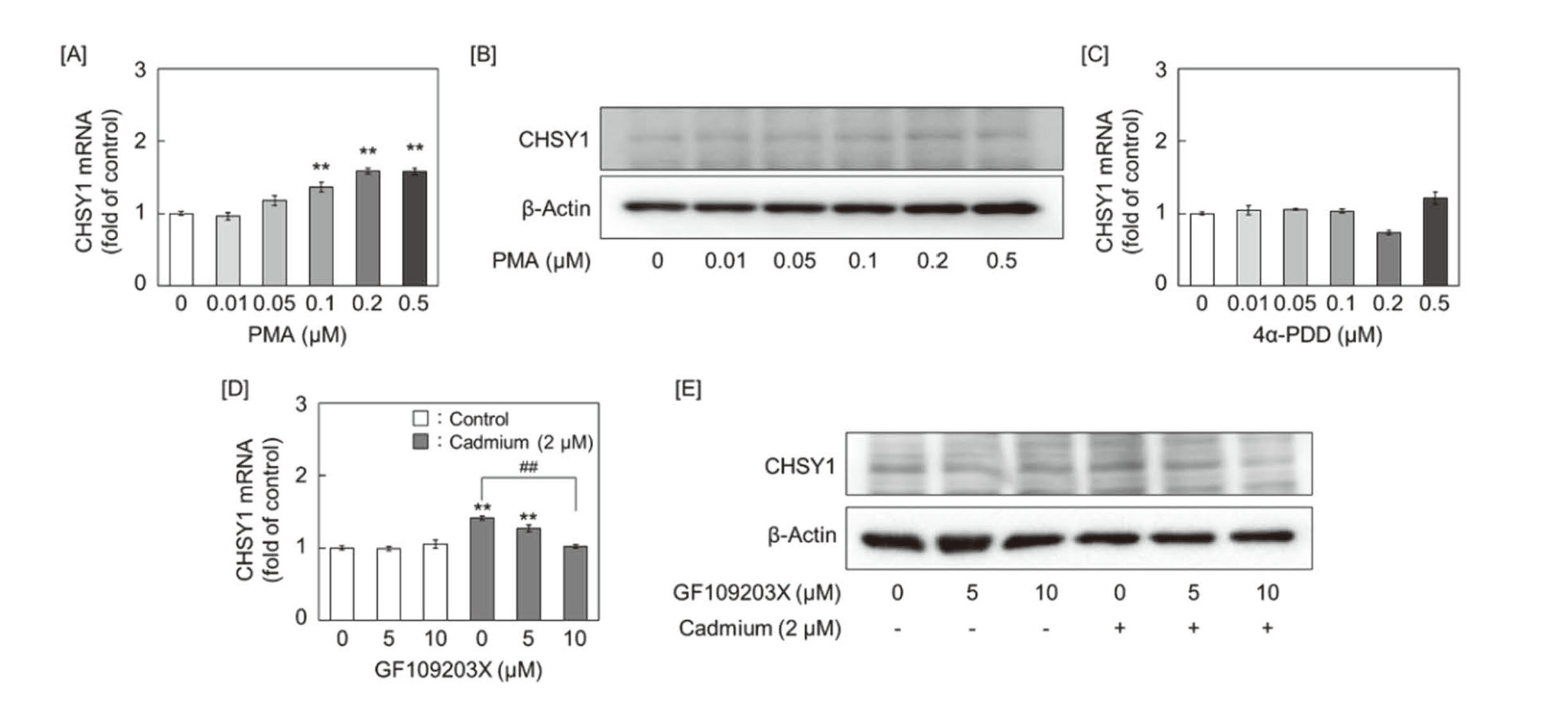
Protein kinase C (PKC) is involved in cadmium-mediated CHSY1 induction in vascular endothelial cells. Vascular endothelial cells were treated with [A and B] phorbol 12-myristate 13-acetate (PMA) at 0.01, 0.05, 0.1, 0.2, or 0.5 µM for [A] 6 and [B] 24 hr, or [C] 4α-phorbol 12,13-didecanoate (4α-PDD) at 0.01, 0.05, 0.1, 0.2, or 0.5 µM for 6 hr. [D and E]. Vascular endothelial cells were pretreated with GF109203X at 5 or 10 µM for 2 hr and then treated with cadmium at 2 µM for 24 hr. Values are means ± S.E. of three technical replicates. **p < 0.01 vs. the corresponding control.
Finally, we analyzed the PKC isoforms that contribute to the induction of CHSY1 by cadmium. Protein expression of PKC isoforms expressed in endothelial cells was examined using antibodies recognizing the common region of PKCα/β/γ. A considerable band attenuation was observed only in the PKCα siRNA transfected cells (Fig. 8A), indicating that PKCα is the major isoform in vascular endothelial cells. In addition, the induction of CHSY1 by cadmium was significantly suppressed when PKCα expression was suppressed (Figs. 8B and 8C).

Cadmium upregulates CHSY1 via PKCα in vascular endothelial cells. [A] Vascular endothelial cells were transfected with PKCα, PKCβ, or PKCγ siRNA for 24 hr and western blotting was performed using an antibody to react with PKCα/β/γ. [B] Vascular endothelial cells were treated with cadmium at 1 and 2 µM for 24 hr after transfecting with PKCα siRNA for 24 hr.
PGs, particularly CS/DSPGs, have an ability to bind LDL and elongation of the CS/DS chains allows for more LDL binding (Sambandam et al., 1991; Mourão and Bracamonte, 1984; Little et al., 2002). Importantly, the formation of the LDL-PG complexes increases the accumulation of LDL in macrophages (Salisbury et al., 1985) and accelerates the progression of atherosclerotic lesions. We characterized the CS/DS chains of biglycan synthesized by vascular endothelial cells after exposure to cadmium and observed the following results: (1) cadmium elongated CS/DS chains of biglycan secreted into the conditioned medium from vascular endothelial cells, (2) biglycan core protein expression was not induced by cadmium, (3) cadmium induced CHSY1 expression in a concentration- and time-dependent manner, and (4) the induction of CHSY1 expression by cadmium was mediated by PKCα. These results clearly indicated that cadmium elongates the CS/DS chains of biglycan via induction of CHSY1 expression mediated by PKC. The mechanisms underlying the involvement of cadmium in atherosclerosis may include the intimal accumulation of LDL caused by biglycan with elongated CS/DS chains.
In vivo atherosclerosis model experiments in rabbits showed a higher activation of PKCα in the vessel wall of the high-fat diet group (Li et al., 2015). Moreover, in vitro experiments using human aortic endothelial cells, aortic smooth muscle cells, and monocyte-derived THP-1 cells have shown that the activation of PKCα is increased by oxidized LDL cholesterol treatment (Li et al., 2015). In the light of these observations, we expect a series of plaque-forming processes in atherosclerotic vessel walls, such as an increase in CHSY1 via PKCα activation by oxidized LDL cholesterol and accelerated oxidation and accumulation of LDL cholesterol via elongated CS/DS chains. Moreover, some studies have reported that endothelial hyperpermeability occurs in a PKCα-dependent manner (Mehta et al., 2001; Sandoval et al., 2001). These reports indicate that monocyte subendothelial invasion is facilitated and that the exacerbation of atherosclerotic lesions with plaque formation (Wight, 2018) is further accelerated.
Cadmium is not only a risk factor for atherosclerosis, but also for hypertension (Pinheiro Júnior et al., 2020; Martins et al., 2021). Angiotensin-II, a hypertensive protein, promotes the synthesis of proteoglycans that are highly bound to LDL (Figueroa and Vijayagopal, 2002). Angiotensin-II causes activation of PKCs and mitogen activate protein kinases (MAPKs) after binding to angiotensin II type I receptor (AT1R) (Verma et al., 2021), and cadmium also activates PKCs and MAPKs pathways (Park et al., 2012). Therefore, we hypothesized that cadmium would disturb the downstream AT1R and focused on the molecules in the present study. Although MAPK-mediated CHSY1 induction was not observed (data not shown), this is the first report to show that PKCα induces CHSY1 and to suggest its contribution to CS/DS chain synthesis.
Cadmium induced metal transporter ZIP-8 via NFkB and JNK pathways further accelerates cadmium accumulation in vascular endothelial cells and exacerbates cell injury (Fujie et al., 2022). Additionally, cadmium-mediated plasminogen activator inhibitor type 1 upregulation via Smad2/3 suppresses the fibrinolytic system (Hara et al., 2021b). Throughout these previous studies of ours, the mechanisms for the destruction of endothelial cell function by cadmium have been elucidated. The present study revealed that cadmium elongates CS/DS chains synthesized by endothelial cells via activation of PKCα and induction of CHSY1, and provides new evidence that cadmium contributes to the development of atherosclerotic lesions by disturbing the environment in which endothelial cells live.
This research was funded by JSPS KAKENHI Grant Numbers JP 19K19418 and JP 22K17355 (to T.H.), The Research Foundation for Pharmaceutical Sciences (to T.H).
Conflict of interestThe authors declare that there is no conflict of interest.